Abstract
BACKGROUND AND AIMS
Considerable evidence suggests a low-folate diet increases colorectal cancer risk, although a recent randomized trial indicates that folate supplementation may not reduce the risk of adenoma recurrence. In laboratory models, folate deficiency appears to induce p53 mutation.
METHODS
We immunohistochemically assayed p53 expression in paraffin-fixed colon cancer specimens in a large prospective cohort of women with 22 years of follow-up, to examine the relationship of folate intake and intake of other one-carbon nutrients to risks by tumor p53-mutational status.
RESULTS
A total of 399 incident colon cancers accessible for p53 expression were available. The effect of folate differed significantly according to p53 mutational status (Pheterogeneity = 0.01). Compared with women reporting less than 200 μg of folate per day, the multivariate relative risks (RRs) for p53 overexpressing (mutated) cancers were 0.54 (95% CI, 0.36-0.81) for women who consumed 200-299 μg per day, 0.42 (95% CI, 0.24-0.76) for those who consumed 300-399 μg per day, and 0.54 (95% CI, 0.35-0.83) for ≥ 400 μg per day. In contrast, total folate intake had no influence on wild-type tumors (RR, 1.05; 95% CI, 0.73-1.51, comparing ≥ 400 to < 200 μg per day). Similarly, high vitamin B6 intake conferred a protective effect on p53-mutated cancers (top versus bottom quintile, RR, 0.57; 95% CI, 0.35-0.94; Pheterogeneity = 0.01) but had no effect on p53 wild-type tumors.
CONCLUSIONS
We found that low folate and vitamin B6 intake was associated with an increased risk of p53 mutated colon cancers but not wild-type tumors.
Keywords: methylgroup donors, one-carbon nutrients, folate, vitamin B6, p53, colon cancer
INTRODUCTION
Folic acid and related vitamins B2, B6 and B12, are essential for DNA methylation and the production of purine and pyrimidine nucleotides required for DNA synthesis. Considerable epidemiological evidence suggests that a low-folate diet is associated with an increased risk of colorectal cancer [1-3]. Animal data support an antineoplastic effect of folate; however, in some animal studies, folate deficiency protects against, and supplementation increases, experimental carcinogenesis [1]. In addition, a distinction between natural folate and synthetic folic acid must be made given the higher bioavailability of folic acid and a growing body of evidence suggesting that too much of the synthetic folic acid might be harmful [4]. Nevertheless, on the whole, the biological and epidemiological evidence is consistent with the hypothesis that folate deficiency increases the risk of colorectal neoplasia in humans.
The mechanism by which folate deficiency influences colon cancer risk remains less well understood. Folate deficiency leads to mutations and chromosomal damage−effects that are also central to the efficacy of antifolate chemotherapeutic agents (such as methotrexate). Specifically, animal studies suggest that folate deficiency can induce DNA strand breaks in a highly conserved region of the p53 tumor suppressor gene [5-7]. In human colon cell lines and lymphocytes, folate depletion increased p53 expression and p53 strand breaks [8,9]. Further, folate-depletion induced hypomethylation of colon cells specifically in the region of the p53 gene was fully reversed by folic acid supplementation [10], suggesting that p53 mutations may play an important role in folate's effect on colon cancer risk. The reported frequency of p53 mutations in colorectal cancer varies; whereas early reports suggested that 75% of colorectal harbored 53 mutations, more recent estimates suggest that 40% to 50% of tumors possess alterations in p53 [11-13].
We assessed whether the influence of folate on colon cancer risk differed according to the presence of p53 mutations. For this purpose, we used tumor specimens from a prospective cohort study that has previously shown that folate intake was inversely associated with the risk of colon cancer [14]. The availability of detailed and updated information on folate intake and tumor specimens within this cohort permitted a more comprehensive examination of the effect of folate intake on p53 mutational status in colon cancers. Specificity in the association between folate and colon cancer to particular tumor markers would further enhance the case for causality and would provide important insights into the carcinogenic mechanisms of folate deficiency.
MATERIALS AND METHODS
Study Subjects
The Nurses' Health Study (NHS) was established in 1976 when 121,701 U.S. female registered nurses, 30 to 55 years of age, completed a mailed questionnaire [15]. Follow-up within the cohort currently exceeds 92%. We mailed biennial questionnaires to update information and identify newly diagnosed cases of cancer. In 1980, the NHS questionnaire was expanded to include a validated assessment of diet, and updated dietary assessments have been conducted every four years [16]. The institutional review boards at Brigham and Women's Hospital and the Harvard School of Public Health approved this study.
Assessment of Nutrients
Dietary intake of various nutrients including folate, vitamin B6, B12, and methionine were assessed by self-administered semiquantitative food frequency questionnaires (SFFQ), which were completed in 1980, 1984, 1986, 1990, 1994, and 1998. Nutrient intakes were calculated by multiplying the reported frequency of consumption of each specified food item by the nutrient content of the specified portion size and then summing these products for all food items. Information on multivitamin use and the use of other supplements was also collected, including details on which brand name and type. An extensive database of supplement formulation was then used to calculate specific nutrient contributions from these supplemental sources. These nutrient contributions were subsequently added to the specific nutrient intake from foods to calculate a daily intake for each woman. This method of dietary assessment has been extensively validated and its reliability evaluated [16]. The correlation coefficient was 0.55 for the correlation between total folate intake calculated from the 1980 questionnaire and erythrocyte folate concentrations measured in 1987 in this cohort [17]. Moreover, vitamin B6 intake as assessed by 1980, 1984, and 1986 SFFQs has been shown to correlate with one-week diet records, with correlations ranging from 0.54 to 0.58 [16,18].
Assessment of Other Covariates
Alcohol consumption was the sum of the values for three types of beverages: beer, wine, and spirits. We assumed an ethanol content of 13.1 g for a 12-ounce (38-dl) can or bottle of beer, 11.0g for a 4-ounce (12-dl) glass of wine, and 14.0 g for a standard portion of spirits. In validation studies, the correlation coefficient derived for the correlation between alcohol consumption derived from the 1980 SFFQ and that derived from the average of four one-week diet records was 0.90 [19]. Other risk factors for colon cancer such as physical exercise and body mass index have generally been assessed biennially on the main questionnaires.
