Abstract
Primordial Germ Cell (PGC) migration in zebrafish is guided by SDF-1a. Binding of this chemokine to its receptor CXCR4b activates downstream signalling cascades leading to cell polarization and directed migration towards the attractant source. Despite the detailed information available concerning the role of SDF-1 in guiding the PGCs to their targets, little was known regarding the molecular mechanisms controlling the distribution of SDF-1a within the tissue. We have recently shown that the activity of a second SDF-1/CXCL12 receptor, CXCR7 is crucial for proper migration of PGCs. Although CXCR4 and CXCR7 are structurally related and serve as receptors for the same ligand, they appear to serve very different functions during PGC migration. Here we discuss a model according to which CXCR4b translates the polarized distribution of SDF-1 into directed PGC migration, while CXCR7 acts as a high-affinity decoy receptor and facilitates the migration of PGCs by shaping the distribution of the chemokine in the environment.
Key words: cell migration, CXCR4, CXCR7, SDF-1, chemokine, chemotaxis
Chemokine-guided cell migration is central for many processes in normal development and homeostasis (e.g., embryogenesis) as well as in pathological conditions (e.g., inflammation). Zebrafish primordial germ cells (PGCs) serve as a useful model for studying chemokine-controlled cell migration in vivo as the migrating PGCs sense and respond to the dynamic distribution of the chemokine SDF-1a through its receptor CXCR4b.1,2
Recent reports identified CXCR7 as a receptor for SDF-13,4 that controls processes such as cell adhesion, survival and tumor progression. A role for this receptor in regulating cell migration during development was demonstrated in the zebrafish lateral line.5,6 The zebrafish lateral line primordium migrates directionally on a stripe of uniform sdf-1a expression to deposit a set of sensory organs along the fish tail. While the authors raised the hypothesis that antagonistic interactions between CXCR4b and CXCR7 polarize the developing organ to allow its migration, the precise function of CXCR7 in this process remained unclear.
To address this question in an in vivo context, we examined the role CXCR7 plays in zebrafish PGC migration.7 Our experiments revealed that knockdown of cxcr7 translation using morpholino antisense oligo nucleotides results in impaired polarity and aberrant migration of PGCs. Unlike cxcr4b, cxcr7 is not specifically expressed in the PGCs but is initially uniformly distributed throughout the embryo. Furthermore, in contrast to activity of CXCR4, CXCR7 function was found to be required in tissues surrounding the migrating cells rather than in the PGCs themselves.
To examine the function of CXCR7 in somatic cells we determined the subcellular localization of the protein as compared with that of CXCR4b and SDF-1a. Interestingly, while CXCR4b is predominantly localized to the plasma membrane, CXCR7 is found primarily in intracellular structures. The fact that SDF-1α and CXCR7 colocalized in the cell and that SDF-1α was found in vesicles that contained the lysosomal marker LAMP-1 suggested that the prime role of CXCR7 is to bind and internalize SDF-1a thereby controlling the level of the diffusible chemokine in the extracellular space. Indeed, observing PGCs expressing CXCR4b on their membrane we detected strong receptor internalization when CXCR7 function was knocked down. The enhanced internalization, a typical response to high levels of SDF-1a8 could be reversed by concomitant removal of SDF-1.
These findings provided an explanation for the CXCR7 knock-down phenotype as abnormally high levels of SDF-1a in the environment have been shown before to interfere with cell motility.1,2 Indeed, PGCs in CXCR7 knocked-down embryos displayed strong inhibition of motility manifested in short migration tracks—a phenotype that could be reversed by simultaneous removal of CXCR7 and SDF-1.
The implication of the results presented above is that the sole function of CXCR7 in the context of PGC migration is ligand sequestration. Consistent with this idea, two typical signalling responses acting downstream of chemokine receptors namely, elevation of intracellular calcium levels and PI3K activation9–13 were not altered in cells knocked down for CXCR7. Thus, consistent with other reports,4,14 our results imply that CXCR7 signalling is not required for PGC migration.
