Abstract
Reduced energy intake, or caloric restriction (CR), is known to extend life span and to retard age-related health decline in a number of different species, including worms, flies, fish, mice and rats. CR has been shown to reduce oxidative stress, improve insulin sensitivity, and alter neuroendocrine responses and central nervous system (CNS) function in animals. CR has particularly profound and complex actions upon reproductive health. At the reductionist level the most crucial physiological function of any organism is its capacity to reproduce. For a successful species to thrive, the balance between available energy (food) and the energy expenditure required for reproduction must be tightly linked. An ability to coordinate energy balance and fecundity involves complex interactions of hormones from both the periphery and the CNS and primarily centers upon the master endocrine gland, the anterior pituitary. In this review article we review the effects of CR on pituitary gonadotrope function and on the male and female reproductive axes. A better understanding of how dietary energy intake affects reproductive axis function and endocrine pulsatility could provide novel strategies for the prevention and management of reproductive dysfunction and its associated comorbidities.
Keywords: Caloric restriction, Neuroendocrine pulsatility, Pituitary
1. Introduction
The availability of energy in the form of food is a critical factor in the maintenance of the reproductive capacity of animals. The apparent availability of food and the actions of intaken and digested food have profound and complex effects upon numerous neuroendocrine axes in the body. In experimental paradigms in various species, it has been shown that specific alterations in available dietary energy, e.g. mild caloric restriction (CR) or intermittent fasting (IF), can have beneficial and long-lasting effects on animal physiology (for review, see Martin et al., 2006). Low, moderate and high levels of dietary energy intake can affect reproductive function in different ways. CR and IF have been demonstrated to modulate the release patterns of many important trophic hormones required for development, growth and general metabolism. The reproductive axis in mammals is controlled by a complex series of hormones secreted from multiple organs. The canonical pathways involving these hormones that control reproduction takes place across the hypothalamic-pituitary-gonadal axis (HPG). This HPG axis will be discussed in several other reviews in this special issue, and therefore we will illuminate a specific hormonal loop within this axis, i.e. the functioning of the anterior pituitary gland, in paradigms where available food energy is limited either by CR or by IF. The issues that we will discuss in this review are concerned with the understanding of how CR and IF exert their effects upon whole body physiology and reproduction through actions on the pituitary.
2. Hormonal regulation of reproductive function
Regulation of normal reproductive development and physiology is a complex process involving the coordinated interaction of neurotransmitter systems, hypothalamic releasing factors, pituitary hormones, gonadal sex steroid hormones and various growth factors. The reproductive system is part of the endocrine system, which contains an elegant feedback system with control centers at the level of the hypothalamus and the pituitary gland, and with target organs such as the testes or ovaries. There are also smaller local feedback loops involving paracrine and autocrine signals at the levels of the pituitary, testes and ovaries, which maintain organ or cell homeostasis.
The pituitary gland, or hypophysis as it is also known, consists of two major subdivisions, the anterior lobe (or adenohypophysis) and the posterior lobe (or neurohypophysis). The posterior lobe is further divided into the infundibular process and the infundibular stem (or pituitary stalk) which connects to the median eminence. The posterior lobe is made up of neural tissue and is connected to the rest of the brain via the stalk. The infundibular process contains axon terminals of neurons whose cell bodies reside in the hypothalamus, and there is therefore a direct neural link between the posterior pituitary and the brain (Knobil, 1981). The major hormones released into the blood from axon terminals in the posterior pituitary are vasopressin and oxytocin. The anterior lobe of the pituitary is further subdivided into the pars distalis, pars intermedia, and pars tuberalis. The pars tuberalis surrounds the infundibular stem like a cuff and extends upwards to lie beneath a portion of the median eminence. Unlike the posterior lobe, the anterior pituitary contains no nerve fibres and terminals and so is not in direct neuronal contact with the hypothalamus. Instead, it is connected to the brain by a vascular connection, the hypothalamo-hypophyseal-portal system. Most of the blood supplied to the anterior lobe comes from this portal system. In some species, it accounts for over 90% of the total blood supply (Knobil et al., 1980). In addition to blood flowing down the stalk from the brain to the anterior pituitary, a small proportion of blood flows up the pituitary stalk. This provides a direct vascular link from the anterior pituitary back to the hypothalamus (Bergland and Page, 1978).
Secretion of the gonadotrophin hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary is under the control of gonadotropin-releasing hormone (GnRH). These two gonadotrophin hormones (LH, FSH) play a primary role in the regulation of peripheral reproductive tissue function. GnRH is synthesized and secreted in a pulsatile manner from the hypothalamus and carried by the hypothalamo-hypophyseal-portal system to the gonadotrope cells of the anterior pituitary. Binding of GnRH to receptors on the pituitary gonadotropes causes the release of LH and some FSH. Direct measurement of GnRH in the hypothalamo-hypophyseal-portal blood of sheep has shown that each rise in serum LH is preceded by a GnRH pulse in the portal blood (Clarke and Cummins, 1982). Recent studies in which the release patterns of LH and FSH were investigated in hypophyseal portal (Padmanabhan et al., 1997) or cavernous sinus (Clarke, 2002) blood samples (i.e. samples collected very close to pituitary in vivo) have shown that while there is a high degree of synchrony between the pulsatile release of GnRH and LH, FSH release on the other hand is only associated with a small proportion of GnRH pulses. Additionally, it has been demonstrated that the episodic secretion of FSH continues when GnRH input is blocked by GnRH antagonist treatment (Padmanabhan et al., 2003). Since LH and FSH are present within the same gonadotrope cell (Taragnat et al., 1998; Crawford and McNeilly, 2002), this asynchrony between the release of FSH and LH has been proposed to be due to the fact that LH and FSH are released by separate mechanisms from the gonadotrope cell (McNeilly, 1988; Farnworth, 1995; McNeilly et al., 2003), and while GnRH is known to be crucial for the pulsatile release of LH, basal secretion of LH and the majority of FSH secretion occurs independently of signals arising from the GnRH receptor to the release mechanisms. Gonadal hormones can decrease gonadotropin release both by decreasing GnRH release from the hypothalamus and by affecting the ability of GnRH to stimulate gonadotropin secretion from the pituitary itself. For example, administration of exogenous testosterone has been shown to lead to a marked slowing in GnRH pulse frequency in men (Matsumoto et al., 1984). Testosterone administration has also been shown to inhibit LH and FSH by a direct pituitary effect (Sheckter et al., 1989).
The pulsatile mode of GnRH secretion reflects the synchronised activity of many of the 1500 or so GnRH neurons that form a network within the hypothalamus. Each burst of GnRH secretion is followed by a prominent pulse of LH release into the systemic circulation. Such LH pulses occur at intervals of 30–120 min in various species, and about once per hour in humans. Decreases in the rate of stimulation by GnRH, or sustained treatment with GnRH, are associated with impaired secretion of both LH and FSH (Knobil, 1989; Millar et al., 1989). The coordinated activity of the scattered GnRH neurons is attributed to a neural pacemaker that is termed the GnRH pulse generator (Knobil, 1989). The nature of the pulse generator has yet to be established, but its activity reflects a pattern of electrical activity in the mediobasal hypothalamus that is correlated with episodic increases in circulating LH levels in the rhesus monkey (Knobil, 1981; Sealfon et al., 1997). Thus, the pulsatile nature of GnRH secretion is essential for the physiological maintenance of normal gonadotrope function, and ultimately for normal reproductive capacity (Millar et al., 1987). Slow GnRH pulse input preferentially increases FSH secretion while fast frequency pulses, e.g. hourly, favor LH secretion (Padmanabhan and McNeilly, 2001). Thus, GnRH is crucial for the activation of both the LHβ and FSHβ genes, but only directly controls the pulsatile secretion of LH, and a minimal number of co-incidental FSH episodes of secretion. Alteration of the expression and release of GnRH will then impact upon the pituitary gonadotrope cells as GnRH receptor activation controls not only GnRH receptor expression but also expression of the LH-β subunit (Maudsley et al., 2007; Lopez de Maturana et al., 2007).
3. Dietary restriction and reproductive pituitary function
For any organism to survive the rigorous caloric demands of reproduction, there must be a well-maintained balance with other metabolic requirements needed for basic sustenance, e.g. thermoregulation, locomotor activity, innate immunity and sensory functions. Food energy is typically stored as fat or glycogen which can then be mobilised for reproductive functions such as pubertal maturation and reproduction itself. Due to the extra constraints that reproductive functionality places on the basic necessary energy reserves, there needs to exist an endogenous ‘energy sensor’ that restricts the activity of the reproductive system in times of limited caloric intake. In this review we shall investigate the hormonal nature of the body's ‘energy sensor’ that regulates the relationship between energy availability and the ability of the organism to remain fecund.
Energy balance, appetite and reproductive function are tightly linked and alterations in energy and appetite endocrine axes will lead to altered feedback and feed-forward in the HPG axis.