Ascertainment of Colon Cancer and Deaths
We included colon cancers reported on the biennial questionnaires between the return of the 1980 questionnaire and June 1, 2002. With permission from study participants, colon cancer was confirmed through physicians' review of the nurses' medical records. If permission was denied, we attempted to confirm the self-reported cancer with an additional letter or phone call. We also searched the National Death Index to identify deaths among the nonrespondents to each two-year questionnaire. The computerized National Death Index is a highly sensitive method for identifying deaths in this cohort [20].For all deaths attributable to colon cancer, we requested permission from family members (subject to state regulation) to review the medical records. Colon cancer was considered the cause of death if the medical records or autopsy reports confirmed fatal colon cancer or if colon cancer was listed as the underlying cause of death without another more plausible cause.
Assessment of p53 Mutations
Beginning in 2001, we began retrieving, from treating hospital pathology departments, representative pathological specimens from participants whom we confirmed to have developed colon cancer. We successfully obtained specimens for 58% of cases (n=527) over 22 years of follow-up in NHS. To assess for p53 mutations, we examined specimens for abnormalities of p53 protein accumulation by immunohistochemistry. This technique takes advantage of the phenomenon that mutant p53 proteins have a prolonged half-life in the nucleus due to a decreased rate of degradation [21,22]. Therefore, the increased nuclear staining is correlated with point mutations, which involve over 80% of p53 alterations [23]. In contrast, immunohistochemistry will not detect frameshifts or mutations generating stop codons, which may involve only 8% of the p53 defects in human colorectal cancers [23]. Although the concordance between p53 overexpression and mutations is not perfect, the immunohistochemical assay is a rapid and cost-effective evaluation. In comparative studies, p53 overexpression is a good approximation to the real mutation rate with approximately 90% concordance between immunostaining and gene analysis[22] and 91% specificity [21]. We limited the current analysis to those participants for whom we were able to obtain sufficient amounts of tumor tissue paraffin blocks (N = 399; 76% of those for whom we successfully obtained specimen). A random sample of 58 cancers was reread by the same pathologist 2 years apart, and the concordance was 0.95 (κ=0.90, P<0.001). Baseline characteristics among 908 participants with colon cancer who we did and did not analyze for p53 expression were largely similar (all were Caucasian; mean age, 50.3 years vs. 50.8 years; former or current smoker, 58.7% vs. 58.2%; mean body mass index, 25.1 vs. 24.9; mean metabolic equivalents score per week, 12.0 vs. 13.2; current multivitamin use, 31.3% vs. 29.3%; folate intake, 352 μg/day vs. 363 μg/day; calcium intake, 718 mg/day vs. 707 mg/day; alcohol intake, 6.7 g/day vs. 7.0 g/day; p>0.35 for all comparisons).
Tissue Microarray (TMA) and Immunohistochemistry for p53
Tissue microarray (TMA) blocks were constructed for p53 analysis as previously described [24], using an automated arrayer (Beecher Instruments, Sun Prairie, WI). We examined two to four tumor tissue cores for p53 immunohistochemistry. A previous validation study has shown that examining two TMA cores can yield comparable results to examining whole-tissue sections in more than 95% of cases [25].
We have previously described in detail our method for p53 staining (immunohistochemistry, IHC) [26]. We visually estimated the fraction of tumor cells with unequivocal strong nuclear staining for p53, by examining at least two tissue cores in TMAs, or the whole-tissue section in each case for which there was not enough tissue for TMAs or results were equivocal in TMAs. The presence of p53 mutation (p53-positivity) was defined as 50% or more of tumor cells with unequivocal moderate/strong nuclear staining, as recommended for improved specificity [27]. The absence of p53 mutation (p53 negativity) was defined as either absent/weak staining or <50% of tumor cells with moderate/strong staining. Appropriate positive and negative controls were included in each run of immunohistochemistry. All immunohistochemically stained slides were interpreted by a pathologist (S.O.) blinded to any other clinical or laboratory data. The results of the p53 assays on these tumors have been previously correlated with other molecular features in tumors [24,26,28]. Moreover, the categorization we used in this paper has previously been shown to correlate sufficiently well with TP53 sequencing analyses (sensitivity 0.85; specificity 0.77 with a chosen cut point of 50% or more positive cells) [29,30].
Statistical Analysis
We excluded women who did not complete the baseline 1980 dietary questionnaire or recorded implausible dietary data (n=29,279), reported a baseline history of cancer (except non-melanoma skin cancer; n=3,627), inflammatory bowel disease, hereditary nonpolyposis colon cancer, or a familial polyposis syndrome (n=103), or had died prior to baseline (n=1). After these exclusions, 88,691 women were eligible for analysis and accrued follow-up time beginning on the month of return of their baseline questionnaire and ending on the month of diagnosis of colon cancer, death from other causes, or June 2002, whichever came first. In a previous analysis of this cohort, folate intake was significantly associated with the risk of colon cancer but had no influence on the risk of rectal cancer [14]; as a result, we did not consider incident rectal cancer among the study participants in this analysis. Like rectal cancer cases, cases of colon cancer for which we were unable to assay tumoral p53 expression were censored from the analyses at their date of diagnosis and were not included as endpoints.
We calculated incidence rates of colon cancer for participants in a specific category of folate intake by dividing the number of incident cases by the number of person-years. We computed relative risks (RR) by dividing the incidence rate in one category by the incidence rate in the reference category and used Cox proportional hazards modeling to control for multiple variables simultaneously and to compute 95% confidence intervals (CI). With the exception of folate, vitamin B6, methionine, and alcohol, for which we used baseline information in our primary analyses, we used the most updated information for all covariates prior to each two-year interval. In secondary analyses, as previously described [31], to reduce in-person variation, and to estimate long-term intake better, we also used the cumulative average intake of folate as reported on all available questionnaires up to the start of each two-year follow-up interval. In subanalyses, we evaluated the risk of colon cancer by p53 mutational status among women low both in folate and vitamin B6 intake. To examine a potential effect of folate on preneoplastic lesions, we further conducted analyses restricting to colon tumors that occurred within five years after baseline nutrient assessment.
