Abstract
Mammalian DAI (DNA-dependent activator of IFN-regulatory factors), an activator of the innate immune response, senses cytosolic DNA by using 2 N-terminal Z-DNA binding domains (ZBDs) and a third putative DNA binding domain located next to the second ZBD. Compared with other previously known ZBDs, the second ZBD of human DAI (hZβDAI) shows significant variation in the sequence of the residues that are essential for DNA binding. In this article, the crystal structure of the hZβDAI/Z-DNA complex reveals that hZβDAI has a similar fold to that of other ZBDs, but adopts an unusual binding mode for recognition of Z-DNA. A residue in the first β-strand rather than residues in the β-loop contributes to DNA binding, and part of the (α3) recognition helix adopts a 310 helix conformation. The role of each residue that makes contact with DNA was confirmed by mutational analysis. The 2 ZBDs of DAI can together bind to DNA and both are necessary for full B-to-Z conversion. It is possible that binding 2 DAIs to 1 dsDNA brings about dimerization of DAI that might facilitate DNA-mediated innate immune activation.
Keywords: circular dichroism, hydrogen bonding, interferon induction, X-ray crystallography, innate immunity
The innate immune response is essential for protection from foreign invasion, acting as an immediate cellular defense mechanism. Nucleic acids are known as one of the triggers for activation of the innate immune response (1–3). Recent reports have indicated the presence of a new cytosolic DNA sensor that can initiate an innate immune response independent of the endosomic Toll-like receptor 9 (4, 5). Z-DNA binding protein 1 (ZBP1), also known as DLM-1, was identified as the first innate immune activator that senses cytosolic DNAs (4). In response to foreign DNA, this protein activates type I IFN and other immune responses and was therefore named DAI (DNA-dependent activator of IFN-regulatory factors; ref 4). DAI contains 2 tandem Z-DNA binding domains (ZBDs or Zα and Zβ) at its N terminus and a third DNA binding region (D3) located next to the second ZBD. D3 is a novel domain and is reported to bind right-handed B-DNA (4). Upon activation, the C terminus of DAI binds to Tank binding kinase 1 (TBK1), a serine/threonine kinase, and to IFN regulatory factor 3 (IRF3), a transcription factor (4). The N-terminal region, including D3, is thought to be essential for sensing DNA, as shown by its ability to bind to Z-DNA and synthetic B-DNA (4). For the full activation of an in vivo DNA-dependent immune response, all 3 DNA-binding regions are required (5). At the molecular level, it has been suggested that dimerization of DAI results in activation of the innate immune response (5). At the cellular level, it is known that the localization of DAI and its association with stress granules is regulated by ZBDs (6, 7).
ZBDs that are found in DAI are also found in the editing enzyme dsRNA adenosine deaminase (ADAR1) in vertebrates and in fish PKZ protein kinase containing Z-DNA-binding domains and in the E3L of pox viruses (Fig. 1). They all bind tightly and specifically to Z-DNA and usually are able to convert B-DNA to Z-DNA (8–14). Structural studies of ZBDs have shown that they all share an α/β architecture consisting of 3 β-strands and 3 α-helices (9, 15–17). Although they have limited similarity at the amino acid sequence level, they share a common binding mode to the Z conformation of DNA with many conserved residues involved in DNA binding. ZBDs recognize Z-DNA with conformational specificity by using the recognition helix α3 and the wing, which is made up mostly of an antiparallel β-sheet (β2 and β3) and a β-loop (9, 15–17). In their interaction with Z-DNA, positively charged or polar residues play major roles in recognizing the zig-zag conformation of the Z-DNA backbone. There are many direct ionic interactions or water-mediated hydrogen-bonding interactions with the DNA backbone phosphate groups (15). In addition, the CH–π interaction between Tyr and the C8 of guanine in the syn conformation is known to be a key interaction for Z-DNA specific binding (15). The β-sheet wing also contributes to the interaction through water-mediated hydrogen bonds and hydrophobic interaction involving Trp, Thr, and Pro residues (15).
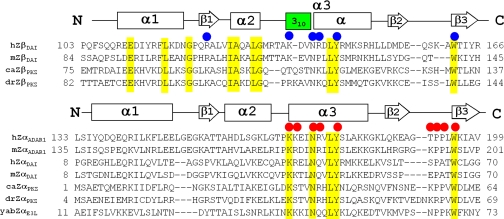
Fig. 1.