The most basic form of reduced energy intake in laboratory experiments is glucose deprivation, as this is the prime source of energy for most mammals. It has been demonstrated in mammalian models (mostly rodents, sheep or primates) that fasting or glucose deprivation affects reproductive function by suppressing pulsatile LH release from the pituitary gonadotropes. The glucose energy deficit is therefore rapidly detected and is probably conveyed to the hypothalamus where GnRH secretion is disrupted to attenuate the pulsatile LH release, and to ultimately conserve energy in times of low glucose availability. Several metabolic hormones important for general energy homeostasis, e.g. leptin, insulin, ghrelin and thyroid hormones, have also been shown to exert profound effects on the function of the reproductive axis. In addition to these humoral factors connecting energy balance to reproduction, it has recently been posited that there is a specific glucose-sensing mechanism in the CNS itself that is mediated via noradrenergic stimulation of the nuclei of the solitary tract (NTS: Kinoshita et al., 2003). Profound nutritional signals such as those imposed by CR or IF exert a wide range of physiological effects across the whole organism subjected to the dietary change (for review see Martin et al., 2006). One of the most profound alterations that take place with these dietary regimes is disruption of the complex neuroendocrine feedback axes centered around the anterior pituitary. Reduced energy intake typically suppresses the reproductive axis, and activates the hypothalamo-pituitary-adrenal axis and/or somatotropic axes (Faria et al., 1992; Hartmann et al., 1996).
The metabolic status of an animal, dependent on its access to food, controls multiple diverse physiological factors in both males and females (Martin et al., 2007). The ability to generate and maintain functional gametes has been shown to be connected to the ability of the organism to secure and consume energy in the form of food. This connectivity between food intake and reproduction is especially evident in the female, where pregnancy and lactation are linked to the considerable energetic drain needed for the nurture of embryos and newborns (Casanueva and Dieguez, 1999). The physiological basis for such a joint regulation of energy balance and reproduction has begun to be unveiled only recently, in a phenomenon that involves multiple common regulatory signals, acting at different levels of the reproductive system. As we have previously stated, the prime controller of reproductive function at the level of the anterior pituitary is the hormone GnRH (which controls the secretion of the gonadotropins, LH and FSH). However in recent years it has been demonstrated that there are many other hormonal factors that exert feedback from diverse peripheral and central organs to the pituitary to connect the energy status of the organism to its reproductive capacity. As one would imagine it appears that many of the hormones that are involved in food sensation and appetite control can directly feedback and control the reproductive axis at the level of the hypothalamic-pituitary interaction. In the next section we will discuss how such hormonal systems link energy balance to reproduction and describe their actions on pituitary gland function.
Reproduction is a physiologically costly process that consumes significant amounts of energy, and the mechanisms controlling energy balance are therefore closely linked to fertility. This close relationship ensures that pregnancy and lactation occur only in favorable conditions with respect to energy. The primary metabolic cue that modulates reproduction is the availability of oxidizable fuel. An organism's metabolic status is transmitted to the brain through metabolic fuel detectors. There are many of these detectors at both the peripheral (e.g. leptin, insulin, ghrelin) and central (e.g. neuropeptide Y, melanocortin, orexins) levels. When oxidizable fuel is scarce, the detectors function to inhibit the release of GnRH and LH, thereby altering steroidogenesis, reproductive cyclicity, and sexual behavior. Infertility can also result when resources are abundant but food intake fails to compensate for increased energy demands. Examples of these conditions in women include anorexia nervosa and exercise-induced amenorrhea. Infertility associated with obesity appears to be less related to an effect of oxidizable fuel on the hypothalamic-pituitary-ovarian axis. Instead, impaired insulin sensitivity may play a role in the etiology of these conditions, but their specific causes remain unresolved. Research into the metabolic regulation of reproductive function has implications for elucidating mechanisms of impaired pubertal development, nutritional amenorrhea, and obesity-related infertility. A better understanding of these etiologies has far-reaching implications for the prevention and management of reproductive dysfunction and its associated comorbidities.
4. Leptin
The adipocyte-derived circulating hormone leptin, is a satiety factor that signals the amount of body energy (as fat) stores not only to the neural pathways involved in food intake (Friedman and Halaas, 1998; Rosenbaum and Leibel, 1998; Ahima et al., 2000), but also to the reproductive neuroendocrine axis. Plasma levels of leptin are directly proportional to the existing fat reserves. Reductions in plasma levels of leptin activate feeding behavior, slow the metabolism and help conserve energy stores. Most of the effects of leptin in the control of food intake are mediated at the level of the hypothalamus, where activation of its cognate receptor results in modulation of expression of a wide variety of neurotransmitters and hormones. Leptin receptor activation in the hypothalamus stimulates orexigenic neurons expressing neuropeptide Y (NPY) and agouti-related peptide as well as anorexigenic neurons expressing pro-opiomelanocortin and cocaine- and amphetamine-regulated transcript (Sahu, 2004; Zigman and Elmquist, 2003).
The primary locus of leptin action upon reproduction is generally considered to be at the level of the hypothalamus (Cheung et al., 2000; Cunningham et al., 1999). In animals with a normal energy balance, i.e. similar levels of energy intake and energy expenditure, the effects of leptin levels upon reproductive axis function are minimal. However during times of CR or fasting, leptin can exert strong effects on the reproductive axis (Henry et al., 2001). Loss of the leptin receptor from experimental animals prevents normal pubertal maturation and renders the animals infertile (Henry et al., 2001). Acute dietary fasting also profoundly suppresses serum leptin levels and attenuates the leptin pulse area and incremental pulse amplitude (Bergendahl et al., 2000). In humans, as in mice, congenital absence of leptin or functional leptin receptor causes severe obesity accompanied by neuroendocrine abnormalities (Montague et al., 1997; Strobel et al., 1998; Ozata et al., 1999; Clement et al., 1998). Interestingly, the neuroendocrine defects that are present in leptin-deficient rodents and in leptin-resistant humans differ from each other, which indicates that the role of leptin in mediating the neuroendocrine response to starvation may be different in humans versus rodents (Montague et al., 1997; Strobel et al., 1998; Ozata et al., 1999; Clement et al., 1998). Data from Chan et al. (2003) has suggested that a reduction of circulating leptin levels in lean men regulates the acute fasting-induced changes that occur in the HPG axis and, in part, the changes that occur in the hypothalamic-pituitary-thyroid (HPT) axis and in IGF-1-binding capacity, but it is not responsible for changes in the HPA, rennin–aldosterone, and GH–IGF-1 axes associated with acute fasting (Chan et al., 2003). Additionally, leptin replacement in healthy men during fasting has been shown to have a significant effect on the HPG axis, with full restoration of LH pulsatility and circulating testosterone levels (Chan et al., 2003).
Intrahypothalamic infusion of leptin has been shown to elicit GnRH release in vivo (Watanobe, 2002). Central application of leptin to animals that possess a fasting-disrupted GnRH/LH secretion profile was able to normalize this imbalance and re-instate reproductive function (Barash et al., 1996; Chehab et al., 1996; Nagatani et al., 1998). These findings suggest that the body can interpret circulating levels of leptin as an indicator of its metabolic state, which may then act as a gate to control the activity of the reproductive axis. Although the hypothalamic actions of leptin in connecting the metabolic state to reproductive function seem primary, there is some evidence that additional actions of leptin on the HPG axis occur. For example, functional leptin receptors have been reported in the pituitary itself (Jin et al., 1999; Iqbal et al., 2000). However, despite this seemingly direct control of GnRH function, it is still unclear whether there is a significant amount of leptin receptor expression in hypothalamic GnRH neurons (Chan and Mantzoros, 2001; Watanobe, 2002). Another possible mechanism by which leptin regulation controls GnRH functionality is through its ability to activate kisspeptin-positive neurons (Smith et al., 2006). Kisspeptin, the gene product of the Kiss-1 gene, binds to and signals through the GPR54 G protein-coupled receptor expressed in high levels in GnRH-positive neurons in the hypothalamus (Irwig et al., 2004; Messager et al., 2005). We shall discuss the potential impact of kisspeptin's actions during CR in the next section.
5. Kisspeptin
Kisspeptin was first identified by several independent groups in 2001 as a high affinity RF-amide (Arg-Phe-NH2) peptide ligand for the G protein-coupled receptor GPR54 (Muir et al., 2001; Kotani et al., 2001; Ohtaki et al., 2001; Clements et al., 2001). Centrally administered kisspeptin stimulates GnRH neurons in the mouse, rat, sheep, and primate (Gottsch et al., 2004a; Irwig et al., 2004; Shahab et al., 2005). The integrity of the kisspeptin/GPR54 signaling system is necessary for normal reproduction. Mutations in GPR54 result in complete disruption of reproductive function in both humans and mice (De Roux et al., 2003; Seminara et al., 2003). Kiss1 mutant female mice have underdeveloped uteri and ovaries, do not progress through the estrous cycle and do not produce mature Graffian follicles (d'Anglemont de Tassigny et al., 2007). Mutant males have small testes, and deficient spermatogenesis. Levels of circulating LH and FSH, and testosterone and estradiol are reduced in both sexes.