To compare the specific effect of intake of folate and other nutrients on colon cancer risk according to p53 mutational status, we employed a previously described method of competing risk analysis utilizing duplication method Cox regression [32,33]. This methodology permits estimation of separate regression coefficients for nutrient intake stratified by the type of outcome (e.g. p53-mutant cancer vs. p53-wild-type cancer). We assessed the statistical significance of the difference between the risk estimates according to tumor type using a likelihood ratio test that compared the model that allowed for separate associations of folate and other nutrients according to p53 mutational status with a model that assumed a common association. We examined the possibly non-linear relation between folate, vitamin B6, and the RR of colon cancer by p53 expression status non-parametrically with restricted cubic splines [34]. Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. We used SAS version 9.1.3 (Cary, NC) for all analyses. All P values are two-sided.
RESULTS
Among 88,691 women included in these analyses, those with a baseline folate intake of less than 200 μg/day were slightly younger and more likely to smoke and to be sedentary, compared to women with 400 μg daily folate intake or more (Table 1). They were also less likely to use aspirin or postmenopausal hormones regularly. In addition, only 8% of women with less than 200 μg/day folate intake used multivitamins, whereas among those with 400 μg folate or more daily, 84% reported current multivitamin use.
Table 1.
Baseline Characteristics of the Nurses' Health Study Cohort*
| Energy-adjusted Folate Intake, μg/day | |||||
|---|---|---|---|---|---|
| <200 | 200-299 | 300-399 | ≥400 | ||
| Characteristic* | N=20,907 | N=28,882 | N=12,997 | N=25,905 | |
| Dietary intake ψ | |||||
| Folate (μg/day) | 159 | 246 | 341 | 678 | |
| Beef, pork, or lamb as a main dish (servings per week) |
3.1 | 2.6 | 2.3 | 2.3 | |
| Vitamin B6 (mg/day) | 1.59 | 2.05 | 2.76 | 5.15 | |
| Vitamin B12 (mg/day) | 5.55 | 6.45 | 7.78 | 15.1 | |
| Alcohol (g/day) | 6.7 | 6.4 | 6.0 | 6.3 | |
| Methionine (mg/day) | 1.74 | 1.86 | 1.95 | 1.93 | |
| Calcium (mg/day) | 607 | 723 | 798 | 812 | |
| Beef, pork, or lamb as a main dish (servings/week) | 3.1 | 2.6 | 2.3 | 2.3 | |
| Other characteristics* | |||||
| Median age (yr) | 45.5 | 46.8 | 47.5 | 47.0 | |
| Former or current smoker (%) | 60 | 56 | 54 | 55 | |
| Pack-yr † | 23.3 | 20.4 | 18.7 | 19.2 | |
| Regular aspirin user | 31 | 32 | 32 | 35 | |
| Body mass index (kg/m2)‡ | 24.4 | 24.5 | 24.3 | 24.0 | |
| Physical activity, METS/wk (%) § | 11.1 | 13.8 | 15.8 | 15.6 | |
| Post-menopausal (%) ¶ | 44 | 44 | 44 | 44 | |
| Never used hormones (%) | 64 | 62 | 61 | 59 | |
| Past use of hormones (%) | 18 | 19 | 19 | 19 | |
| Current use of hormones (%) | 18 | 19 | 20 | 22 | |
| Current multivitamin use (%) | 8 | 13 | 24 | 84 | |
| Prior lower endoscopy (%) | 2 | 2 | 2 | 2 | |
| Colorectal cancer in a parent or sibling (%) | 8 | 8 | 7 | 8 | |
Dietary intake and other characteristics at baseline questionnaire in 1980 (mean value, unless otherwise indicated). All values have been directly standardized according to the age distribution of the cohort.
Pack-years were calculated for former and current smokers only.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
METS are metabolic equivalents. This was calculated based on the frequency of a range of physical activities (such as jogging) in 1986.
Hormones are defined as post-menopausal estrogen or estrogen/progesterone preparations. Percent of never, past, and current use was calculated among post-menopausal women only.
Nutrient values (folate, vitamin B6, B12, methionine, and calcium) represent the mean of energy-adjusted intake.
We documented 399 incident cases of colon cancer accessible for p53 expression data during 1,861,916 person-years. Using immunohistochemical analysis of these 399 colon cancers in our tissue microarrays, 143 (35.8%) tumors were found to be p53 mutated (p53 overexpression). As in our previous studies [14,35-37], we observed an inverse association between folate and vitamin B6 intake and colon cancer risk among all cases in our cohort, independent of p53 mutational status (Tables 2 and 3). The multivariate relative risk of colon cancer was 0.80 (95% CI, 0.61 to 1.06) for a daily folate intake of 400 μg or greater, compared to less than 200 μg folate per day. This RR did not change materially in analyses where we included all 908 colon cancer cases documented over follow-up, irrespective of the availability of p53 expression analysis (multivariate RR, 0.86; 95% CI, 0.72 to 1.04), although associations were generally not statistically significant. Of note for both folate and B6 intake, risk was principally elevated among participants in the lowest category, whereas the risk did not appear to decline substantially beyond the second category of exposure.
Table 2.
Relative risk of colon cancer according to p53 status and baseline folate intake, among 88,691 women from the Nurses' Health Study with 399 colon cancer cases¶.
| Energy-adjusted Folate Intake, μg/day | P trend‡ | |||||
|---|---|---|---|---|---|---|
| <200 | 200-299 | 300-399 | ≥400 | |||
| All cancer | ||||||
| No. cases / Person-years | 105 / 439,200 | 126 / 606,366 | 58 / 273,310 | 110 / 543,040 | ||
| Age-adjusted RR (95% CI) | 1.0 | 0.78 (0.60-1.01) | 0.75 (0.55-1.04) | 0.74 (0.57-0.97) | 0.04 | |
| Multivariate RR (95% CI)* | 1.0 | 0.81 (0.62-1.05) | 0.80 (0.58-1.10) | 0.80 (0.61-1.06) | 0.14 | |
| p53-positive cancer† | ||||||
| No. cases / Person-years | 51 / 439248 | 41 / 606442 | 15 / 273345 | 36 / 543103 | ||
| Age-adjusted RR (95% CI) | 1.0 | 0.52 (0.35-0.79) | 0.40 (0.23-0.71) | 0.50 (0.33-0.77) | 0.003 | |
| Multivariate RR (95% CI)* | 1.0 | 0.54 (0.36-0.81) | 0.42 (0.24-0.76) | 0.54 (0.35-0.83)** | 0.008 | |
| p53-negative cancer† | ||||||
| No. cases / Person-years | 54 / 439237 | 85 / 606398 | 43 / 273323 | 74 / 543072 | ||
| Age-adjusted RR (95% CI) | 1.0 | 0.98 (0.69-1.37) | 1.09 (0.73-1.62) | 0.97 (0.68-1.38) | 0.70 | |
| Multivariate RR (95% CI)* | 1.0 | 0.95 (0.67-1.33) | 1.15 (0.77-1.72) | 1.05 (0.73-1.51)** | 0.94 | |
P for trend from Wald statistic, modeling log-transformed folate continuously
P for heterogeneity of the association for folate intake and p53-positive colon cancer and folate intake and p53-negative colon cancers = 0.012 (χ2 = 10.88, 3 d.f.)