Multiple sequence alignments of the first ZBDs (Zα) and the second ZBDs (Zβ). Zαs include human ADAR1 (hZαADAR1), mouse ADAR1 (mZαADAR1), human DAI (hZαDAI), mouse DAI (mZαDAI), goldfish PKZ (caZαPKZ), zebrafish PKZ (drZαPKZ), and Yaba-like disease poxvirus E3L (yabZαE3L). Zβs include human DAI (hZβDAI), mouse DAI (mZβDAI), goldfish PKZ (caZβPKZ), and zebrafish PKZ (drZβPKZ). The secondary structures of hZβDAI and hZαADAR1 are shown by a rectangle indicating a helix or an arrow for a β-strand. The 310 helix is shown in green. Residues of hZβDAI and hZαADAR1 involved in DNA interactions are marked by blue and red circles, respectively. Highly conserved residues are highlighted in yellow. The residue numbers of both N and C termini are shown.
In DNA-mediated activation of the innate immune response, the N-terminal ZBDs are believed to be essential for the full activity of DAI (4, 5). The first DNA binding domain of DAI (ZαDAI, also known as ZαDLM-1 or ZαZBP1) was well characterized as a canonical ZBD in structural and biochemical studies (9). The second ZBD (ZβDAI) was also shown to bind Z-DNA based on its binding specificity for Z-DNA and its ability to convert B-DNA to Z-DNA, as measured in the CD spectrum (6, 14). However, some residues that are known to mediate ZBD/DNA interactions in other systems are not present in hZβDAI, suggesting that the binding mode of ZβDAI with DNA might be different from that of other canonical ZBDs (Fig. 1). In particular, the conserved residues in the β-loop of the wing region are missing in hZβDAI (Fig. 1). Therefore, an intriguing question emerges as to how this second ZBD recognizes Z-DNA despite the limited sequence similarity to canonical ZBDs. In addition, it would also be interesting to explore how hZαβDAI, which consists of hZαDAI, hZβDAI, and a linker, recognizes DNA and to determine the role of DNA binding by hZαβDAI in the innate immune response.
To address these questions about the binding mode of hZβDAI to Z-DNA, we have solved the crystal structure of hZβDAI complexed with dsDNA. In addition, we have carried out Z-DNA converting assays of hZβDAI and mutants that lack some of the residues involved in Z-DNA binding found in other structures. Although hZβDAI has the common overall fold of ZBDs, its binding mode to DNA is clearly distinguishable from that of other ZBDs. In particular, residues in the β-loop of the wing do not engage Z-DNA at all and are not directly involved in its recognition. Furthermore, biochemical studies of hZαβDAI reveal that both ZBDs of DAI are involved in DNA binding, and such binding could play a role in the proposed oligomerization and activation of DAI (5).
Results
Overall Structure of the hZβDAI/Z-DNA Complex.
The structure of hZβDAI (residues 103–166) bound to dsDNA in the Z conformation, d(TCGCGCG)2, was solved at 1.45-Å resolution by using multiwavelength anomalous dispersion (MAD) data with an R factor of 15.3% and an Rfree of 19.3% (Table S1). The asymmetric unit contains 2 hZβDAI molecules called chain A and chain B, and 1 duplex DNA in the Z conformation (Fig. 2A). The DNA strands facing protein chains A and B are designated as C and D, respectively (Fig. 2A). The 2 hZβDAI/ssDNA complexes are related by pseudo noncrystallographic 2-fold symmetry in the asymmetric unit and are nearly identical with 0.68-Å rmsd between 63 Cα atoms of the protein and all atoms of the 6 nucleotides (pCpGpCpGpCpG). The 5′-deoxythymine of chain C and R166 residues in chains A and B were not modeled because of structural flexibility.
Fig. 2.
