Abstract
Arf6 and its effector AMAP1 are overexpressed in malignant breast cancer cells, and are involved in their invasion and metastasis. We recently revealed that GEP100, a guanine nucleotide exchanging factor, is responsible for the activation of Arf6 which induces invasion and metastasis. GEP100 associated directly with ligand-activated epidermal growth factor receptor (EGFR) to be activated. Disruption of E-cadherin-mediated cell-cell adhesion is one of the major steps involved in acquisition of invasive phenotypes of most carcinomas. The EGFR-GEP100-Arf6 pathway not only activated matrix invasion activity but also perturbed E-cadherin function. GEP100 was found to be expressed in more than 80% of invasive ductal carcinomas. However, 60% of ductal carcinomas in situ were also positive for GEP100, in which GEP100 was preferentially coexpressed with EGFR in their malignant cases. Microenvionments have been highly implicated in the development of tumor malignancy. Our results reveal an aspect of the precise molecular mechanism of cancer invasion and metastasis, in which full invasiveness is not acquired just by alterations of cancer cells themselves, but their microenvironments may also play pivotal roles.
Key words: Arf6, breast cancer, E-cadherin, epidermal growth factor, epidermal growth factor receptor, GEP100, invasion, metastasis, tumor-associated macrophage, tumor microenvironment
The Arf-family of small GTPases regulate membrane trafficking and remodeling.1,2 There are six isoforms of Arf GTPases in mammals (Arf1–6), although Arf2 has been lost in humans. Arf6 is the most divergent of the Arf isoforms and primarily functions at cell peripheries by regulating endocytosis and the recycling-back of plasma membrane components as well as certain types of cell surface receptors, through its GTPase cycle.1
Years ago we provided the first evidence supporting that Arf6 activity is crucially involved in the motile phenotype of epithelial cells.3 We subsequently found that the Arf6 protein, as well as its effector, AMAP1/ASAP1/DEF1,4,5 are both highly overexpressed in malignant breast cancer cells and contribute greatly to their invasive and metastatic activities.6,7 Analysis of surgical specimens of human breast cancer also revealed a high correlation between their malignant phenotypes and AMAP1 protein levels, while AMAP1 protein expression in non-cancerous components is only marginal7 (also see later). The importance of Arf6 and its signaling in invasion has also been implicated in other types of tumors, such as lung cancer,8 glioblastoma8 and melanoma.9 For tumor cell invasion, complex formation of AMAP1 with several other protein, including cortactin and paxillin, is necessary7 (and our unpublished results). The precise mechanisms as to how this AMAP1-mediated signalsome functions in invasion is under investigation.
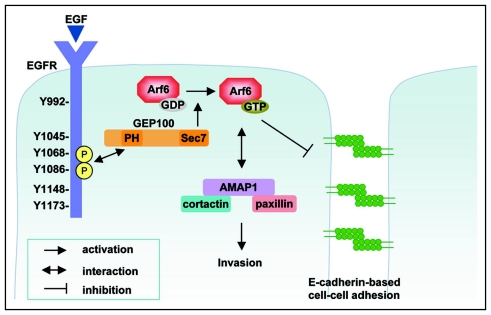
Following these findings, we recently found that GEP100, a guanine nucleotide exchanging factor (GEF),10 is primarily responsible for Arf6 activation to induce breast cancer cell invasion and metastasis.11 On the other hand, other ArfGEFs, including ARNO and EFA6 which are both known to be robust GEFs against Arf6, were not immediately involved in invasive activities.11 These results suggest that Arf6 becomes engaged in different cellular functions, even within a single cell, depending on the specific type of GEF that activates this small GTPase (and hence depending on the external stimuli activating the GEFs). We then found that GEP100 is associated with ligand-activated epidermal growth factor receptor (EGFR), an association which is mediated via a direct interaction of the pleckstrin homology (PH) domain of GEP100 with either the phosphorylated Tyr1068 or Tyr1086 of EGFR. This is the first demonstration that a PH domain binds to phosphotyrosine. We have also shown that the invasive activities of breast cancer cells utilizing the EGFR-GEP100-Arf6 pathway fully depend on the ligand-activation of their EGFR. Therefore, our results indicate that such invasiveness utilizing the EGFR-GEP100-Arf6 pathway does not arise only by alterations of breast cancer cells themselves (i.e., in this case, by expression of EGFR, GEP100, Arf6 and AMAP1, and perhaps also other alterations), but need other exogenous factors.