Presented as relative risks (RRs) and 95% Confidence Intervals (95% CI).
Multivariate RRs are adjusted for age, smoking before age 30 (0, 1-4, 5-10, 11-15, or >15 pack-years), body mass index (in quintiles), regular vigorous exercise (in quintiles of metabolic equivalent task score per week), colorectal cancer in a parent or sibling (yes or no), history of previous endoscopy (yes or no), history of previous polyp (yes or no), current multivitamin use (yes or no), beef, pork, or lamb as a main dish (0-3 per month, 1 per week, 2-4 per week, or ≥5 per week), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15 g per day), and post-menopausal hormone use (premenopausal, never, past, current).
p53 status was determined by immunohistochemistry.
Table 3.
Relative risk of colon cancer according to p53 status and baseline vitamin B6 intake, among 88,691 women from the Nurses' Health Study with 399 colon cancer cases¶.
| Energy-adjusted vitamin B6 intake, mg/day | P trend‡ | ||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| ≤1.30 | 1.31-1.60 | 1.61-2.00 | 2.01-3.50 | ≥3.51 | |||
| All cancer | |||||||
| No. cases / Person-years | 95 / 375526 | 77 / 372929 | 73 / 374308 | 69 / 371263 | 85 / 367890 | ||
| Age-adjusted RR (95% CI) | 1.0 | 0.75 (0.56-1.02) | 0.65 (0.48-0.88) | 0.64 (0.47-0.87) | 0.77 (0.57-1.03) | 0.45 | |
| Multivariate RR (95% CI)* | 1.0 | 0.76 (0.56-1.03) | 0.68 (0.49-0.94) | 0.69 (0.49-0.98) | 0.88 (0.62-1.24) | 0.27 | |
| p53-positive cancer† | |||||||
| No. cases / Person-years | 48 / 375571 | 22 / 372971 | 20 / 374358 | 25 / 371298 | 28 / 367940 | ||
| Age-adjusted RR (95% CI) | 1.0 | 0.43 (0.26-0.71) | 0.35 (0.21-0.60) | 0.46 (0.28-0.74) | 0.50 (0.31-0.80) | 0.15 | |
| Multivariate RR (95% CI)* | 1.0 | 0.43 (0.26-0.71) | 0.37 (0.22-0.63) | 0.50 (0.30-0.82) | 0.57 (0.35-0.94) ** | 0.45 | |
| p53-negative cancer† | |||||||
| No. cases / Person-years | 47 / 375566 | 55 / 372944 | 53 / 374322 | 44 / 371283 | 57 / 367915 | ||
| Age-adjusted RR (95% CI) | 1.0 | 0.92 (0.62-1.36) | 0.96 (0.65-1.42) | 0.82 (0.54-1.24) | 1.04 (0.70-1.53) | 0.95 | |
| Multivariate RR (95% CI)* | 1.0 | 0.91 (0.61-1.35) | 1.00 (0.67-1.50) | 0.89 (0.58-1.39) | 1.19 (0.78-1.83) ** | 0.44 | |
P for trend from Wald statistic, modeling log-transformed vitamin B6 continuously
P for heterogeneity of the association for vitamin B6 intake and p53-positive colon cancer and vitamin B6 intake and p53-negative colon cancers = 0.011 (χ2 = 13.09, 4 d.f.)
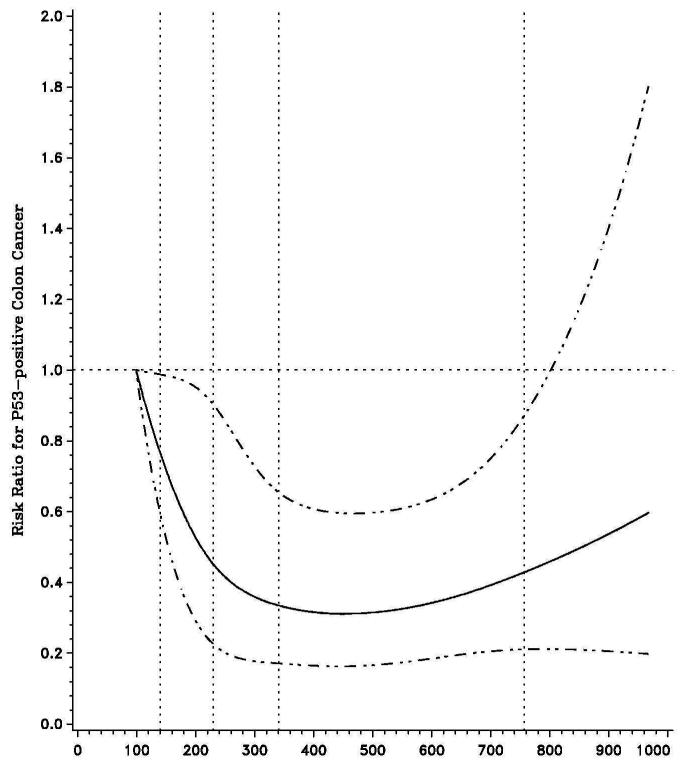
We further evaluated the influence of one-carbon nutrients on colon cancer risk according to p53 status in colon cancer. For total folate intake (Table 2), the benefit of consumption beyond 200 μg per day was restricted to cancers with p53 overexpression (p53 mutation). Compared to women with less than 200 μg folate per day, the multivariate RR for the development of p53-mutated colon cancer among those reporting 400 μg or more of folate intake per day was 0.54 (95% CI, 0.35 to 0.83; P for trend = 0.008). In contrast, total folate intake had no influence on the risk of p53 wild-type (p53-negative) tumors (P for trend = 0.94). A test for heterogeneity for the effect of folate intake on risk of colon cancer by p53 expression status was statistically significant (Pheterogeneity = 0.01). Overall, the effect of folate intake on p53 mutated cancer appeared to follow a U-shaped distribution with maximum benefits at levels around 400μg per day (Figure 1).