Structural comparison of hZβDAI and hZαADAR1 and their interactions with Z-DNA. (A) Overall structure of the hZβDAI/Z-DNA complex. The protein and DNA are drawn as a ribbon diagram and stick model, respectively. The N and C termini, the secondary structure elements of hZβDAI, and 5′ and 3′ of DNA are labeled. (B) The structural overlap of hZβDAI and hZαADAR1 near the Z-DNA binding site. The ribbon diagrams of hZβDAI (blue) and hZαADAR1 (green) are overlapped, and the stick model of a double-strand Z-DNA is drawn in brown and gray. Structural deviation near α3 and the β-wing are labeled and marked by red circles. (C) Stereoviews of the protein–DNA interfaces of hZβDAI (chain A)/Z-DNA (Left) and hZβDAI(Chain B)/Z-DNA (Right). The Cα chains of the ZBDs are colored sky blue, and the residues involved in DNA contact are depicted in sky blue stick models. The Z-DNA backbone is drawn as a red stick model, and bases are drawn as gray stick models. Water molecules are shown in green. Hydrogen bonds are drawn as dashed lines. (D) Schematic diagrams of the protein–Z-DNA interactions in hZβDAI (Left) and hZαADAR1 (Right). Hydrogen bonds and van der Waals contacts are represented by dashed black lines and pink lines, respectively. The CH–π interaction between the conserved Tyr and the C8 of a syn-guanine is indicated by black circles, and waters are shown by green circles. The protein–DNA interactions in hZαADAR1 are identical on both sides of the Z-DNA, thus only 1 side is shown.
Similar to other structures of ZBDs bound to Z-DNA (9, 15, 16), hZβDAI also has α/β topology with 3 helices (α1, α2, and α3) packed against 3 β-stands (β1, β2, and β3) (Fig. 2A and Fig. S1). However, hZβDAI differs from other ZBDs by the presence of a 310 helix at the N terminus of α3 (Fig. 2 and Fig. S1). Instead of the long continuous α-helix (α3) present in other Zαs, hZβDAI has a kinked helix with a mixed conformation of 310- and α-helices. Furthermore, the most unique conformational difference in hZβDAI is found in the β-sheet wing, where hZβDAI has disengaged the β-loop of the wing from Z-DNA so it no longer makes contact (Fig. 2B and Fig. S1). Thus, the β-loop of hZβDAI has limited interaction with Z-DNA. In the complex, dsDNA adopts a typical Z-DNA conformation, with guanine nucleotides in the syn conformation and C3′-endo sugar puckering, except for G6, which is C2′ -endo. Whereas previous Z-DNA structures have the ZI conformation in their phosphodiester backbones (9, 15, 16), both strands of Z-DNA bound to hZβDAI have a ZII conformation for G4pC5, which further illustrates the different binding mode of hZβDAI compared with other ZBDs (Fig. S2).
Protein–DNA Interactions in the Recognition Helix.
Interactions mediated by the most conserved core residues of ZBDs (Y145, N141, and W162) are maintained in the hZβDAI/Z-DNA complex, although the position of the water molecules involved in hydrogen bonding varies somewhat. In hZβDAI, N141 forms 1 direct and 1 water-mediated interaction with the phosphate backbone of Z-DNA, whereas N173 of hZαADAR1 contacts DNA through 2 water-mediated hydrogen bonds (Fig. 2C and Fig. S3) (15). However, except for those core residues, the other interactions with Z-DNA seem to be different for hZβDAI. It is likely that R142 of hZβDAI plays a role in Z-DNA recognition similar to R174 of hZαADAR1, but hZβDAI does not form a bond with the ribose ring of G6, as found in hZαADAR1 (Fig. 2 C and D and Fig. S3). Also, K138 of hZβDAI is located in a region that is between K169 and K170 of hZαADAR1. However, K138 of hZβDAI plays a significantly different role than the 2 positively charged residues in other ZBDs (Fig. 2 C and D and Fig. S3). K138 forms a bond to the phosphate atom of the DNA strand and has only limited additional interactions, whereas the 2 positively charged residues of hZαADAR1 contact the 4 phosphate groups of 1 DNA strand by using hydrophobic and coulombic interactions. It is interesting that K138 spans across the Z-DNA molecule and interacts with the C5 phosphate group on the opposite DNA strand. Cross-interactions of this type are not found in ZαADAR1 (Fig. 2D). For K138 to point to and contact DNA in that way, a conformational change would be necessary in the recognition helix (α3) and the following loop. Indeed, the N-terminal end of the recognition helix is changed to a 310 conformation and the following β-loop is moved away from the DNA and toward α2 (Fig. 2C and Fig. S3).
Protein–DNA Interactions in the Wing.