Kiss1 neurons are also direct targets for the action of sex steroids, which regulate the expression of Kiss1 mRNA (Smith et al., 2005a,b). It had been demonstrated that in mice, kisspeptin neurons (i.e. those that express Kiss1 mRNA) are most numerous in the arcuate nucleus (Arc), but significant numbers are also seen in the periventricular nucleus (PeN) and the anteroventral periventricular nucleus (AVPV) (Gottsch et al., 2004a; Smith et al., 2005a,b). The Arc and AVPVare thought to play important roles in the feedback regulation of GnRH and gonadotropin secretion by estradiol and testosterone, however the exact circuitry that mediates this phenomenon is unclear (Kalra and Kalra, 1989). The normal pulsatile secretion of GnRH is thought to be controlled by kisspeptin neurons in the ARC nucleus, while a different set of kisspeptin neurons in the AVPVare thought to regulate the GnRH pulse surge. Interestingly, these Kiss neurons are thought to be the primary manner in which hypothalamic neuronal inputs are relayed to the GnRH neurons, which do not appear to express many of the necessary receptors that are required for the induction of the appropriate responses to hormones and factors such as leptin, ghrelin, dopamine, estradiol, and other factors that are related to the nutritional status of the organism (Dungan et al., 2006; Maeda et al., 2007). The activation of Kiss1 gene expression is likely to play an important role in timing the onset of puberty, sexual differentiation of the GnRH/LH surge mechanism, and the preovulatory GnRH/LH surge itself (in females) (Gottsch et al., 2004a; Shahab et al., 2005; Han et al., 2005, Navarro et al., 2004).
Dietary restriction, e.g. fasting, is associated with an inhibition in the expression of Kiss1 mRNA along with the well-described decreased circulating levels of leptin (Castellano et al., 2005). Leptin-deficient ob/ob mice have reduced levels of Kiss1 mRNA compared to wild-type controls, and the central administration of leptin partially reduces this effect (Smith et al., 2006). Further support of the notion that the cells are regulated by leptin is the observation that 40% of Kiss1 cells in the arcuate nucleus express the signaling version of the leptin receptor, ObRb (Smith et al., 2006).
Not only can central injections of kisspeptin reverse the fasting-induced inhibition of GnRH secretion, but kisspeptin administration has also been shown to rescue the GnRH decline in rats that are treated with leptin antibodies (Castellano et al., 2006). These findings suggest that kisspeptin is involved in relaying metabolic signals to the neuroendocrine reproductive axis. Kisspeptin also interacts with the insulin signaling pathway in the hypothalamus. Rats that are rendered diabetic, thus having systemic glucose insufficiency, have diminished expression of Kiss1 mRNA, which can be reversed with insulin therapy (Castellano et al., 2006). Thus, together with galanin-like peptide (GALP), pro-opiomelanocortin (POMC) and NPY neurons, Kiss1 neurons are likely to serve as cellular conduits for relaying information about circulating levels of leptin and insulin to the neuroendocrine reproductive axis.
The cellular and molecular basis for the integration of metabolism and reproduction involves a complex interaction of hypothalamic neuropeptides with metabolic hormones, fuels, and sex steroids. Kisspeptin is unlikely to be the last of the neuropeptides discovered having relevance to both metabolic regulation and reproductive function—just as leptin, insulin, and thyroid hormone are not the full cast of metabolic hormones with actions on the neuroendocrine reproductive axis (Fig. 1). Understanding this integrative process will require careful mapping of hypothalamic and brainstem circuitry, cataloguing of receptor expression profiles within these circuits, and a detailed analysis of the action of metabolic hormones on these pathways.
Fig. 1.
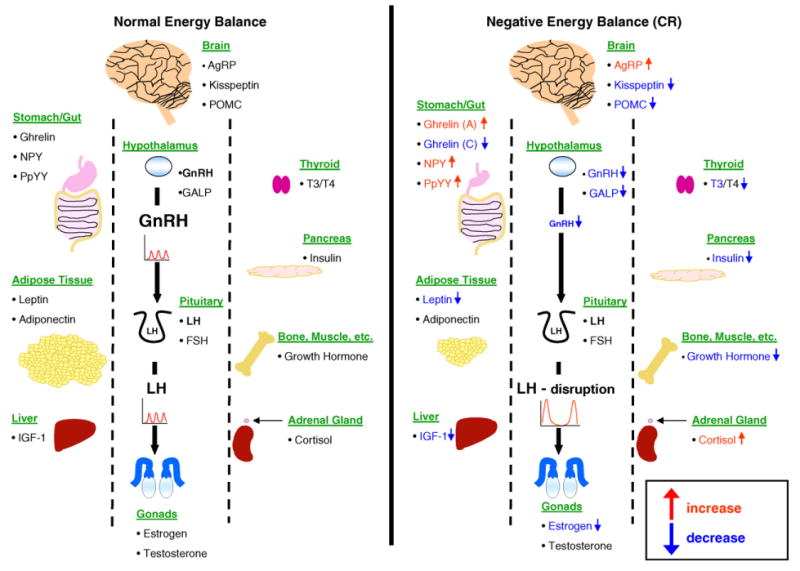
The effects of negative energy balance (caloric restriction)on pituitary function. The secretion of luteinizing hormone (LH) from the pituitary gonadotropes is controlled by gonadotropin-releasing hormone (GnRH) secreted from the hypothalamus. Under normal energy balance conditions, both are secreted in a pulsatile manner. The release patterns of GnRH and LH are themselves controlled by multiple feedback hormones (Ghrelin, NPY, polypeptide YY (PpYY), leptin, adiponectin, insulin-like growth factor 1 (IGF-1), agouti-related peptide (AgRP), kisspeptin, pro-opiomelanocortin (POMC), Galanin-like peptide (GALP), thyroid hormones 3 and 4 (T3/T4), insulin, growth hormone (GH), cortisol, estrogen and testosterone) that control the hypothalamic secretion of GnRH and the activity of gonadotropes in the anterior pituitary. In times of negative energy balance, i.e. caloric restriction, pulsatile GnRH secretion is reduced, resulting in a subsequent suppression of pulsatile LH release. A variety of metabolic hormones and neuropeptides important for energy homeostasis have been shown to influence the function of this hypothalamic-pituitary axis during caloric restriction. With reduction of visceral fat, the adipose-derived appetite hormone leptin is greatly reduced during CR. While leptin has little effect on the reproductive axis during normal energy conditions, during times of limited intake it appears to play a direct role in reduction of GnRH and thus LH secretion. While leptin levels are decreased with CR, both NPY and PpYY levels are reportedly increased, which also disrupts the GnRH/LH pulsatile release. Other peptide hormones that are altered by CR and appear to exert complex effects upon the pulsatile GnRH/LH secretion at the level of the hypothalamus and the pituitary itself are AgRP, kisspeptin, GALP and POMC. Insulin, produced in the pancreas, and IGF-1 produced in the liver, are also reduced in CR. Each has been shown to regulate hypothalamic GnRH secretion and therefore LH release, thus their reduction may be another mechanism through which these hormones are reduced during CR. Ghrelin, which is involved directly at the pituitary level, is differentially affected in CR, becoming increased with acute fasting (indicating hunger), and decreased with chronic fasting. Additionally, GH release disrupted by CR seems to control reproductive capacity as well. The thyroid hormones T3 and T4, which are involved with growth and the setting of the metabolic rate, seem to play a role in CR-mediated pituitary disruption, as abnormal levels of these hormones are associated with reproductive deficiency. In response to CR, T3 levels decrease while T4 levels remain unchanged. Caloric restriction is known to induce a mild stress response in the organism. Interestingly, GnRH pulsatile secretion (and thus LH secretion) is reduced by increased exposure to stress-related hormones such as corticotrophin-releasing hormone (CRH). One final effect that may lead to the reduction of LH pulsatile secretion during CR is the reported enhancement of negative feedback by estrogen and testosterone onto the pituitary during times of negative energy balance. Additional abbreviation: follicle-stimulating hormone (FSH).
6. Ghrelin and growth hormone
Ghrelin, the endogenous ligand for the GH secretagogue receptor (GHS-R), has been recently identified to be involved in the regulation of reproductive function. Ghrelin has been shown to not only directly control gonadal function but to also affect gonadotropin secretion itself. Similarly to leptin, the primary actions of ghrelin upon reproductive function were long considered to be associated with its activity at the level of the hypothalamus. However the GH-releasing effects of ghrelin are conducted directly at the pituitary level where there are high levels of expression of its cognate receptor in somatotropes (for review, see Van der Lely et al., 2004). The effects of ghrelin upon pituitary function are dependent upon the nature of the model employed. Ghrelin is able to inhibit GnRH-induced LH release from pituitaries from prepubertal animals and adult cyclic female rats regardless of their stage in the estrous cycle (Iqbal et al., 2006; Fernandez-Fernandez et al., 2005). However in ex vivo studies, ghrelin (at high concentrations) has been shown to directly stimulate LH and FSH secretion from pituitaries extracted from prepubertal and adult male and female rats (Iqbal et al., 2006; Fernandez-Fernandez et al., 2005). This dichotomy of function was hypothesized to be indicative of potentially different roles of locally produced ghrelin at the pituitary as opposed to systemically circulating ghrelin. Therefore, high concentrations are only likely to be achieved by locally produced ghrelin where it may be stimulatory, while the systemic ghrelin may have an inhibitory action on pulsatile LH release.