Figure 1.
In secondary analyses, we limited our examination to cases that occurred prior to 1998 (that is, before folate fortification, which began in 1996 and became mandatory in 1998), however, our estimates remained virtually unchanged (RR for the development of p53-mutated colon cancer among those reporting 400 μg or more of folate intake per day, 0.52; 95% CI, 0.31-0.87; RR of p53 wild-type (p53-negative) tumors, 1.04; 95% CI, 0.68-1.60). Moreover, when we restricted the analysis to participants who obtained their folate intake from food sources only, the differential effect of folate intake on p53-mutated vs. wild-type cancers appears somewhat greater. After excluding supplement users, the multivariate RR for p53-mutated cancer for women consuming 400 μg or more per day was 0.44 (95% CI, 0.24 to 0.82) when compared to those who reported less than 200 μg (P for trend = 0.0006). In contrast, the multivariate RR for p53 wild-type cancer was 1.38 (95% CI, 0.82 to 2.32; P for trend = 0.35) for participants reporting more than 400 μg per day. Among supplement users (restricting to baseline multivitamin users only, n=138 cases of colon cancer), the multivariate RR for p53-mutated cancer for women consuming 400 μg or more per day was 0.58 (95% CI, 0.36 to 0.95) when compared to those who reported less than 200 μg association between folate intake; and 1.10 (95% CI, 0.7-1.68) for p53 wildtype tumors.
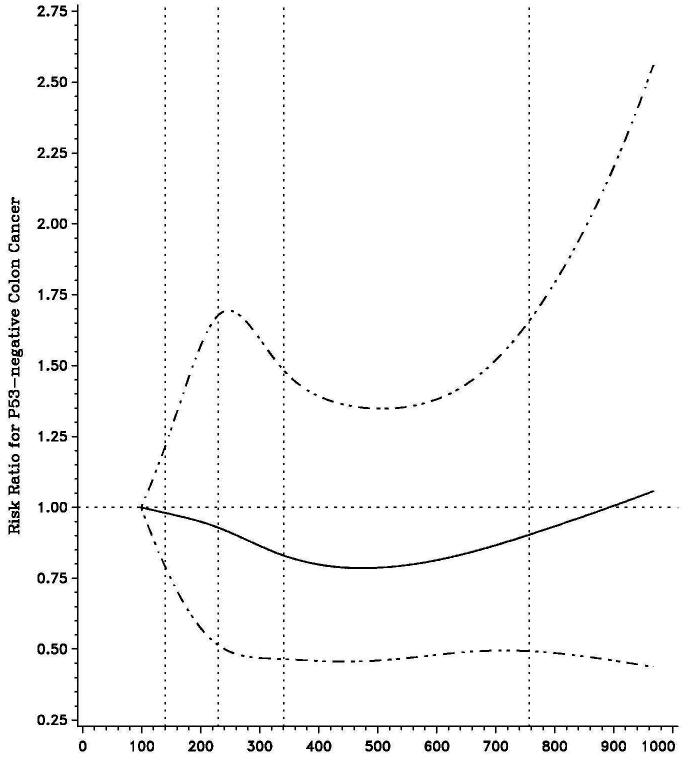
We further examined the influence of vitamin B6 intake according to p53 mutational status (Table 3). The benefit of vitamin B6 intake also appeared confined to cancers with p53 overexpression (p53-mutated) (multivariate RR 0.57; 95% CI, 0.35 to 0.94 comparing extreme quintiles), whereas B6 intake had no influence on the risk of p53 wild-type tumors. A test for heterogeneity for the effect of B6 intake on the risk of colon cancer by p53 expression status was statistically significant (Pheterogeneity = 0.01; Figure 2).
Figure 2.
We also examined the influence of methionine intake and alcohol intake according to p53 mutational status (Table 4). Although there appeared to be a modestly greater reduction in the risk of p53-mutated cancers with increasing methionine intake, a test for heterogeneity for the effect of methionine on the risk of colon cancer by p53 expression status was not statistically significant (Pheterogeneity = 0.67). In contrast, we did not observe a significant influence of alcohol intake on colon cancer risk, and the effect did not appear to differ according to p53 status.
Table 4.
Relative risk of colon cancer according to p53 status and baseline methionine and alcohol intake, among 88,691 women from the Nurses' Health Study with 399 colon cancer cases¶.