Most ZBDs have 1 or 2 Pro residues that contribute to DNA binding through hydrophobic interactions plus polar residues, such as Thr or Asn, that bind the DNA through water-mediated hydrogen bonds (Fig. 2 and Fig. S3) (9, 15, 16). Moreover, Pro residues in this position are known to be essential for the pathogenicity of the vaccinia E3L protein, which has a ZBD in its N-terminal region (18). hZβDAI does not have residues that play a role equivalent to Pro or Thr in the wing region (Fig. 1), but it still has nearly the same Z-DNA-converting activity as hZαADAR1 (6, 14). Therefore, it was anticipated that there would be another residue in the wing region that must bind to Z-DNA. Recent mutagenesis data suggest that K160 might play this role, because mutation of K160 into Glu reduced Z-DNA binding activity (19). Although the β-loop in the wing of hZβDAI is disengaged from the DNA compared with other ZBDs (Fig. 2), K160, located in the tip of the loop, might reach the backbone phosphate group of Z-DNA. But, in the current crystal structure, K160 is unlikely to be involved in DNA binding because the side chain of K160 in each subunit is disordered (Fig. 3). Thus, neither K160 nor any other residue in the β-loop of hZβDAI, systematically participates in DNA recognition.
Fig. 3.
The Z-DNA binding mode of hZβDAI. (A) Z-DNA recognition by R124 of hZβDAI. A magnified view near the wing region is drawn for the interfaces between hZβDAI and Z-DNA [chains A and C (Left) and chains B and D (Right)]. A σ-weighted 2 Fo − Fc omit map contoured at 1.2 σ was generated by omitting R124, R160, and the waters involved in the R124-mediated interaction. Possible hydrogen bonds among R124, water and the phosphate group of G2 are shown by dashed lines, and their distances are indicated in Å. (B) Surface charge distributions of the chain A (Left) and chain B (Right) viewed along the DNA binding cleft. The red and blue areas represent negatively and positively charged surfaces, respectively. Z-DNA chains are shown as stick models, and R124, K160 and each nucleotide are labeled.
However, an unusual Z-DNA binding mode is found in the hZβDAI wing. ZBDs usually recognize the zig-zag backbone of Z-DNA, but hZβDAI, lacking binding interactions through the β-loop of the wing, uses a different method. R124, which is located in the first β-stand of hZβDAI, plays that role (Figs. 2 and 3). The Nε of R124 from chain A forms a water-mediated hydrogen bond with O6 of G2; NH1 of R124 forms direct hydrogen bonds with O1P and O2P of the G2 phosphate group. In chain B, NH1 of R124 is connected to O1P of the G2 phosphate group through water (Fig. 3).
It is interesting that all ZBDs lacking Pro residues in the wing region have a conserved positively-charged residue corresponding to R124 of hZβDAI (Fig. 1). For example, the second ZBDs of zebrafish PKZ (drZβPKZ) and goldfish PKZ (caZβPKZ) lack the Pro residues in the wing, but have a Lys residue at the same site as R124 of hZβDYI (Fig. 1). In this regard, ZBDs can be divided into 2 groups on the basis of the presence of the Pro residues in the wing region, and accordingly, the binding mode to Z-DNA. Group I ZBDs, which have the Pro residue in the wing, can recognize Z-DNA by using a recognition helix and the β-loop of the wing, whereas group II ZBDs have a positively charged residue in the β1 strand, instead of the Pro residue in the wing. In Z-DNA binding, it is expected that the positively charged residue in the first β-stand (β1) of other group II ZBDs will play the same role in Z-DNA binding as the residues in the β-loop of the wing of group I ZBDs.
The Alternative Binding Mode Is Supported by Z-DNA Converting Activity.