It has been highly implicated that tumor-associated macrophages, rather than carcinoma cells, are the major source of epidermal growth factor (EGF) in mammary tumors.12 Tumor-associated macrophages correlate with poor prognosis in human breast cancer,13–16 and the crucial roles of macrophages in the development of invasive and metastatic events of mammary tumors have been documented in a mouse model experiment.17 GEP100 was found to be expressed in more than 80% of invasive ductal carcinomas (n = 30), in which more than 90% of the EGFR-positive cases were also positive for GEP100.11 However, about 60% of ductal carcinomas in situ, which are non-invasive, were also found to express GEP100 (n = 72), in which GEP100 was preferentially coexpressed with EGFR in their malignant cases.11 This observation may also support the above notion that the invasiveness of some breast cancers may not be acquired only by their own alterations. Moreover, these results simultaneously imply that induction of malignancy through microenvironments or EGF may occur in a substantially large population of primary ductal cancers of the human breast.
Biomarkers that specifically correlate with invasive phenotypes have not been clearly identified in mammary tumors, in spite of extensive research, including that on genomic mutations and gene expression.18–20 Our results may also provide an interpretation for this lack of biomarkers of invasiveness in most mammary tumors. We have previously shown that both highly invasive and noninvasive breast cancer cells express comparable amounts of Arf6 mRNA and AMAP1 mRNA, and hence overexpression of these two proteins in highly invasive cells appears to be regulated post-transcriptionally.6,7 The 5′-untranslated regions of these mRNAs, which are thought to be primarily responsible for their translational control, exhibit complicated structures with very high levels of free energy changes. Our preliminary results suggest that the translation of AMAP1 mRNA in some breast cancer cells is upregulated by EGF stimulation (our unpublished results). On the other hand, we still do not know the mechanism by which Arf6 protein levels is regulated post-transcriptionally.
Overexpression of c-Met and ErbB2 correlate with the malignancy of breast tumors.21–23 c-Met and ErbB2 possess tyrosine phosphorylation sites corresponding to Tyr1068 and Tyr1086 of EGFR.22,23 We previously reported that ErbB2, expressed at a moderate level in MDA-MB-231 cells, is not complexed with GEP100.11 These results, however, do not deny the possibility that ErbB2 associates with GEP100, when ErbB2 is overexpressed at very high levels and heavily tyrosine phosphorylated. Whether GEP100 can make a complex with receptors other than EGFR awaits to be clarified.
Disruption of E-cadherin-mediated cell-cell adhesion is a major step in the acquisition of invasive phenotypes of most carcinomas.24–26 Arf6 activity has been shown to play pivotal roles in E-cadherin-mediated epithelial cell-cell adhesion, in which an active form of Arf6, Arf6Q67L, causes disassembly of E-cadherin-mediated adherens junctions, while its inactive form, Arf6T27N, blocks hepatocyte growth factor (HGF)-induced internalization of E-cadherin-based junctional components.27,28 Our results indicate that co-overexpression of Arf6 and GEP100 in MCF7 cells interferes with their E-cadherin-mediated cell-cell adhesion and induces their invasion.11 Consistently, it has been shown that siRNA-mediated knockdown of GEP100 evokes resistance to HGF-induced disruption of adherens junctions.29 On the other hand, overexpression of ARNO or EFA6B together with Arf6 in MCF7 cells did not block their E-cadherin-mediated cell-cell adhesion nor caused invasion, while this co-overexpression evoked active membrane ruffles when cells were cultured under a sparse density and were hence not adhered to each other11 (and our unpublished results). Therefore, Arf6 activated by GEP100, but not other GEFs, appears to be specifically involved in the regulation of E-cadherin function. The biochemical properties of GEP100 that enable its association with α-catenin29 may also play a role in its regulation of E-cadherin. Understanding the precise mechanism of this GEP100-Arf6-mediated regulation of E-cadherin function will greatly contribute to the further advancement of the field of cancer cell biology.
Figure 1.