When hypothalamic explants from 15-day-old male rats were studied 90 min after an intraperitoneal injection of leptin, ghrelin or agouti-related protein (AgRP), the GnRH interpulse interval was significantly increased by ghrelin and AgRP and decreased by leptin. In 50-day-old animals, an increase in GnRH interpulse interval was also caused by ghrelin and AgRP (Lebrethon et al., 2007). When the peptides were directly incubated with the explants, the effects of leptin and AgRP in vitro were consistent with those seen after in vivo administration. By contrast, ghrelin resulted in a reduction of GnRH interpulse interval and this was observed in 15-day-old rats only. However, it is important to note that the role(s) of growth hormone and ghrelin in mediating neuroendocrine responses could potentially differ between rodents and humans.
GnRH release interpulse intervals from hypothalamic explants are significantly decreased after incubation with cocaine and amphetamine-regulated transcript (CART), and NPY. On the other hand, alpha-melanocyte-stimulating hormone and corticotrophin-releasing hormone (CRH) typically repress GnRH pulsatility. Application of an NPY-Y5 receptor antagonist failed to affect the action of leptin on GnRH pulsatility, whereas that antagonist totally prevented the decrease in GnRH interpulse interval caused by ghrelin. The ghrelin-induced reduction of GnRH interpulse interval was partially prevented by SHU 9119 (melanocortin MC3/MC4 receptor antagonist). The NPY-Y5 receptor antagonist resulted in increased GnRH interpulse intervals in pre- and post-pubertal males alike. Interestingly therefore, leptin and ghrelin show opposing effects on pulsatile GnRH secretion after administration in vivo whereas they both have stimulatory effects in vitro. The melanocortigenic system appears to mediate the effects of both leptin and ghrelin. The effects of ghrelin also involve NPY receptors and operate effectively before and at sexual maturity (Lebrethon et al., 2007).
Malnutrition, and more specifically protein restriction, often results in growth retardation. Restriction of dietary protein (for as little as 4 days) can result in reduced GH pulse amplitude, attenuated plasma GH concentrations as well as a reduction in pituitary mass and GH content (Harel and Tannenbaum, 1993). It seems that both GH and one if its peripheral mediators of function, insulin-like growth factor-1 (IGF-1) are both implicated in the growth retardation associated with chronic caloric restriction (Harel and Tannenbaum, 1993). Protein restriction in animal models reduces circulating levels of IGF-1 (Fliesen et al., 1989) and also increases IGF-1 clearance from circulation (Thissen et al., 1992). Recovery of growth retardation in such animals though is not achieved with simple IGF-1 replacement, which demonstrates that disruption of GH pulsatility through CR is probably the prime mediator of the growth effects.
Many reports have demonstrated that decreased food intake can severely inhibit normal reproductive function in both men and women (for review see Bergendahl and Veldhuis, 1995). CR-induced suppression of the pulsatile release of GnRH from the hypothalamus is considered to be the central mechanism by which this reproductive perturbation occurs (Veldhuis et al., 1993; Cameron et al., 1991). It has been shown that GnRH receptor content (which is controlled by GnRH levels in the hypophyseal portal system) in the pituitary is sensitive to CR and fasting (Bergendahl et al., 1989; Greuenwald and Matusmoto, 1993). The resultant effects of short-term fasting, i.e. decline of circulating LH and testosterone, however can be reversed rapidly upon feeding, suggesting that there is a calorie-dependent regulation of GnRH pulse-generator output (Aloi et al., 1997). Further linking GnRH function, there is evidence of not only GnRHs role in generic neuronal excitability during fasting (Röjdmark, 1987), controlling the desire behind food intake (Kauffman et al., 2005a), but there is also evidence that food intake itself directly controls the mRNA and protein levels of GnRH in mammalian brains (Kauffman et al., 2006).
It has been shown that even short periods of dietary energy deprivation (24–72 h) can have dramatic effects upon the hypothalamic-pituitary-gonadal (HPG) axis in humans and animal models. The effects of CR upon the HPG axis normally manifest themselves in a decrease in the pulsatility of LH and a concomitant diminution of serum testosterone levels (Cameron et al., 1991; Parfitt et al., 1991). Acute fasting (72 h) in normal-weight females induces disruption of LH peak amplitudes and frequency (Olson et al., 1995) but this degree of perturbation has not been shown to affect follicle development or circulating estradiol levels. Voluntary and chronic CR is often self-imposed in humans, most usually in a syndrome termed anorexia nervosa (AN). Anorexia nervosa is typically associated with chronic malnutrition resulting in a body mass index (BMI) lower than 17.5 kg/m2 (American Psychiatric Association, 1994). One neuroendocrine alteration that is seen in AN patients are that basal and GHRH-stimulated serum GH levels are increased (Stoving et al., 1999). Interestingly there does not appear to be any change in GH half-life in AN patients, however the pituitary GH secretory burst frequency, burst mass and burst duration are significantly increased in AN women compared to women with a normal BMI. In addition to these changes in secretion volume in AN patients, a marked irregularity in the GH pulses was observed (Stoving et al., 1999). It is generally considered that the changes in GH secretion seen in AN probably reflects altered hypothalamic-pituitary feedback.
7. Polypeptide YY and NPY
Neuropeptide Y, the most abundant neuropeptide in the brain (Allen et al., 1983), plays a pivotal role in the central control of food intake, energy balance and the modulation of neuroendocrine function. It has been implicated in the regulation of the hypothalamo-pituitary-somatotropic axis and the hypothalamo-pituitary-gonadotropic axis. Elevated hypothalamic neuropeptide Yexpression and eventual secretion, such as that occurs during fasting or CR, is known to inhibit both of these axes (Kalra et al., 1991; Brady et al., 1990; Sahu et al., 1988; Adam et al., 1997). In times of CR, NPYexpression in the brain typically increases and participates in the inhibition of reproductive function. Chronic low dietary energy intake reduces LH pulse frequency but increases the circulating levels of LH by virtue of an increase in pulse amplitude. This effect of CR also seems to concomitantly increase hypothalamic NPY gene expression (Adam et al., 1997).
Development and normal function of the reproductive axis requires a precise degree of body energy stores. Polypeptide YY-(3–36) [PYY-(3–36)] is a gastrointestinal secreted molecule recently shown to be involved in the control of food intake with agonistic activity on NPY receptor subtypes Y2 and Y5. Notably, PYY-(3–36) has been recently demonstrated to be a putative regulator of gonadotropin secretion in the rat. Central administration of PYY(13-36) (which is an agonist of Y(2) receptors), significantly decreased the circulating levels of both gonadotropins, an effect that was observed in both prepubertal and adult rats. A dual action of the Y(2) receptors in the control of the male gonadotropic axis has been demonstrated as their activation induces stimulation of gonadotropin responses to GnRH at the pituitary, but inhibition of GnRH secretion at the hypothalamus. Antagonism of Y(2) receptors failed to modify basal LH secretion in intact males either after being fed ad libitum or after being fasted. In contrast, their central blockade in orchidectomized rats evoked a significant increase in circulating LH and FSH levels, suggesting the constitutive activation of Y(2) receptor in such stimulated conditions. These data provide evidence for a complex mode of action of Y(2) receptors in the control of the gonadotropic axis, with stimulatory and inhibitory actions at different levels of the system that are sensitive to the gonadal status (Pinilla et al., 2007).
The neuropeptide Y system seems to be intricately involved in connecting energy balance to the state of the pituitary and reproductive status. For example the Y2 subtype of the NPY receptor seems to regulate CR-induced changes in hypothalamic GHRH expression and circulating IGF-1 levels (Lin et al., 2007). When the somatic NPY Y4 receptor subtype is knocked out, this prevents CR-induced changes in hypothalamic GnRH expression and peripheral testosterone levels (Lin et al., 2007). In addition to the Y4 receptor subtype, it seems that the integration of energy intake and reproductive function also relies in part on Y1 type receptor activation, as Y1 receptor knock-out animals have also been shown to possess a reproductive axis that is resistant to mild chronic CR (Gonzales et al., 2003). As well as being involved in responses to chronic energy shortages, NPY Y1 receptor activation also seems to integrate acute fasting to short-term disruption of the gonadotrope axis (Pralong et al., 2002). With respect to this particular study, it is of interest to note that this relationship was maintained in animals with no leptin receptor, therefore suggesting that Y1 receptor stimulation is inhibitory upon the GnRH axis in times of CR and is independent of leptin levels as well. It is therefore likely that hypothalamic GnRH neurons themselves express Y1 receptors that can mediate these leptin-independent effects.
8. Androgens
A variety of studies have indicated that testicular negative feedback on LH secretion is enhanced during food restriction (Dong et al., 1994). These studies reveal that undernutrition both enhances tonic, androgen receptor-mediated feedback suppression of GnRH secretion and increases in pituitary (but not hypothalamic) androgen receptor numbers to cause inhibition of LH secretion.