The role of each residue in Z-DNA recognition was investigated by a B-DNA-to-Z-DNA CD converting activity assay of various mutants. Residues directly involved in Z-DNA binding are known to stabilize the Z-DNA conformation and enhance Z-DNA conversion in the CD (18, 20, 21). The above results suggest that the kinked recognition helix and R124 of β1 rather than the β-loop of the wing are important in Z-DNA binding of hZβDAI. It has been demonstrated that K160 does not have a critical role in Z-DNA recognition because mutation of K160 to Ala (19) has only a slight effect on Z-DNA converting activity (Fig. 4A). The contribution of R124 was confirmed by this method because Z-DNA converting activity was diminished to 70% when it was replaced with Ala (Fig. 4A). It is clear that K160 is not systematically involved in Z-DNA binding in the current complex structure, but from its proximity to Z-DNA, it can be assumed that K160 may participate in this process in some way (Fig. 3). The reduced Z-DNA converting activity of the K160E (19) mutant suggests that K160 is in close proximity to DNA, at least in terms of charge (Fig. 3B), although a direct interaction was not observed. From these results it can be postulated that R124 has a dominant role and K160 is supplementary in Z-DNA binding. It is tempting to hypothesize that K160 performs a role in Z-DNA binding in the absence of R124, and the 70% remaining activity of the R124A mutant comes from the contribution of K160. To verify this hypothesis, both were mutated to Ala, and the activity of this double mutant was tested. As shown in Fig. 4A, Z-DNA converting activity was dramatically reduced, lower than that of either R124A or K160A mutants. CD spectroscopy (Fig. S4) and dynamic light scattering experiments (data not shown) revealed that WT and mutant hZβDAI R124AK160A have similar secondary structure contents and homogeneous solution conformations, suggesting that the reduced activity is not caused by the structural instability or denaturation of the mutant. These results suggest that the presence of a positively charged residue near G2 of DNA is critical for Z-DNA binding. The fact that a K138A mutant retained ≈80% activity suggests that K138 may significantly participate in this process. The K138A counterparts in hZαADAR1, K169, and K170, have also proven to be important for Z-DNA binding affinity by mutagenesis experiments (20). However, K138 does not seem to be as critical or indispensable when compared with other core residues in the recognition helix, such as Y145, for which an Ala mutant showed no Z-DNA converting activity (Fig. 4A).
Fig. 4.
B-to-Z-DNA conversion activity of hZβDAI and hZαβDAI. (A) The Z-converting activities were estimated by monitoring the CD signal at 255 nm for 40 min by using 60 μg/ml of double-stranded (dCdG)6. The molar ratio of hZβDAI or its alanine mutants to ds(dCdG)6 was 4:1. D139A was randomly chosen as a negative control. (B) WT hZαβDAI and 2 tyrosine mutants, hZαβDAI Y50A and hZαβDAI Y145A, were mixed with 15 μM double-stranded (dCdG)6, at 2:1 and 4:1 molar ratios, and their CD signals were monitored at 255 nm for 60 min. As a control, the Z-DNA converting activity of a single ZBD (hZαADAR1) at a 4:1 molar ratio to DNA was also measured under the same condition.
Z-DNA Binding and Oligomerization of hZαβDAI.
From structural and biochemical studies on the first ZBD of DAI (6, 9, 14, 19), it was expected that hZαDAI shared a canonical ZBD fold and a similar binding mode with other members of what we call group I ZBDs. This study reveals that hZβDAI belongs to what we call group II ZBDs and has a distinct Z-DNA binding mode. The next questions are how the 2 tandem ZBDs contribute to Z-DNA binding and how Z-DNA binds to hZαβDAI. Structural studies will be required to get a firm answer. We would like to know how it may contribute to a possible oligomerization of DAI and ultimately, how this binding contributes to the immune response. We may, however, get clues from solution studies.
It has already been established that each of the 2 ZBDs of DAI individually possesses Z-DNA converting activity and can bind to Z-DNA independently (6, 14, 19). To investigate their joint binding modes, Z-DNA converting activities were measured for hZαβDAI, hZαβDAI Y50A, and ZαβDAI Y145A (Fig. 4B). It is known that the Tyr residue located in the α3 recognition helix of these domains plays a pivotal role in the conversion to Z-DNA, and substitution with Ala sharply lowers the activity of hZαADAR1 (18) and abolishes the activity of hZβDAI (Fig. 4B; ref. 19).