| Energy-adjusted methionine intake, mg/day | P trend‡ | ||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| ≤1.50 | 1.51-1.70 | 1.71-1.90 | 1.91-2.20 | ≥2.2 | |||
| All cancer | |||||||
| No. cases / Person-years | 84 / 371361 | 93 / 371698 | 65 / 375390 | 67 / 372420 | 90 / 371047 | ||
| Age-adjusted | 1.0 | 1.09 (0.81-1.47) | 0.74 (0.53-1.02) | 0.75 (0.54-1.04) | 0.95 (0.70-1.28) | 0.78 | |
| Multivariate* | 1.0 | 1.09 (0.81-1.46) | 0.73 (0.53-1.02) | 0.75 (0.54-1.04) | 0.95 (0.70-1.29) | 0.85 | |
| p53-positive cancer† | |||||||
| No. cases / Person-years | 35 / 371403 | 34 / 371746 | 24 / 375428 | 21 / 372460 | 29 / 371101 | ||
| Age-adjusted | 1.0 | 0.96 (0.60-1.54) | 0.65 (0.39-1.10) | 0.57 (0.33-0.97) | 0.73 (0.45-1.20) | 0.34 | |
| Multivariate* | 1.0 | 0.95 (0.59-1.53) | 0.65 (0.38-1.09) | 0.56 (0.33-0.97) | 0.74 (0.45-1.21)** | 0.37 | |
| p53-negative cancer† | |||||||
| No. cases / Person-years | 49 / 371390 | 59 / 371733 | 41 / 375409 | 46 / 372431 | 61 / 371066 | ||
| Age-adjusted | 1.0 | 0.84 (0.58-1.23) | 0.80 (0.53-1.21) | 0.88 (0.59-1.32) | 1.10 (0.76-1.60) | 0.72 | |
| Multivariate* | 1.0 | 0.85 (0.58-1.24) | 0.79 (0.52-1.20) | 0.88 (0.58-1.32) | 1.11 (0.75-1.62) ** | 0.67 | |
| Alcohol intake in g/day | |||||||
| No alcohol | <5 g/day | 5-14.9 g/day | ≥15 g/day | ||||
| All cancer | |||||||
| No. cases / Person-years | 115 / 595224 | 147 / 629279 | 84 / 416133 | 53 / 221280 | |||
| Age-adjusted | 1.0 | 1.28 (1.00-1.63) | 1.05 (0.79-1.39) | 1.19 (0.86-1.65) | 0.72 | ||
| Multivariate* | 1.0 | 1.30 (1.01-1.66) | 1.07 (0.80-1.43) | 1.19 (0.85-1.67) | 0.67 | ||
| p53-positive cancer† | |||||||
| No. cases / Person-years | 43 / 595285 | 49 / 629363 | 30 / 416179 | 21 / 221311 | |||
| Age-adjusted | 1.0 | 1.14 (0.75-1.71) | 1.00 (0.63-1.59) | 1.26 (0.75-2.12) | 0.60 | ||
| Multivariate* | 1.0 | 1.16 (0.77-1.74) | 1.02 (0.64-1.64) | 1.26 (0.74-2.14) ** | 0.69 | ||
| p53-negative cancer† | |||||||
| No. cases / Person-years | 72 / 595259 | 98 / 629314 | 54 / 416159 | 32 / 221297 | |||
| Age-adjusted | 1.0 | 0.74 (0.54-1.00) | 1.08 (0.76-1.53) | 1.15 (0.76-1.75) | 0.99 | ||
| Multivariate* | 1.0 | 0.73 (0.53-0.99) | 1.10 (0.77-1.58) | 1.15 (0.75-1.76) ** | 0.89 | ||
P for trend from Wald statistic, modeling methionine and alcohol intake continuously
P for heterogeneity of the association for methionine intake and p53-positive colon cancer and methionine intake and p53-negative colon cancers = 0.667 (χ2 = 2.376, 4 d.f.), and for alcohol intake = 0.838 (χ2 = 0.848, 3 d.f.)
Because previous analyses have suggested larger effects on colon carcinogenesis when examining dietary contrasts of total vitamin B6 and folate availability [17,37], we further evaluated the risk of colon cancer by p53 mutational status among women low both in folate and vitamin B6 intake. Among women with a daily folate intake of less than 200 μg and vitamin B6 consumption in the lowest quintile, the relative risk of p53-positive colon cancer was 2.61 (95% CI, 1.73 to 3.95) compared to women with higher intakes in both nutrients (that is, women with a folate intake of greater or equal to 200 μg/day and in the top four quintiles of vitamin B6 intake); by contrast, the comparable risk for p53-negative colon tumors was 0.78 (95% CI, 0.48 to 1.25).
Concerns have been voiced regarding the potential dual role of folate in colon carcinogenesis, with suggestions of a potentially harmful effect of excessive folate on preneoplastic lesions [38]. To examine this possibility, we repeated our analyses restricting them to colon tumors that occurred within five years after baseline nutrient assessment – a plausible time frame for preneoplastic lesions already being present at baseline. Due to the age distribution of our cohort at study baseline, only 21 colon tumors were diagnosed within these first five years of follow-up. Nonetheless, compared to women reporting less than 200 μg per day of folate, participants who consumed 400 μg per day or more experienced a RR for p53-wild-type colon cancers of 2.87 (95% CI, 0.60 to 13.8), whereas the risk of p53-mutant (overexpressing) tumors was 1.23 (95% CI, 0.21 to 7.37).
DISCUSSION
In this large prospective cohort study, we found that both low folate and vitamin B6 intakes were associated with an increased risk of p53-mutated (p53 overexpressing) colon cancers but not p53-wild-type tumors. The elevation in risk was principally limited to participants with the lowest levels of folate and vitamin B6 intake, and no additional risk reduction was observed for intake beyond the second lowest category of consumption. Specifically, supraphysiologic doses of either vitamin did not appear to confer any additional benefit.
To our knowledge, no previous study has assessed the influence of one-carbon nutrients on colon cancer according to p53 mutational status. A prior cross-sectional case-control study evaluated dietary associations with p53 status of colon cancers but did not directly examine nutrients like folate or vitamin B6 [39]. However, that study reported a higher risk of p53-mutated cancers associated with a Western diet, which is presumed to be low in one-carbon nutrients (OR = 2.03 for p53-mutated tumors vs. OR = 1.57 for p53-wild-type colon tumors). In addition, one other study reported that folate supplementation protected against the development of p53 mutations in individuals with ulcerative colitis, a well-established predisposing condition for colon cancer [40].
Mechanistically, it appears plausible that chronic folate deficiency may be involved in p53 expression, by inducing methylation within the p53 gene [10] or by upregulating p53 expression [8,9]. In addition to upregulating p53 expression, experimental studies suggest that folate deficiency also induces DNA strand breaks in a highly conserved region of the p53 tumor suppressor gene [5,8,9]. Folic supplementation, on the other hand, fully reversed the hypomethylation induced by folate depletion in the region of the p53 gene [10].
However, the role of folate supplementation in reducing colorectal neoplasia risk has been conflicting [41,42], and, given an apparent threshold effect in our cohort, one may speculate that pharmacological folate supplementation may not confer any real benefit. In a recent randomized placebo-controlled trial of 1 mg daily supplementation of folic acid among a population with a history of colorectal adenomas, folate conferred no reduction in the risk of adenoma recurrence [42]. Moreover, at six-year follow-up, there was a 67% increased risk of advanced lesions. This finding may be consistent with experimental studies showing increased colorectal neoplasia when folic acid is administered after lesions are present [1]. There is also suggestive evidence from ecologic data for a potential harmful effect of folate fortification, given the reversal of a downward trend in colorectal cancer rates in the US and Canada shortly after the introduction of mandatory folate fortification in 1998 [43].