Growth retardation induced by dietary restriction in the lamb results in a low frequency of episodic LH secretion and, thus, delayed puberty. Such lambs respond normally to physiological doses of GnRH, indicating that the pituitary gland can function adequately during diet-induced hypogonadotropism. This suggests that central mechanisms controlling the release, rather than synthesis, of GnRH are limiting LH secretion when sexual maturation is delayed by growth retardation (Ebling et al., 1990). These findings indicate that dietary restriction in the developing female lamb depresses gonadotropin secretion without reducing other anterior pituitary gland secretions, such as prolactin (PRL) and GH. That these changes occur in the absence of the ovaries implies that metabolic and growth-related modulation of neuroendocrine function can occur independently of changes in sensitivity to the feedback actions of ovarian steroids and polypeptides (Foster et al., 1989).
9. Insulin and IGF-1
Along with the multiple neuropeptides derived from the hypothalamus, insulin also appears to mediate a humoral connection between energy intake levels and reproduction. Insulin is released from the pancreatic beta cells according to the input food status of the organism. However the resting insulin levels are also indicative of the status of stored energy reserves. During dietary restriction these reserves will rapidly become depleted and thus resting insulin levels will be attenuated (Martin et al., 2007). As the steady state of insulin is correlated with the existing energy levels in the animal, these values may be used to communicate information about long-term metabolic conditions to the reproductive axis. For example, it has been shown that circulating insulin can directly regulate GnRH and subsequently LH secretion (Miller et al., 1995; Hileman et al., 1993). Central administration of insulin as well has also been shown to directly stimulate GnRH neurons, resulting in LH pulsatile release (Kovacs et al., 2002; Kotani et al., 2002). Recent studies of in vitro gonadotrope function have also shown that insulin can directly enhance the mRNA levels of LH itself (Dorn et al., 2004). In situations of caloric deficit, e.g. CR or fasting, where insulin levels are low, the pulsatile release patterns of LH are effectively inhibited. Recovery of the circulating levels of insulin in these cases can rapidly reverse the perturbation of the pulsatile LH release. Excessive caloric intake, resulting in obesity and diabetes, also can disrupt the reproductive system as diabetic animals have been shown to display delayed pubertal maturation, reduced ovulation, incomplete estrous cycles, disrupted pulsatile GnRH and LH release as well as loss of reproductive desire (Karkanias et al., 1997; Katayama et al., 1984; Kovacs et al., 2002). As with leptin receptor deficiency, experimental animal models lacking proper insulin signaling become obese and possess a disrupted metabolism and reproductive capacity.
IGF-1 appears to directly possess the capacity to regulate gonadotrope functionality (Pazos et al., 2004). Exposure of pituitary cells to IGF-I can stimulate basal LH and FSH release without significantly altering their mRNA levels. These gonadotropins are composed of a specific beta-subunit protein (LH- or FSH-β) and a common alpha-subunit. In contrast to IGF-1s actions on LH and FSH, IGF-1 treatment of pituitaries has been shown to elicit a functional secretion of α-subunit protein from the cells and to augment its mRNA levels. Exposure to IGF-I consistently has been shown to reduce GH release from pituitary cells (Pazos et al., 2004).
10. Thyroid hormone
Thyroid-derived hormones, T3 and T4 are critical for growth and reproduction as well as their primary role of setting the general metabolic rate of the organism (Dellovade et al., 1996). Abnormal plasma levels of the thyroid hormones are typically associated with reproductive deficiency (Ortega et al., 1990). Excessive levels of plasma thyroid hormones stunts growth and development, inhibits sexual behaviour (Morgan et al., 2000), decreases plasma LH levels and thus impairs gonadal function (Chandrasekhar et al., 1985). Hypothyroidism as well as hyperthyroidism also can modulate the reproductive capacity of experimental animals, e.g. low plasma levels of thyroid hormones is associated with abnormal menstrual cycles and disrupted follicular development (Ortega et al., 1990; Vriend et al., 1987). From experimental data it appears that in response to protracted CR there is a decrease in serum T3 concentrations but little change in T4 (Roth et al., 2002; Fontana et al., 2006). The effect of CR upon TSH however seems quite subtle as there appears to be little change in TSH levels during acute CR, but with long-term CR TSH levels may be elevated (Roth et al., 2002).
11. Stress hormone-related pathways
Considering the complexity of gonadal feedback to the pituitary in times of caloric alteration, many experimental paradigms investigating the link between energy balance and reproduction take place in ovariectomized animals (supplemented with estrogen). Many groups have reported that CR, either chronically or through IF results in activation of stress pathways, e.g. increases in circulating cortisol and norepinephrine (Martin et al., 2007; Cagampang et al., 1992; Luque et al., 2007). The initiation of this stress-related reproductive feedback has been suggested to emanate from the upper digestive tract. CR-induced activation of vagal afferents stimulates regions of the medulla oblongata, notably the NTS, to activate noradrenergic input to the hypothalamic paraventricular nucleus (PVN: Cagampang et al., 1992). At the PVN, the noradrenergic stimulation of hypothalamic α2-adrenoceptors results in an increase in secretion of CRH onto GnRH positive neurons. CRH stimulation of GnRH-positive neurons suppresses the GnRH release on to the pituitary resulting in an attenuation of the pulsatile LH release (Gong, 2002).
12. Galanin-like peptide (GALP)
Galanin-like peptide (GALP) was originally isolated from porcine hypothalamus and was shown to possess a capacity to stimulate the galanin type 2 G protein-coupled receptor (Ohtaki et al., 1999). GALP is specifically expressed in the arcuate nucleus of the hypothalamus. GALP expression is tightly linked to the metabolic state of the organism as its expression is regulated by both circulating levels of leptin and insulin (Kumano et al., 2003; Fraley et al., 2004). GALP-positive neurons appear to be directly stimulated by leptin as they possess the ObRb leptin receptor. It has been demonstrated that central administration of GALP stimulates GnRH release and subsequently LH secretion in numerous mammalian models (Gottsch et al., 2004b; Kauffman et al., 2005b). GALP expression, as one would expect by its leptin/insulin control, is profoundly reduced by implementation of CR regimes. It is also of interest to note that the deleterious effects of diabetic states upon reproductive function can be partially restored with administration of the GALP peptide (Krasnow et al., 2003; Matsumoto et al., 2001).
13. Pro-opiomelanocortin
Pro-opiomelanocortin (POMC) is a precursor polypeptide (241 residues) that is synthesized in the pituitary, hypothalamus, brainstem and in melanocytes. POMC can be differentially cleaved by proteases to generate an array of hormones (adrenocorticotrophic hormone [ACTH], lipotropin, corticotrophin-like intermediate peptide [CLIP], melanocyte-stimulating hormone [MSH] and β-endorphin) that exert multiple effects crucial in linking the organism's metabolic state to its reproductive capacity. The expression of POMC mRNA is significantly reduced by CR implemented upon rats, mice and also primates (Bergendahl et al., 1992; Koegler et al., 2001; Schwartz et al., 1997; Mizuno et al., 1998). The proteolytic cleavage products themselves of POMC have differential effects on the reproductive axis, e.g. α-MSH reduces appetite but stimulates reproductive behavior in female rats (Gonzales et al., 1993; Scimonelli et al., 2000), while β-endorphin stimulates appetite and inhibits GnRH and LH secretion (Leadem and Kalra, 1985; Wardlaw and Ferin, 1990).
14. Conclusions
A mammal's metabolic state and its reproductive capacity are tightly linked. Dietary regimes such as CR and IF can affect multiple neuroendocrine systems, including hormones involved in energy balance, appetite, and reproduction. The maintenance of reproductive function requires a considerable amount of free available energy. Thus during times when food availability and energy supplies are low, it would make evolutionary sense to re-direct any available energy to maintaining brain function and cognition. In the wild, food scarcity imposes a stress on the animal, motivating them to seek food elsewhere. This may be particularly important in females as they must obtain sufficient energy to support the survival and development of their offspring as well as themselves (Martin et al., 2007). Diverting energy from reproduction to brain and neuromuscular activity would be expected to increase the probability of survival. One consideration to take into account when investigating the effects of CR is that different variations of the caloric restriction paradigm can affect neuroendocrine systems in different manners. The effects of reducing caloric intake upon whole body physiology will depend upon when the CR paradigm is initiated (e.g. pre-pubertal, post-pubertal, middle age, old age), the degree of CR (e.g. 15% CR, 25% CR, 40% CR or IF), and whether overall caloric intake is decreased or whether caloric intake of one particular food-component (e.g. protein, fat or carbohydrate) is reduced. Caloric restriction probably exerts its effects through a myriad of highly complex systems, including neuroendocrine feedback, inflammatory response, etc. Fully understanding and teasing out the effects of CR upon neuroendocrine function and pulsatility could lead to better strategies for maintaining reproductive health.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging.