In Fig. 4B, 100% of the converting activity (B-DNA to Z-DNA) of 15 μM ds(dCdG)6 is found with 30 μM hZαβDAI, which is equivalent in activity to 60 μM hZαADAR1. This indicates that both Zα and Zβ domains of DAI are active in binding Z-DNA. Mutating Y145 of 30 μM hZαβDAI to alanine lowers the converting activity to 35%. The analogous mutant in the Zα domain (Y50A) lowers the converting activity to nearly 23%. These results suggest that the binding of ZαDAI is equal to or slightly more important for converting activity than ZβDAI. When the concentration of the mutant (ZαβDAI Y150A or ZαβDAI Y145A) is doubled to 60 μM to compensate for the loss of Z binding by the mutations, the converting activity approximately doubles but is still below the activity of unmutated 30 μM ZαβDAI. There may be many possible explanations for this phenomenon, including various types of steric hindrance. However, it suggests the 2 ZBDs act largely independently of each other. It is known that Zα domains bind to 6 bp of Z-DNA, with a domain largely bound to 1 DNA strand. To get some insight into how ZαβDAI domains bind, sizing gel experiments were carried out by using 12-bp fragments that can form Z-DNA (Fig. S5). These support the idea that the dsDNA can recruit 2 ZαβDAI domains.
Discussion
Since the first identification of a ZBD (8), there has been great interest in the role and the specificity at their N terminus for the Z conformation of DNA or RNA (22). Recent studies of poxvirus E3L demonstrated that deletion of the ZBD or mutations that eliminate Z-DNA binding resulted in loss of viral pathogenicity. The ZBD is thus essential for virulence (18). It has been proposed that E3L may compete with cellular Z-DNA binding proteins (23). There is also evidence that E3L can act through gene transactivation and conversion of the host cell to an antiapoptotic state (24). Recently, ZBP1 (DLM-1) was reported to be a cytosolic B-DNA binding sensor that regulates the DNA-mediated innate immune response. It was thus renamed DAI (4). It has also been suggested that a DNA-mediated multimerization of DAI may be involved in activation of the innate immune response (5). It is possible that poxvirus E3L competes with DAI for the binding to Z-DNA, thereby preventing activation of the innate immune response. An understanding of the binding mode and spatial arrangements of the ZBDs of DAI is essential for understanding the mechanism of DAI as an IFN activator in the innate immune response.
The second ZBD of DAI shows distinctive features in sequence and structure compared with other ZBDs of defined structure (Fig. 1). The crystal structure analysis supported by mutagenesis and a B- to Z-DNA converting assay showed that hZβDAI recognizes Z-DNA by an unusual binding mode. It has a kinked recognition helix containing a 310-helix segment and a positively charged residue in the β1 strand that play key roles (Figs. 2 and 3). In contrast with other ZBDs, the β-loop in the wing of hZβDAI is short and positioned away from the Z-DNA. We conclude that ZBDs can be categorized into 2 groups based on the residues involved in DNA binding. A study of splicing patterns of DAI (ZBP1) reveals considerable complexity (23). The first ZBD (Zα) is spliced out in ≈50% of DAI mRNA transcripts, whereas the second ZBD (Zβ) is present in nearly all splicing variants. Recent work (25) reports that human DAI activity can also be found when Zα is spliced out, which suggests a major role of ZβDAI in Z-DNA recognition and immune activation. Substantial sequence variation in the second ZBDs (Zβ) of most Z-DNA binding proteins could also result in the loss of Z-DNA binding activity. For instance, the Z-DNA binding protein ADAR1, which has RNA editing activity, also has 2 tandem ZBDs at its N terminus, but the Zβ domain does not bind to Z-DNA as it is missing the crucial Tyr residue (11, 18). However, hZβDAI retains Z-DNA binding activity, despite the considerable sequence variation that leads to alterations in the DNA binding mode.
The results of this study, combined with previous biochemical analyses, indicate that each ZBD of DAI binds to DNA in a distinctive mode, but both act together in inducing the conversion to Z-DNA. DAI also has a DNA binding region (D3) located at the C terminal to hZβDAI. It has been shown that all 3 DNA-binding domains of DAI are required for full activation of the mouse DNA-dependent immune response (5). The linker between hZαDAI and hZβDAI may help position the 2 ZBDs in close enough proximity to allow for their full accessibility to Z-DNA. It has been suggested that the linker may be involved as it enhances the DNA binding activity of ZBDs (6). Interestingly, artificial dimerization of DAI was shown to activate the innate immune response, even in the absence of DNA (5), which suggests the possibility that DNA-mediated multimerization of DAI might also act to evoke the innate immune response. Some data presented here may provide clues for the molecular mechanism underlying such multimerization. Two ZBDs (ZαβDAI) are able to bind dsDNA in tandem or by encircling the DNA. If the Z-forming region is ≥12 bp, then 2 DAIs can be attached in close proximity, and such dimerization could result in activation. An analogous example is the dsRNA-induced dimerization of PKR by binding 2 tandemly arranged RNA binding domains, which results in phosphorylation of the C-terminal domain (26). It has also been proposed that PKZ is activated in a DNA-dependent manner by using its 2 tandemly arranged ZBDs (27).