The absence of an association between folate and p53 wild-type tumors in the current analysis may, in part, explain the lack of benefit of folate in the adenoma recurrence trial, given the relative infrequency of p53 mutations in adenomas [42]. However, both folate supplementation and deficiency can have diverse effects on tumor development, depending on the various stages of carcinogenesis; and much more work is needed to fully delineate the roles of different folate concentrations and its specific role in cancer prevention. Of note, we did examine the effect of folate on colon tumors that arose within five years of study initiation—a plausible time frame for the assumption that precarcinogenic lesions may already have been present at the time at which we assessed folate intake. Within this subanalysis, there was no effect of folate intake on p53-mutated cancers, however, increased folate intake appeared to more than double the risk of p53-wild-type cancers.
Our study has several important strengths. First, because we collected detailed, updated information on a number of dietary and lifestyle covariates relevant to colon carcinogenesis over 22 years of follow-up and with high follow-up rates, we were able to examine long-term exposures to one-carbon nutrients and to take into consideration important confounding factors. Second, our study is prospective, eliminating concerns about differential recall bias, particularly with regard to our dietary assessments. Any remaining bias from exposure misclassification would thus be nondifferential by nature, biasing our results only toward the null. Further, we have successfully linked these nutrients—as assessed via SFFQ—to other relevant endpoints in prior analyses, indicating that measurement error is not large enough to hide any real associations.
Limitations of note relate to folate fortification, which became mandatory in 1998 [44]. We did obtain multiple assessments of one-carbon nutrient intakes prior to fortification. In addition, since the development of colon cancer likely requires some induction period before the onset of a clinically apparent tumor, it is unlikely that the post-fortification folate exposure would substantially influence colon cancer risk through 2002. However, if, as supported by our own data, folate independently promotes the growth of preneoplastic lesions, folate fortification may still have lead to an increase in colon tumors between 1998 and 2002; nonetheless, our results remained unchanged when restricted to cases that arose prior to folate fortification (1998).
Several methods are available for assessment of p53 mutational status. Although the concordance between p53 overexpression and mutations is not perfect, p53 overexpression appears to be a good approximation to the real mutation rate with approximately 90% concordance between immunostaining and gene analysis[22] and 91% specificity [21]. Our classification scheme of p53 expression resulted in a similar proportion of p53-positive tumors as other investigators have found [39,45]. Moreover, we assessed p53 expression through central, blinded review of tumor specimens [24,28]. Any substantial misclassification of p53 status would presumably bias our results toward finding no significant difference in the effects of the one-carbon nutrients on colon tumors according to p53 status. Finally, tumor specimen retrieval became increasingly more difficult with more time between diagnosis and collection having passed. However, a real drop off in success rates occurred if time between start of collection (2001) and diagnosis exceeded 10 years (e.g., for cases diagnosed within 5 years of collection the yield was 78%, 68% for cases diagnosed within 5 to 7 years, 62% for cases diagnosed within 7 to 10 years, and 38% for those diagnosed more than 10 years from start of collection). With a mean time between diagnosis and collection in our cohort of 8.6 years, however, we anticipate this potential survivor effect to be minimal.
We cannot exclude the possibility of residual confounding as a potential explanation for our findings; nonetheless, in our multivariate analyses which included many known or suspected risk factors for colon cancer, the multivariate risk estimates did not materially differ from the age-adjusted results. Further, we were unable to obtain tumor tissue from all cases of confirmed colon cancer detected in the Nurses' Health Study cohort, but the risk factors in these cases did not appreciably differ from those in cases with tumor tissue available. Finally, even prior to mandated fortification in 1998 [44], our participants still had relatively high folate and vitamin B6 levels. It is therefore possible that we might have found even stronger associations in the present study if our folate levels would have included an even lower range.
In summary, we demonstrate that the reduced risk of colon cancer associated with replete folate status is limited to p53-mutated cancers. Our study offers a potential mechanism by which relative deficiency may promote colon carcinogenesis and highlights the importance of continued investigation into the role of dietary methyl groups in colorectal neoplasia prevention. Ultimately, they suggest that p53 may constitute an important molecular marker for tailored chemoprevention.
ACKNOWLEDGMENTS
All of the authors declare no relevant conflict of interest. This work is supported by National Institutes of Health research grants CA70817, CA87969, CA55075, CA42812, CA58684, CA90598, and the Lustgarten Foundation for Pancreatic Cancer Research as well as the Bennett Family Fund and Entertainment Industry Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank the participants of the Nurses' Health Study for their cooperation and participation. The authors are grateful to Ryan Lee for technical assistance.