References
- Adam CL, Findlay PA, Kyle CE, Young P, Mercer JG. Effect of chronic food restriction on pulsatile luteinizing hormone secretion and hypothalamic neuropeptide Y gene expression in castrated male sheep. J Endocrinol. 1997;152:329–337. doi: 10.1677/joe.0.1520329. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- Aloi JA, Bergendahl M, Iranmanesh A, Veldhuis JD. Pulsatile intravenous gondaotropin-releasing hormone administration averts fasting-induced hypogonadotropism and hypoandrogenemia in healthy, normal weight men. J Clin Endocrinol Metab. 1997;82:1543–1548. doi: 10.1210/jcem.82.5.3947. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Barash I, Cheung CC, Weigle DS, Ren H, Kabigting E, Kuijper J, Clifton D, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Iranmanesh A, Evans WS, Veldhuis JD. Short-term fasting selectively suppresses leptin pulse mass and 24-hour rhythmic leptin release in healthy midluteal phase women without disturbing leptin pulse frequency or its entropy control (pattern orderliness) J Clin Endocrinol Metab. 2000;85:207–213. doi: 10.1210/jcem.85.1.6325. [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Perheentupa A, Huhtaniemi I. Effect of short-term starvation on reproductive hormone gene expression, secretion and receptor levels in male rats. J Endocrinol. 1989;121:409–417. doi: 10.1677/joe.0.1210409. [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Veldhuis JD. Altered pulsatile gonadotropin signalling in nutritional deficiency in the male. Trends Endocrinol Metab. 1995;6:145–149. doi: 10.1016/1043-2760(95)00081-r. [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Wiemann J, Clifton DK, Huhtaniemi I, Steiner R. Short-term starvation decreases POMC mRNA but does not alter GnRH mRNA in the brain of adult male rats. Neuroendocrinology. 1992;56:913–920. doi: 10.1159/000126324. [DOI] [PubMed] [Google Scholar]
- Bergland RM, Page RB. Can the pituitary secrete directly to the brain? (Affirmative anatomical evidence) Endocrinology. 1978;102:1325–1338. doi: 10.1210/endo-102-5-1325. [DOI] [PubMed] [Google Scholar]
- Brady LS, Smith MA, Gold PW, Harkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52:441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Maeda K, Ota K. Involvement of the gastric vagal nerve in the suppression of pulsatile luteinizing hormone release during acute fasting in rats. Endocrinology. 1992;130:3003–3006. doi: 10.1210/endo.130.5.1572309. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Weltzin TE, McConaha C, Helmreich DL, Kaye W. Slowing of pulsatile luteinizing hormone secretion in men after forty-eight hours of fasting. J Clin Endocrinol Metab. 1991;73:35–41. doi: 10.1210/jcem-73-1-35. [DOI] [PubMed] [Google Scholar]
- Casanueva FF, Dieguez C. Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol. 1999;20:317–363. doi: 10.1006/frne.1999.0187. [DOI] [PubMed] [Google Scholar]
- Castellano J, Navarro V, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez M, Vigo E, Casanueva F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Castellano J, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- Chan JL, Mantzoros CS. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary. 2001;4:87–92. doi: 10.1023/a:1012947113197. [DOI] [PubMed] [Google Scholar]
- Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar Y, Holland MK, D'Occhio M, Setchell B. Spermatogenesis, seminal characteristics and reproductive hormone levels in mature rams with induced hypothyroidism and hyperthyroidism. J Endocrinol. 1985;105:39–46. doi: 10.1677/joe.0.1050039. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- Cheung C, Clifton D, Steiner R. Perspectives on leptin's role as a metabolic signal for the onset of puberty. Front Horm Res. 2000;26:87–105. doi: 10.1159/000061017. [DOI] [PubMed] [Google Scholar]
- Clarke IJ. Multifarious effects of estrogen on the pituitary gonadotrope with special emphasis on studies in the ovine species. Arch Physiol Biochem. 2002;110:62–73. doi: 10.1076/apab.110.1.62.898. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Clements MK, McDonald TP, Wang R, Xie G, O'Dowd BF, George SR, Austin CP, Liu Q. FMRFamide-related neuropeptides are agonists of the orphan G protein-coupled receptor GPR54. Biochem Biophys Res Commun. 2001;284:1189–1193. doi: 10.1006/bbrc.2001.5098. [DOI] [PubMed] [Google Scholar]
- Crawford JL, McNeilly AS. Co-localization of gonadotrophins and granins in gonadotrophs at different stages of the oestrous cycle in sheep. J Endocrinol. 2002;174:179–194. doi: 10.1677/joe.0.1740179. [DOI] [PubMed] [Google Scholar]
- Cunningham M, Clifton DK, Steiner RA. Leptin's actions on the reproductive axis: perspectives and mechanisms. Biol Reprod. 1999;60:216–222. doi: 10.1095/biolreprod60.2.216. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roux N, Genin E, Carel J, Matsuda F, Chaussain J, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellovade T, Zhu Y, Krey L, Pfaff D. Thyroid hormone and estrogen interact to regulate behaviour. Proc Natl Acad Sci USA. 1996;93:12581–12586. doi: 10.1073/pnas.93.22.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Rintala H, Handelsman DJ. Androgen receptor function during undernutrition. J Neuroendocrinol. 1994;6:397–402. doi: 10.1111/j.1365-2826.1994.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Dorn C, Mouillet JF, Yan X, Qu Q, Sadovsky Y. Insulin enhances the transcription of luteinizing hormone-beta gene. Am J Obstet Gynecol. 2004;191:132–137. doi: 10.1016/j.ajog.2004.01.054. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Wood RI, Karsch FJ, Vannerson LA, Suttie JM, Bucholtz DC, Schall RE, Foster DL. Metabolic interfaces between growth and reproduction. III. Central mechanisms controlling pulsatile luteinizing hormone secretion in the nutritionally growth-limited female lamb. Endocrinology. 1990;126:2719–2727. doi: 10.1210/endo-126-5-2719. [DOI] [PubMed] [Google Scholar]
- Faria AC, Beckenstein LW, Booth RA, Jr, Vaccaro VA, Asplin CM, Veldhuis JD, Thorner MO, Evans WS. Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol. 1992;36:591–596. doi: 10.1111/j.1365-2265.1992.tb02270.x. [DOI] [PubMed] [Google Scholar]
- Farnworth PG. Gonadotrophin secretion revisited, how many ways can FSH leave a gonadotroph? J Endocrinol. 1995;145:387–395. doi: 10.1677/joe.0.1450387. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E, Pinilla L. Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in female adult rats: in vivo and in vitro studies. Neuroendocrinology. 2005;82:245–255. doi: 10.1159/000092753. [DOI] [PubMed] [Google Scholar]
- Fliesen T, Maiter D, Gerard G, Underwood LE, Maes M, Ketelslegers JM. Reduction of serum insulin-like growth factor-1 by dietary protein restriction is age dependent. Pediatr Res. 1989;26:415–419. doi: 10.1203/00006450-198911000-00010. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Foster DL, Ebling FJ, Micka AF, Vannerson LA, Bucholtz DC, Wood RI, Suttie JM, Fenner DE. Metabolic interfaces between growth and reproduction. I. Nutritional modulation of gonadotropin, prolactin, and growth hormone secretion in the growth-limited female lamb. Endocrinology. 1989;125:342–350. doi: 10.1210/endo-125-1-342. [DOI] [PubMed] [Google Scholar]
- Fraley G, Scarlett J, Shimada I, Teklemichael DN, Acohido B, Clifton DK, Steiner R. Effects of diabetes and insulin on the expression of galanin-like peptide in the hypothalamus of the rat. Diabetes. 2004;53:1237–1242. doi: 10.2337/diabetes.53.5.1237. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and regulation of body weight in mammals. Nature. 1998;39:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gong JG. Influence of metabolic hormones and nutrition on ovarian follicle development in cattle: practical implications. Domest Anim Endocrinol. 2002;23:229–241. doi: 10.1016/s0739-7240(02)00159-5. [DOI] [PubMed] [Google Scholar]
- Gonzales C, Voirol MJ, Giacomini M, Gaillard RC, Pedrazzini T, Pralong FP. The neuropeptide Y1 receptor mediates NPY-induced inhibition of the gonadotrope axis under poor metabolic conditions. FASEB J. 2003;18:137–139. doi: 10.1096/fj.03-0189fje. [DOI] [PubMed] [Google Scholar]
- Gonzales M, Celis M, Hole D, Wilson C. Interaction of oestradiol, α-melanotrophin and noradrenaline within the ventromedial nucleus in the control of female sexual behaviour. Neuroendocrinology. 1993;58:218–226. doi: 10.1159/000126536. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Clifton DK, Steiner RA. Galanin-like peptide as a link in the integration of metabolism and reproduction. Trends Endocrinol Metab. 2004b;15:215–221. doi: 10.1016/j.tem.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004a;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Greuenwald DA, Matusmoto AM. Reduced gonadotropin-releasing hormone gene expression with fasting in the male rat brain. Endocrinology. 1993;132:480–482. doi: 10.1210/endo.132.1.8419144. [DOI] [PubMed] [Google Scholar]