B-DNA activates DAI for IFN production. It is possible that ZαβDAI recognizes and binds to a transient Z-conformation in a long B-DNA. It is also possible but less likely that ZαβDAI, in concert with the third DNA binding domain, may bind to B-DNA. We have observed that hZαADAR1 has a binding affinity for B-DNA with a dissociation constant (Kd) of the order of 10−5 M (data not shown), and this binding is likely to be nonspecific. In contrast, hZαADAR1 has a Kd near 10−8 M to Z-DNA (8). It will be of interest to make similar measurements for hZαDAI and its subunits. It is likely that possession of 2 ZBD domains and a linker maximizes DNA binding activity and possibly facilitates dimerization of DAI, leading to IFN induction.
This study contributes to the understanding of the structure and function of DAI and provides a foundation for elucidating DAI function in the innate immune response. However, it is clear that more work will be required to uncover the detailed mode of dsDNA binding to DAI that leads to IFN production.
Materials and Methods
Protein Preparation.
hZβDAI (residues 103–166) and hZαβDAI (residues 8–166) were prepared as described (14, 19). Point mutations were introduced by using QuikChange according to the manufacturer's instructions (Stratagene). All mutations were confirmed by DNA sequencing, and mutant proteins were purified in the same way as WT hZβDAI.
CD.
The conversion of double-stranded (dCdG)6 from B-DNA to Z-DNA was monitored by its CD spectrum, which was measured with a Jasco J-810 CD spectrometer at 25 °C in a 1-mm quartz cell. For kinetic comparisons of hZβDAI and its mutants, changes in the CD signal at 255 nm were measured for 40 min by using 60 μg/ml of ds(dCdG)6 in the CD buffer [10 mM Hepes-NaOH (pH 7.5), 10 mM NaCl, and 0.1 mM EDTA]. Similarly, the CD signal of hZαβDAI and its mutants were monitored for 60 min. The volume of the added protein did not exceed 5% of the total reaction volume.
Structure Determination.
The crystallization and data collection of wild and selenomethionine-substituted hZβDAI were carried out as described (14). The structure was solved via Se MAD phasing and model building and refinement (SI Text). The final model refined at 1.45 Å with Rwork and Rfree of 15.5% and 19.3%, respectively, contains 2 domains of hZβDAI and a DNA duplex with the Z conformation in the asymmetric unit. The refinement statistics are summarized in Table S1.
Supplementary Material
Acknowledgments.
We thank Dr. Ky Lowenhaupt for helpful discussions. This work was supported by Korean National Research Laboratory Program Grant NRL-2006-02287.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3EYI).
This article contains supporting information online at www.pnas.org/cgi/content/full/0810463106/DCSupplemental.