Footnotes
The authors declare no conflict of interest relevant to this article
References
- 1.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen. 2004;44:10–25. doi: 10.1002/em.20025. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132:2350S–2355S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 3.Harnack L, Jacobs DR, Jr., Nicodemus K, et al. Relationship of folate, vitamin B-6, vitamin B-12, and methionine intake to incidence of colorectal cancers. Nutr Cancer. 2002;43:152–158. doi: 10.1207/S15327914NC432_5. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr. 2007;86:271–273. doi: 10.1093/ajcn/86.2.271. [DOI] [PubMed] [Google Scholar]
- 5.Kim YI, Shirwadkar S, Choi SW, et al. Effects of dietary folate on DNA strand breaks within mutation-prone exons of the p53 gene in rat colon. Gastroenterology. 2000;119:151–161. doi: 10.1053/gast.2000.8518. [DOI] [PubMed] [Google Scholar]
- 6.Crott JW, Choi SW, Ordovas JM, Ditelberg JS, Mason JB. Effects of dietary folate and aging on gene expression in the colonic mucosa of rats: implications for carcinogenesis. Carcinogenesis. 2004;25:69–76. doi: 10.1093/carcin/bgg150. [DOI] [PubMed] [Google Scholar]
- 7.Sohn KJ, Stempak JM, Reid S, et al. The effect of dietary folate on genomic and p53-specific DNA methylation in rat colon. Carcinogenesis. 2003;24:81–90. doi: 10.1093/carcin/24.1.81. [DOI] [PubMed] [Google Scholar]
- 8.Crott JW, Liu Z, Keyes MK, et al. Moderate folate depletion modulates the expression of selected genes involved in cell cycle, intracellular signaling and folate uptake in human colonic epithelial cell lines. J Nutr Biochem. 2007 doi: 10.1016/j.jnutbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crott JW, Liu Z, Choi SW, Mason JB. Folate depletion in human lymphocytes up-regulates p53 expression despite marked induction of strand breaks in exons 5-8 of the gene. Mutat Res. 2007;626:171–179. doi: 10.1016/j.mrgentox.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Wasson GR, McGlynn AP, McNulty H, et al. Global DNA and p53 region-specific hypomethylation in human colonic cells is induced by folate depletion and reversed by folate supplementation. J Nutr. 2006;136:2748–2753. doi: 10.1093/jn/136.11.2748. [DOI] [PubMed] [Google Scholar]
- 11.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 12.Baker SJ, Preisinger AC, Jessup JM, et al. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990;50:7717–7722. [PubMed] [Google Scholar]
- 13.Hollstein M, Rice K, Greenblatt MS, et al. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannucci E, Stampfer MJ, Colditz GA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med. 1998;129:517–524. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Giovannucci E, Hankinson SE, et al. A prospective study of methylenetetrahydrofolate reductase and methionine synthase gene polymorphisms, and risk of colorectal adenoma. Carcinogenesis. 1998;19:2129–2132. doi: 10.1093/carcin/19.12.2129. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Stampfer MJ, Colditz GA, et al. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993;85:875–884. doi: 10.1093/jnci/85.11.875. [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Stampfer MJ, Colditz GA, et al. Moderate alcohol consumption and the risk of breast cancer. N Engl J Med. 1987;316:1174–1180. doi: 10.1056/NEJM198705073161902. [DOI] [PubMed] [Google Scholar]
- 20.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119:837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 21.Melhem MF, Law JC, el-Ashmawy L, et al. Assessment of sensitivity and specificity of immunohistochemical staining of p53 in lung and head and neck cancers. Am J Pathol. 1995;146:1170–1177. [PMC free article] [PubMed] [Google Scholar]
- 22.Nishio M, Koshikawa T, Kuroishi T, et al. Prognostic significance of abnormal p53 accumulation in primary, resected non-small-cell lung cancers. J Clin Oncol. 1996;14:497–502. doi: 10.1200/JCO.1996.14.2.497. [DOI] [PubMed] [Google Scholar]
- 23.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 24.Ogino S, Brahmandam M, Kawasaki T, et al. Combined analysis of COX-2 and p53 expressions reveals synergistic inverse correlations with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Neoplasia. 2006;8:458–464. doi: 10.1593/neo.06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80:1943–1949. doi: 10.1038/labinvest.3780204. [DOI] [PubMed] [Google Scholar]
- 26.Ogino S, Kawasaki T, Kirkner GJ, et al. Loss of nuclear p27 (CDKN1B/KIP1) in colorectal cancer is correlated with microsatellite instability and CIMP. Mod Pathol. 2007;20:15–22. doi: 10.1038/modpathol.3800709. [DOI] [PubMed] [Google Scholar]
- 27.Hall PA, McCluggage WG. Assessing p53 in clinical contexts: unlearned lessons and new perspectives. J Pathol. 2006;208:1–6. doi: 10.1002/path.1913. [DOI] [PubMed] [Google Scholar]
- 28.Ogino S, Brahmandam M, Cantor M, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- 29.Curtin K, Slattery ML, Holubkov R, et al. p53 alterations in colon tumors: a comparison of SSCP/sequencing and immunohistochemistry. Appl Immunohistochem Mol Morphol. 2004;12:380–386. doi: 10.1097/00129039-200412000-00017. [DOI] [PubMed] [Google Scholar]
- 30.de Jong KP, Gouw AS, Peeters PM, et al. P53 mutation analysis of colorectal liver metastases: relation to actual survival, angiogenic status, and p53 overexpression. Clin Cancer Res. 2005;11:4067–4073. doi: 10.1158/1078-0432.CCR-04-2389. [DOI] [PubMed] [Google Scholar]
- 31.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 32.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 33.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162:975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
- 34.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 35.Wei EK, Giovannucci E, Selhub J, et al. Plasma vitamin B6 and the risk of colorectal cancer and adenoma in women. J Natl Cancer Inst. 2005;97:684–692. doi: 10.1093/jnci/dji116. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs CS, Willett WC, Colditz GA, et al. The influence of folate and multivitamin use on the familial risk of colon cancer in women. Cancer Epidemiol Biomarkers Prev. 2002;11:227–234. [PubMed] [Google Scholar]
- 37.Giovannucci E, Rimm EB, Ascherio A, et al. Alcohol, low-methionine--low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst. 1995;87:265–273. doi: 10.1093/jnci/87.4.265. [DOI] [PubMed] [Google Scholar]
- 38.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev. 2006;15:189–193. doi: 10.1158/1055-9965.EPI-152CO. [DOI] [PubMed] [Google Scholar]
- 39.Slattery ML, Curtin K, Ma K, et al. Diet activity, and lifestyle associations with p53 mutations in colon tumors. Cancer Epidemiol Biomarkers Prev. 2002;11:541–548. [PubMed] [Google Scholar]
- 40.Lashner BA, Shapiro BD, Husain A, Goldblum JR. Evaluation of the usefulness of testing for p53 mutations in colorectal cancer surveillance for ulcerative colitis. Am J Gastroenterol. 1999;94:456–462. doi: 10.1111/j.1572-0241.1999.877_f.x. [DOI] [PubMed] [Google Scholar]
- 41.Ulrich CM, Potter JD. Folate and cancer--timing is everything. Jama. 2007;297:2408–2409. doi: 10.1001/jama.297.21.2408. [DOI] [PubMed] [Google Scholar]
- 42.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. Jama. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 43.Mason JB, Dickstein A, Jacques PF, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16:1325–1329. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]
- 44.Food and Drug Administration Food standards: amendment of standards of identity ro enriched grain products to require addition of folic acid. Final rule. Fed Regist. 1996:8797–8807. [Google Scholar]
- 45.Ghavam-Nasiri MR, Rezaei E, Ghafarzadegan K, Seilanian-Toosi M, Malekifard H. Expression of p53 in colorectal carcinoma: correlation with clinicopathologic features. Arch Iran Med. 2007;10:38–42. [PubMed] [Google Scholar]