The GEP100-Arf6 pathway to invasion. The Arf6 pathway, which involves its activation by GEP100 and employs the AMAP1-mediated signalsome for its downstream signaling constitutes a robust and central pathway for the induction of the invasion and metastasis of some breast cancer cells. GEP100 associates with tyrosine-phosphorylated EGFR for its activation. Therefore, activation of EGFR is required for the activation of this pathway. This pathway also appears to perturb the formation and maintenance of E-cadherin-based cell-cell adhesion.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6191
References
- 1.Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- 2.D'Souza Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 3.Kondo A, Hashimoto S, Yano H, Nagayama K, Mazaki Y, Sabe H. A new paxillin-binding protein, PAG3/Papα/KIAA0400, bearing an ADP-ribosylation factor GTPase-activating protein activity, is involved in paxillin recruitment to focal adhesions and cell migration. Mol Biol Cell. 2000;11:1315–1327. doi: 10.1091/mbc.11.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto S, Hashimoto A, Yamada A, Kojima C, Yamamoto H, Tsutsumi T, Higashi M, Mizoguchi A, Yagi R, Sabe H. A novel mode of action of an ArfGAP, AMAP2/PAG3/Papα, in Arf6 function. J Biol Chem. 2004;279:37677–376784. doi: 10.1074/jbc.M404196200. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto S, Hashimoto A, Yamada A, Onodera Y, Sabe H. Assays and properties of the ArfGAPs, AMAP1 and AMAP2, in Arf6 function. Methods Enzymol. 2005;404:216–231. doi: 10.1016/S0076-6879(05)04021-8. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto S, Onodera Y, Hashimoto A, Tanaka M, Hamaguchi M, Yamada A, Sabe H. Requirement for Arf6 in breast cancer invasive activities. Proc Natl Acad Sci USA. 2004;101:6647–6652. doi: 10.1073/pnas.0401753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onodera Y, Hashimoto S, Hashimoto A, Morishige M, Mazaki Y, Yamada A, Ogawa E, Adachi M, Sakurai T, Manabe T, Wada H, Matsuura N, Sabe H. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24:963–973. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto S, Hirose M, Hashimoto A, Morishige M, Yamada A, Hosaka H, Akagi K, Ogawa E, Oneyama C, Agatsuma T, Okada M, Kobayashi H, Wada H, Nakano H, Ikegami T, Nakagawa A, Sabe H. Targeting AMAP1 and cortactin binding bearing an atypical src homology 3/proline interface for prevention of breast cancer invasion and metastasis. Proc Natl Acad Sci USA. 2006;103:7036–7041. doi: 10.1073/pnas.0509166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tague SE, Muralidharan V, D'Souza Schorey C. ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc Natl Acad Sci USA. 2004;101:9671–9676. doi: 10.1073/pnas.0403531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Someya A, Sata M, Takeda K, Pacheco Rodriguez G, Ferrans VJ, Moss J, Vaughan M. ARF-GEP(100), a guanine nucleotide-exchange protein for ADP-ribosylation factor 6. Proc Natl Acad Sci USA. 2001;98:2413–2418. doi: 10.1073/pnas.051634798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morishige M, Hashimoto S, Ogawa E, Toda Y, Kotani H, Hirose M, Wei S, Hashimoto A, Yamada A, Yano H, Mazaki Y, Kodama H, Nio Y, Manabe T, Wada H, Kobayashi H, Sabe H. GEP100 links epidermal growth factor receptor signalling to Arf6 activation to induce breast cancer invasion. Nat Cell Biol. 2008;10:85–92. doi: 10.1038/ncb1672. [DOI] [PubMed] [Google Scholar]
- 12.Pollard JW. Tumou-reducated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 13.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 14.Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Lin EY, Gouon Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7:147–162. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 16.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, Zhou YX, Varnholt H, Smith B, Gadd M, Chatfield E, Kessler J, Baer TM, Erlander MG, Sgroi DC. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter D, Lahti Domenici J, Keshaviah A, Bae YK, Argani P, Marks J, Richardson A, Cooper A, Strausberg R, Riggins GJ, Schnitt S, Gabrielson E, Gelman R, Polyak K. Molecular markers in ductal carcinoma in situ of the breast. Mol Cancer Res. 2003;1:362–375. [PubMed] [Google Scholar]
- 20.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 21.Blume Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 22.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 23.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 24.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 25.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 26.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 27.Palacios F, Price L, Schweitzer J, Collard JG, D'Souza Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 2001;20:4973–4986. doi: 10.1093/emboj/20.17.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palacios F, Schweitzer JK, Boshans RL, D'Souza Schorey C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol. 2002;4:929–936. doi: 10.1038/ncb881. [DOI] [PubMed] [Google Scholar]
- 29.Hiroi T, Someya A, Thompson W, Moss J, Vaughan M. GEP100/BRAG2: activator of ADP-ribosylation factor 6 for regulation of cell adhesion and actin cytoskeleton via E-cadherin and α-catenin. Proc Natl Acad Sci USA. 2006;103:10672–10677. doi: 10.1073/pnas.0604091103. [DOI] [PMC free article] [PubMed] [Google Scholar]