References
- 1.Eda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 2.Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:525–532. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 4.Takaoka A, et al. DAI(DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–504. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, et al. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci USA. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deigendesch N, Koch-Nolte F, Rothenburg S. ZBP1 subcellular localization and association with stress granules is controlled by its Z-DNA binding domains. Nucleic Acid Res. 2006;34:5007–5020. doi: 10.1093/nar/gkl575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham HT, et al. Intracellular localization of human ZBP1: Differential regulation by the Z-DNA binding domain, Zα, in splice variants. Biochem Biophys Res Commun. 2006;348:145–152. doi: 10.1016/j.bbrc.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 8.Herbert A, et al. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc Natl Acad Sci USA. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz T, Behlke J, Lowenhaupt K, Heinemann U, Rich A. Structure of the DLM-1–Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat Struct Mol Biol. 2001;8:761–765. doi: 10.1038/nsb0901-761. [DOI] [PubMed] [Google Scholar]
- 10.Hu CY, Zhang YB, Huang GP, Zhang QY, Gui JF. Molecular cloning and characterisation of a fish PKR-like gene from cultured CAB cells induced by UV-inactivated virus. Fish Shellfish Immunol. 2004;17:353–366. doi: 10.1016/j.fsi.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Kim YG, et al. Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: Implications for development of a therapy for poxvirus infection. Proc Natl Acad Sci USA. 2004;101:1514–1518. doi: 10.1073/pnas.0308260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha SC, Oh DB, Kim KK, Kim YG. Crystallization and preliminary X-ray crystallographic study of the viral Zα domain bound to left-handed Z-DNA. Protein Pept Lett. 2005;12:391–393. doi: 10.2174/0929866053765608. [DOI] [PubMed] [Google Scholar]
- 13.Rothenburg S, et al. A PKR-like eukaryotic initiation factor 2α kinase from zebrafish contains Z-DNA binding domains instead of dsRNA binding domains. Proc Natl Acad Sci USA. 2005;102:1602–1607. doi: 10.1073/pnas.0408714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha SC, et al. Biochemical characterization and preliminary X-ray crystallographic study of the domains of human ZBP1 bound to left-handed Z-DNA. Biochim Biophys Acta. 2006;1764:320–323. doi: 10.1016/j.bbapap.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz T, Rould MA, Lowenhaupt K, Herbert A, Rich A. Crystal structure of the Zα domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science. 1999;284:1841–1845. doi: 10.1126/science.284.5421.1841. [DOI] [PubMed] [Google Scholar]
- 16.Ha SC, et al. A poxvirus protein forms a complex with left-handed Z-DNA: Crystal structure of a Yatapoxvirus Zα bound to DNA. Proc Natl Acad Sci USA. 2004;101:14367–14372. doi: 10.1073/pnas.0405586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Athanasiadis A, et al. The crystal structure of the Zα domain of the RNA-editing enzyme ADAR1 reveals distinct conserved surfaces among Z-domains. J Mol Biol. 2005;351:496–507. doi: 10.1016/j.jmb.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Kim YG, et al. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci USA. 2003;100:6974–6979. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quyen DV, et al. Binding surface in Zβ domain from human ZBP1 does not require conserved proline residues for Z-DNA binding and B-to-Z-DNA conversion activities. Bull Korean Chem Soc. 2007;28:2539–2542. [Google Scholar]
- 20.Schade M, Turner CJ, Lowenhaupt K, Rich A, Herbert A. Structure-function analysis of the Z-DNA-binding domain Zα of dsRNA adenosine deaminase type I reveals similarity to the (α + β) family of helix-turn-helix proteins. EMBO J. 1999;18:470–479. doi: 10.1093/emboj/18.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quyen DV, et al. Characterization of DNA binding activity of Zα domains from poxviruses and importance of the β-wing regions for Z-DNA flipping activity. Nucleic Acid Res. 2007;35:7714–7720. doi: 10.1093/nar/gkm748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown BA, 2nd, Lowenhaupt K, Wilbert CM, Hanlon EB, Rich A. The zalpha domain of the editing enzyme dsRNA adenosine deaminase binds left-handed Z-RNA as well as Z-DNA. Proc Natl Acad Sci USA. 2000;97:13532–13536. doi: 10.1073/pnas.240464097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothenburg S, Schwartz T, Koch-Nolte F, Haag F. Complex regulation of the human gene for the Z-DNA binding protein DLM-1. Nucleic Acid Res. 2002;30:993–1000. doi: 10.1093/nar/30.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon JA, Rich A. Biological function of the vaccinia virus Z-DNA-binding protein E3L: Gene transactivation and antiapoptotic activity in HeLa cells. Proc Natl Acad Sci USA. 2005;102:12759–12764. doi: 10.1073/pnas.0506011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippmann J, et al. IFN-β responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI) Cell Microbiol. 2008 doi: 10.1111/j.1462–5822.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, et al. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J Biol Chem. 2001;276:24946–24958. doi: 10.1074/jbc.M102108200. [DOI] [PubMed] [Google Scholar]
- 27.Bergan V, Jagus R, Lauksund S, Kileng Ø, Robertsen B. The Atlantic salmon Z-DNA binding protein kinase phosphorylates translation initiation factor 2 α and constitutes a unique orthologue to the mammalian dsRNA-activated protein kinase R. FEBS J. 2008;275:184–197. doi: 10.1111/j.1742-4658.2007.06188.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.