- Han S, Gottsch ML, Lee K, Popa S, Smith J, Jakawich S, Clifton D, Steiner RA, Herbison A. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel Z, Tannenbaum GS. Dietary protein restriction impairs both spontaneous and growth hormone-releasing factor-stimulated growth hormone release in the rat. Endocrinology. 1993;133:1035–1043. doi: 10.1210/endo.133.3.8103447. [DOI] [PubMed] [Google Scholar]
- Hartmann ML, Pezzoli SS, Hellmann PJ, Suratt PM, Thorner MO. Pulsatile growth hormone secretion in older persons is enhanced by fasting without relationships to sleep stages. J Clin Endocrinol Metab. 1996;81:2694–2701. doi: 10.1210/jcem.81.7.8675598. [DOI] [PubMed] [Google Scholar]
- Henry B, Goding J, Tilbrook A, Dunshea FR, Clarke IJ. Intracerebroventricular infusion of leptin elevates the secretion of luteinizing hormone without affecting food intake in long-term food-restricted sheep, but increases growth hormone irrespective of body weight. J Endocrinol. 2001;168:67–77. doi: 10.1677/joe.0.1680067. [DOI] [PubMed] [Google Scholar]
- Hileman S, Schillo KK, Hall J. Effects of acute, intracerebroventricular administration of insulin on serum concentrations of luteinizing hormone, insulin and glucose in ovariectomized lambs during restricted and ad libitum feed intake. Biol Reprod. 1993;48:117–124. doi: 10.1095/biolreprod48.1.117. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Kurose Y, Canny B, Clarke IJ. Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin and cortisol secretion in sheep. Endocrinology. 2006;147:510–519. doi: 10.1210/en.2005-1048. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Pompolo S, Considine RV, Clarke IJ. Localization of leptin receptor-like immunoreactivity in the corticotropes, somatotropes and gonadotropes in the ovine anterior pituitary. Endocrinology. 2000;141:1515–1520. doi: 10.1210/endo.141.4.7433. [DOI] [PubMed] [Google Scholar]
- Irwig M, Fraley G, Smith J, Acohido BV, Popa S, Cunningham M, Gottsch ML, Clifton DK, Steiner R. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, Kulig E, Lloyd RV. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;84:2903–2911. doi: 10.1210/jcem.84.8.5908. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci USA. 1991;88:10931–10935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. Do testosterone and estradiol-17β enforce inhibition or stimulation of luteinizing hormone-releasing hormone secretion? Biol Reprod. 1989;41:559–570. doi: 10.1095/biolreprod41.4.559. [DOI] [PubMed] [Google Scholar]
- Karkanias G, Morales J, Li C. Deficits in reproductive behaviour in diabetic female rats are due to hypoinsulinemia rather than hyperglycemia. Horm Behav. 1997;32:19–29. doi: 10.1006/hbeh.1997.1401. [DOI] [PubMed] [Google Scholar]
- Katayama S, Brownscheidle C, Wootten V, Lee J, Shimaoka K. Absent or delayed pre-ovulatory luteinizing hormone surge in experimental diabetes mellitus. Diabetes. 1984;33:324–327. doi: 10.2337/diab.33.4.324. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Bojkowska K, Wills A, Rissman EF. Gonadotropin-releasing hormone-II messenger ribonucleic acid and protein content in the mammalian brain are modulated by food intake. Endocrinology. 2006;147:5069–5077. doi: 10.1210/en.2006-0615. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Buenzle J, Fraley G, Rissman EF. Effects of galanin-like peptide on locomotion, reproduction and body weight in female and male mice. Horm Behav. 2005b;48:141–151. doi: 10.1016/j.yhbeh.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Wills A, Millar RP, Rissman EF. Evidence that the type-2 gonadotropin-releasing hormone (GnRH) receptor mediates the behavioural effects of GnRH-II on feeding and reproduction in musk shrews. J Neuroendocrinol. 2005a;17:489–497. doi: 10.1111/j.1365-2826.2005.01334.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Moriyama R, Tsukamura H, Maeda KI. A rat model for the energetic regulation of gonadotropin secretion: role of the glucose-sensing mechanism in the brain. Domest Anim Endocrinol. 2003;25:109–120. doi: 10.1016/s0739-7240(03)00050-x. [DOI] [PubMed] [Google Scholar]
- Knobil E. The electrophysiology of the GnRH pulse generator in the rhesus monkey. J Steroid Biochem. 1989;33:669–671. doi: 10.1016/0022-4731(89)90476-7. [DOI] [PubMed] [Google Scholar]
- Knobil E. Patterns of hypophysiotropic signals and gonadotrophin secretion in the rhesus monkey. Biol Reprod. 1981;24:44–49. doi: 10.1095/biolreprod24.1.44. [DOI] [PubMed] [Google Scholar]
- Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207:1371–1373. doi: 10.1126/science.6766566. [DOI] [PubMed] [Google Scholar]
- Koegler F, Grove K, Schiffmacher A, Smith M, Cameron J. Central melanocortin receptors mediate changes in food intake in the rhesus macaque. Endocrinology. 2001;142:2586–2592. doi: 10.1210/endo.142.6.8198. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes Kisspeptins, the natural ligands of the orphan G protein coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Kovacs P, Parlow A, Karkanias G. Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology. 2002;76:357–365. doi: 10.1159/000067585. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Parlow AF, Karkanias GB. Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology. 2002;76:357–365. doi: 10.1159/000067585. [DOI] [PubMed] [Google Scholar]
- Krasnow S, Fraley G, Schuh S, Baumgartner J, Clifton DK, Steiner R. A role for galanin-like peptide in the integration of feeding, body weight regulation and reproduction in the mouse. Endocrinology. 2003;144:813–822. doi: 10.1210/en.2002-220982. [DOI] [PubMed] [Google Scholar]
- Kumano S, Matsumoto H, Takatsu Y, Noguchi J, Kitada C, Ohtaki T. Changes in hypothalamic expression levels of galanin-like peptide in rat and mouse models support that it is a leptin-target peptide. Endocrinology. 2003;144:2634–2643. doi: 10.1210/en.2002-221113. [DOI] [PubMed] [Google Scholar]
- Leadem CA, Kalra S. Effects of endogenous opioid peptides and opiates on luteinizing hormone and prolactin secretion in ovariectomized rats. Neuroendocrinology. 1985;41:342–352. doi: 10.1159/000124199. [DOI] [PubMed] [Google Scholar]
- Lebrethon MC, Aganina A, Fournier M, Gérard A, Parent AS, Bourguignon JP. Effects of in vivo and in vitro administration of ghrelin, leptin and neuropeptide mediators on pulsatile gonadotrophin-releasing hormone secretion from male rat hypothalamus before and after puberty. J Neuroendocrinol. 2007;19:181–188. doi: 10.1111/j.1365-2826.2006.01518.x. [DOI] [PubMed] [Google Scholar]
- Lin S, Lin EJ, Boey D, Lee NJ, Slack K, During MJ, Sainsbury A, Herzog H. Fasting inhibits the growth and reproductive axes via distinct Y2 and Y4 receptor-mediated pathways. Endocrinology. 2007;148:2056–2065. doi: 10.1210/en.2006-1408. [DOI] [PubMed] [Google Scholar]
- Lopez de Maturana R, Martin B, Millar RP, Brown P, Davidson L, Pawson AJ, Nicol MR, Mason JI, Barran P, Naor Z, Maudsley S. GnRH-mediated DAN production regulates the transcription of the GnRH receptor in gonadotrope cells. Neuromol Med. 2007;9:230–248. doi: 10.1007/s12017-007-8004-z. [DOI] [PubMed] [Google Scholar]
- Luque RM, Park S, Kineman RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotrophin-releasing hormone. Endocrinology. 2007;148:300–309. doi: 10.1210/en.2006-0592. [DOI] [PubMed] [Google Scholar]
- Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord. 2007;8:21–29. doi: 10.1007/s11154-007-9032-6. [DOI] [PubMed] [Google Scholar]
- Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–4333. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto AM, Paulsen CA, Bremner WJ. Stimulation of sperm production by human luteinizing hormone in gonadotropin-suppressed normal men. J Clin Endocrinol Metab. 1984;59:882–887. doi: 10.1210/jcem-59-5-882. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Noguchi J, Takatsu Y, Horikoshi Y, Kumano S, Ohtaki T, Kitada C, Itoh T, Onda H, Nishimura O, Fujino M. Stimulation effect of galanin-like peptide on luteinizing hormone-releasing hormone-mediated luteinizing hormone secretion in male rats. Endocrinology. 2001;142:3693–3696. doi: 10.1210/endo.142.8.8432. [DOI] [PubMed] [Google Scholar]
- Maudsley S, Naor Z, Bonfil D, Davidson L, Karali D, Pawson AJ, Larder R, Pope C, Nelson N, Millar RP, Brown P. Proline-rich tyrosine kinase 2 mediated gonadotropin-releasing hormone signaling to a specific extracellularly regulated kinase-sensitive transcriptional locus in the luteinizing hormone beta-subunit gene. Mol Endocrinol. 2007;21:1216–1233. doi: 10.1210/me.2006-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AS. The control of FSH secretion. Acta Endocrinol Suppl (Copenh) 1988;288:31–40. [PubMed] [Google Scholar]
- McNeilly AS, Crawford JF, Taragnat C, Nicol L, McNeilly JR. The differential secretion of LH and FSH: regulation through genes, feedback and packaging. Reprod Suppl. 2003;61:463–476. [PubMed] [Google Scholar]