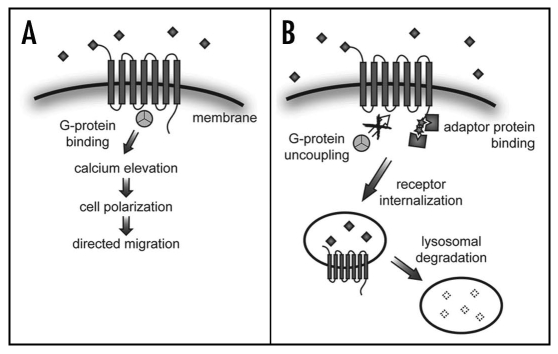
An important outstanding question concerns the molecular basis for the dramatic difference between the activity of CXCR4 and that of CXCR7. Defining domains and amino acids responsible for this difference would provide extensive information regarding chemokine receptor signalling and trafficking within the cell. Whereas random mutagenesis and generation of various CXCR4-CXCR7 chimeric molecules might provide an answer to this question, it is tempting to speculate that known protein motifs are responsible for the differences between the two receptors. For example, an obvious candidate region is that around its DRY motif,14 a motif within the second intracellular loop that is important for Gprotein coupling and signalling.15 Whereas uncoupling downstream signalling in the case of CXCR7 is an interesting research avenue, other non-mutually exclusive options should be examined (Fig. 1). For example, CXCR7 could possess domains that facilitate interaction with components that enhance internalization. Such an interaction could remove the receptor from the location where it normally interacts with the signalling machinery, while effectively internalizing SDF-1a.
Figure 1.
Proposed model for differential functions of CXCR4b and CXCR7. (A) CXCR4b signalling in PGCs controls cell polarization and directional migration in response to SDF-1a binding (squares), through interaction with G-proteins and elevation of calcium levels. (B) Binding of SDF-1a by CXCR7 does not elicit signalling. Endocytosis of the lignad-bound CXCR7 leads to sequestration and degradation of SDF-1a in the somatic environment.
Taken together, we show that proper PGC migration requires a mechanism to remove the guidance cue thereby allowing the formation of an informative chemotactic gradient. It would be very interesting to examine whether the paradigm demonstrated for the PGC migration model applies for other chemokine-guided events in development and disease.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6027
References
- 1.Doitsidou M, Reichman Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, et al. Guidance of Primordial Germ Cell Migration by the Chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 2.Knaut H, Werz C, Geisler R, Nusslein Volhard C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279–282. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- 3.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 4.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dambly Chaudière C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Developmental Biology. 2007;7:23. doi: 10.1186/1471-213X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valentin G, Haas P, Gilmour D. The Chemokine SDF-1a Coordinates Tissue Migration through the Spatially Restricted Activation of Cxcr7 and Cxcr4b. Current Biology. 2007 doi: 10.1016/j.cub.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman Fried M, Blaser H, Minina S, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 8.Minina S, Reichman Fried M, Raz E. Control of receptor internalization, signaling level, and precise arrival at the target in guided cell migration. Curr Biol. 2007;17:1164–1172. doi: 10.1016/j.cub.2007.05.073. [DOI] [PubMed] [Google Scholar]
- 9.Bleul CC, Farzan M, Choe H, Parolin C, Clark Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 10.Blaser H, Reichman Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, et al. Migration of zebrafish primordial germ cells: a role for Myosin contraction and cytoplasmic flow. Dev Cell. 2006;11:613–627. doi: 10.1016/j.devcel.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Andrews S, Stephens LR, Hawkins PT. PI3K class IB pathway. Sci STKE. 2007 doi: 10.1126/stke.4072007cm2. [DOI] [PubMed] [Google Scholar]
- 12.Sotsios Y, Whittaker GC, Westwick J, Ward SG. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J Immunol. 1999;163:5954–5963. [PubMed] [Google Scholar]
- 13.Vicente Manzanares M, Rey M, Jones DR, Sancho D, Mellado M, Rodriguez Frade JM, et al. Involvement of phosphatidylinositol 3-kinase in stromal cell-derived factor-1 alpha-induced lymphocyte polarization and chemotaxis. J Immunol. 1999;163:4001–4012. [PubMed] [Google Scholar]
- 14.Sierro F, Biben C, Martinez Munoz L, Mellado M, Ransohoff RM, Li M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]