- Messager S, Chatzidaki E, Ma D, Hendrick A, Zahn D, Dixon J, Thresher R, Malinge I, Lomet D, Carlton MB, Colledge W, Caraty A, Aparicio S. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar RP, Flanagan CA, Milton RC, King JA. Chimeric analogues of vertebrate gonadotropin-releasing hormones comprising substitutions of the variant amino acids in positions 5, 7, and 8. Characterization of requirements for receptor binding and gonadotropin release in mammalian and avian pituitary gonadotropes. J Biol Chem. 1989;264:21007–21013. [PubMed] [Google Scholar]
- Millar RP, King JA, Davidson JS, Milton RC. Gonadotrophin-releasing hormone—diversity of functions and clinical applications. S Afr Med J. 1987;72:748–755. [PubMed] [Google Scholar]
- Miller D, Blache D, Martin GB. The role of intracerebral insulin in the effect of nutrition on gonadotrophin secretion in mature male sheep. J Endocrinol. 1995;147:321–329. doi: 10.1677/joe.0.1470321. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Kleopoulos S, Bergen H, Roberts JL, Priest C, Mobbs C. Hypothalamic proopiomelanocortin mRNA is reduced by fasting in ob/ob and db/db mice but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Dellovade T, Pfaff D. Effect of thyroid hormone administration on estrogen-induced sex behaviour in female mice. Horm Behav. 2000;37:15–22. doi: 10.1006/hbeh.1999.1553. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CGC, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Nagatani S, Guthikonda P, Thompson RC, Tsukamara H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67:370–376. doi: 10.1159/000054335. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano J, Fernandez-Fernandez R, Barreiro M, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Kumano S, Ishibashi Y, Ogi K, Matsui H, Harada M, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and cDNA cloning of a novel galanin-like peptide (GALP) from porcine hypothalamus. J Biol Chem. 1999;274:37041–37045. doi: 10.1074/jbc.274.52.37041. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Olson BR, Cartledge T, Sebring N, Defensor R, Nieman L. Short-term fasting affects luteinizing hormone secretory dynamics but not reproductive function on normal-weight sedentary women. J Clin Endocrinol Metab. 1995;80:1187–1193. doi: 10.1210/jcem.80.4.7714088. [DOI] [PubMed] [Google Scholar]
- Ortega E, Rodriguez E, Ruiz E, Osorio C. Activity of the hypothalamo-pituitary ovarian axis in hypothyroid rats with or without tri-iodothyronine replacement. Life Sci. 1990;46:391–395. doi: 10.1016/0024-3205(90)90081-2. [DOI] [PubMed] [Google Scholar]
- Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Brown MB, Dahl GE, Evans NP, Karsch FJ, Mauger DT, Neill JD, Van Cleeff J. Neuroendocrine control of follicle-stimulating hormone (FSH) secretion. III. Is there a gonadotrophin-releasing hormone-independent component of episodic FSH secretion on ovariectomized and luteal phase ewes? Endocrinology. 2003;144:1380–1392. doi: 10.1210/en.2002-220973. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, McFadden K, Mauger DT, Karsch FJ, Midgley AR., Jr Neuroendocrine control of follicle-stimulating hormone (FSH) secretion. I. Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology. 1997;138:424–432. doi: 10.1210/endo.138.1.4892. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, McNeilly AS. Is there an FSH-releasing factor? Reproduction. 2001;121:21–30. doi: 10.1530/rep.0.1210021. [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Church KR, Cameron JL. Restoration of pulsatile luteinizing hormone secretion after fasting in rhesus monkey (Macaca Mullatta): dependence on size of the reefed meal. Endocrinology. 1991;129:749–756. doi: 10.1210/endo-129-2-749. [DOI] [PubMed] [Google Scholar]
- Pazos F, Sanchez-Franco F, Balsa J, Escalada J, Cacicedo L. Differential regulation of gonadotropins and glycoprotein hormone alpha-subunit by IGF-1 in anterior pituitary cells from male rats. J Endocrinol Invest. 2004;27:670–675. doi: 10.1007/BF03347501. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Fernández-Fernández R, Roa J, Castellano JM, Tena-Sempere M, Aguilar E. Selective role of neuropeptide Y receptor subtype Y2 in the control of gonadotropin secretion in the rat. Am J Physiol Endocrinol Metab. 2007;293:1385–1392. doi: 10.1152/ajpendo.00274.2007. [DOI] [PubMed] [Google Scholar]
- Pralong FP, Gonzales C, Voirol MJ, Palmiter RD, Brunner HR, Gaillard RC, Seydoux J, Pedrazzini T. The neuropeptide Y Y1 receptor regulates leptin-mediated control of energy homeostasis and reproductive functions. FASEB J. 2002;16:712–714. doi: 10.1096/fj.01-0754fje. [DOI] [PubMed] [Google Scholar]
- Röjdmark S. Increased gonadotropin responsiveness to gonadotropin-releasing hormone during fasting in normal subjects. Metabolism. 1987;36:21–26. doi: 10.1016/0026-0495(87)90057-6. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. Leptin; a molecule integrating somatic energy stores, energy expenditure and fertility. Trends Endocrinol Metab. 1998;9:117–124. doi: 10.1016/s1043-2760(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Roth GS, Handy AM, Mattison JA, Tilmont EM, Ingram DK, Lane MA. Effects of dietary caloric restriction and aging on thyroid hormones of rhesus monkeys. Horm Metab Res. 2002;34:378–382. doi: 10.1055/s-2002-33469. [DOI] [PubMed] [Google Scholar]
- Sahu A. A hypothalamic role in energy balance with a special emphasis on leptin. Endocrinology. 2004;145:2613–2620. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- Sahu A, Kalra PS, Kalra SP. Food deprivation and ingestion induce reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides. 1988;9:83–86. doi: 10.1016/0196-9781(88)90013-7. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Seeley R, Woods S, Weigle D, Campfield L, Burn P, Baskin D. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Scimonelli T, Medina F, Wilson C, Celis M. Interaction of α-melanotropin (α-MSH) and noradrenaline in the median eminence in the control of female sexual behaviour. Peptides. 2000;21:219–223. doi: 10.1016/s0196-9781(99)00191-6. [DOI] [PubMed] [Google Scholar]
- Sealfon SC, Weinstein H, Millar RP. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev. 1997;18:180–205. doi: 10.1210/edrv.18.2.0295. [DOI] [PubMed] [Google Scholar]
- Seminara S, Messager S, Chatzidaki E, Thresher R, Acierno JJ, Shagoury J, Bo-Abbas Y, Kuohung W, Schwinof K, Hendrick A, Zahn D, Dixon J, Kaiser U, Slaugenhaupt SA, Gusella J, O'Rahilly S, Carlton M, Crowley WJ, Aparicio S, Colledge W. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara S, Crowley WF, Ojeda S, Plant T. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheckter CB, Matsumoto AM, Bremner WJ. Testosterone administration inhibits gonadotropin secretion by an effect on the human pituitary. J Clin Endocrinol Metab. 1989;68:397–401. doi: 10.1210/jcem-68-2-397. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham M, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurons are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Stoving RK, Veldhuis JD, Flyvberg A, Vinten J, Hangaard J, Koldkjaer OG, Kristiansen J, Hagen C. Jointly amplified basal and pulsatile growth hormone (GH) secretion and anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-Insulin-like growth factor-1 axis. J Clin Endocrinol Metab. 1999;84:2056–2063. doi: 10.1210/jcem.84.6.5734. [DOI] [PubMed] [Google Scholar]
- Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- Taragnat C, Bernier A, Fontaine J. Gonadotrophin storage patterns in the ewe during the oestrous cycle or after long-term treatment with a GnRH agonist. J Endocrinol. 1998;156:149–157. doi: 10.1677/joe.0.1560149. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Davenport ML, Pucilowska JB, Miles MV, Underwood LE. Increased serum clearance and degradation of 125I-labelled IGF-1 in protein restricted rats. Am J Physiol. 1992;262:E406–E411. doi: 10.1152/ajpendo.1992.262.4.E406. [DOI] [PubMed] [Google Scholar]
- Van der Lely AJ, Tschop M, Heiman ML, Gigo E. Biological, physiological, pathophysiological and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Evans WS, Lizzaralde G, Thorner MO, Vance ML. Amplitude suppression of the pulsatile mode of immunoradiometric LH release in fasting-induced hypoandrogenemia in normal men. J Clin Endocrinol Metab. 1993;76:587–593. doi: 10.1210/jcem.76.3.8445014. [DOI] [PubMed] [Google Scholar]
- Vriend J, Bertalanffy F, Ralcewicz T. The effects of melatonin and hypothyroidism on estradiol and gonadotropin levels in female Syrian hamsters. Biol Reprod. 1987;36:719–728. doi: 10.1095/biolreprod36.3.719. [DOI] [PubMed] [Google Scholar]
- Wardlaw S, Ferin M. Interaction between β-endorphin and α-melanocyte stimulating hormone in the control of prolactin and luteinizing hormone secretion in the primate. Endocrinology. 1990;126:2035–2040. doi: 10.1210/endo-126-4-2035. [DOI] [PubMed] [Google Scholar]
- Watanobe H. Leptin directly acts within the hypothalamus to stimulate gonadotropin-releasing hormone secretion in vivo rats. J Physiol. 2002;545:255–268. doi: 10.1113/jphysiol.2002.023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Elmquist JK. From anorexia to obesity—the Yin and Yang of body weight control. Endocrinology. 2003;144:3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]


