Abstract
The heart is the first organ to function during vertebrate development and cardiac progenitors, are among the first cell lineages to be established from mesoderm cells emerging from the primitive streak during gastrulation. Cardiac progenitors have been mapped in the epiblast of pre-streak embryos. In the early chick gastrula they are located in the mid-primitive streak, from which they enter the mesoderm bilaterally. However, migration routes of cardiac progenitors have never been directly observed within the embryo and the factor(s) controlling their movement are not known. Furthermore, it is not understood how signals controlling cell movement are integrated with those that determine cell fate. Long-term video microscopy combined with GFP labelling and image processing enabled us to observe the movement patterns of prospective cardiac cells in whole embryos in real time. Embryo manipulations and the analysis of explants suggest that Wnt3a plays a crucial role in guiding these cells through a RhoA dependent mechanism involving negative chemotaxis. Wnt3a is expressed at high levels in the amniote primitive streak and ectopic signalling activity caused wider movement trajectories resulting in cardia bifida, which was rescued by dominant-negative Wnt3a. Our studies revealed Wnt3a-RhoA mediated chemo-repulsion as a novel mechanism guiding cardiac progenitors. This activity can act at long-range and does not interfere with cardiac cell fate specification.
Key words: cardiac development, cell migration, Wnt signaling, video microscopy, imaging, chicken
Embryo development involves the coordinated movement of many cells, sometimes over long distances, to reach the final location of cell differentiation and organogenesis. The cells that will become the heart, mesoderm-derived cardiac progenitors, are among the first cells to ingress through the primitive streak during gastrulation. In the chick, future cardiac cells have been identified in the epiblast of pre-streak embryos1 and in the mid-primitive streak at Hamburger-Hamilton (HH) stage 3.2–4 Migration routes of cardiac progenitors have been inferred from fate mapping studies,5 but until recently it has not been possible to observe cell movement in real time and the signals that control directed cell migration were not known.
Recent developments of in vivo imaging techniques have allowed direct observations of cell migration in the chicken embryo. This has enabled us to begin to gain an understanding of the mechanisms involved in guiding cell movements.6–9 We established long-term video microscopy and used this technique to characterize the movement patterns of paraxial and lateral plate mesoderm cells, which emerge from the primitive streak at HH stage 4.6 At this stage in gastrulation, cells are guided by negative and positive chemotaxis in response to FGF-8 and FGF-4 respectively, consistent with phenotypes of mice deficient in FGF signalling where cells remain in the streak.10,11 Cells contributing to the heart gastrulate through the streak prior to paraxial and lateral mesoderm and thus, they were not observed in the initial study. Therefore, we recently examined HH3 chicken embryos and investigated the migration routes of cardiac progenitors and some of the factor(s) involved.12
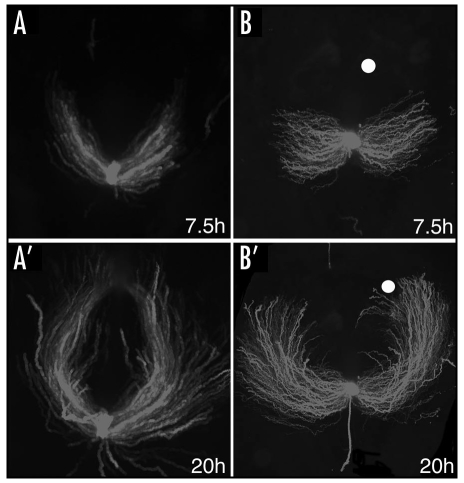
GFP labelled cardiac progenitors were tracked from their origin in the avian primitive streak until they formed a primitive heart tube in the ventral midline. GFP labelled cells contributed to the myocardium and endocardium, and developing hearts showed normal morphogenesis and expressed cardiac specific markers such as Nkx2.5 and ventricular myosin heavy chain, vMHC. Real time imaging demonstrated that prospective heart cells migrate on highly directed trajectories (Fig. 1A and A′). Heterochronic grafts confirmed that cell movement patterns are governed by extrinsic signals; GFP labelled cells always behaved appropriately for their new environment. This was not unexpected since classic mapping experiments have shown that cell fate at these stages is plastic and determined by extrinsic cues. However, in addition this observation suggests that cell fate decisions and cell movement behaviour are intimately linked.
Figure 1.
Long-term video microscopy revealed migration trajectories of cardiac progenitors. (A and A′) GFP labelled cells leave the primitive streak at HH3 and migrate in a anterior-lateral trajectory before moving back toward the midline, where the bilateral heart fields merge to give rise to a single heart tube. (B and B′) Implanting Wnt3a expressing cells (white dots) causes wider cell movement trajectories, many cells failed to reach the midline and cardia bifida was observed frequently. In each panel anterior is to the top and posterior at the bottom. Dark field still images are shown at 7.5 and 20 hours of imaging, see reference12 for more details.
Wnt3a transcripts are highly expressed in the primitive streak of the chick gastrula and implantation of Wnt3a expressing RatB1a fibroblasts into the migration path of cardiogenic cells dramatically changed their movement trajectories. In the majority of embryos cardiac progenitors took a significantly wider path with more pronounced lateral migration (Fig. 1B and B′). Often the two heart fields failed to merge and cardia bifida was observed frequently (75%). Similar results were obtained after directed expression of Wnt3a in the primitive streak.12 We speculate that this phenotype is primarily due to more extensive lateral migration but may also be the result of impaired movement back towards the midline. Expression of a dominant negative Wnt3a (DN-Wnt3a-IRES-GFP)13 restored the movement patterns of cardiac progenitors in the presence of an ectopic source of Wnt3a in vivo and reduced the incidence of cardia bifida. This suggests that Wnt3a levels are crucial in directing cell movements.
To investigate the mechanism by which high levels of Wnt3a present in the primitive streak control the migration patterns of prospective cardiac cells, GFP labelled explants from the anterior primitive streak of HH3 embryos were exposed to different Wnt expressing cells or to the parental RatB1a-LNCX fibroblasts in explant culture. In response to Wnt3a expressing cells HH3 primitive streak cells migrated away from the source of Wnt3a in a highly directed manner. This chemotactic behaviour was inhibited when cells expressed a dominant negative mutant of Wnt3a.12 These experiments suggest that HH3 primitive streak cells can respond directly to Wnt3a, however, we cannot exclude the presence of additional guidance cues acting in parallel, such as for example FGF-8.6
Wnt3a is typically thought to act through β-catenin dependent mechanisms to affect cell fate decisions. However, in CHO cells Wnt3a dependent cell motility involves non-canonical signalling through RhoA.14 RhoA transcripts are enriched in the primitive streak and are upregulated during early heart development in chick.12,15 Small GTPases, including RhoA, are involved in the dynamic organization of the actin cytoskeleton, which provides the force for cell motility. In addition, Rho GTPases may be responsible for the initial polarization of the microtubule cytoskeleton, thus maintaining the stable polarization of a directionally migrating cell.16 To determine whether RhoA is involved in cardiac progenitor cell migration HH3 streak cells were electroporated with constitutively active (RhoA-V14) or dominant negative forms (RhoA-N19).17 In the explant assay the RhoA-V14 expressing cells were still repelled by Wnt3a, while RhoA-N19 expressing cells were not able to respond. In vivo, RhoA-N19-IRES-GFP expressing cells moved more slowly and embryos developed cardia bifida. Movement trajectories of cardiac progenitors expressing RhoA-V14-IRES-GFP were affected during the later phase of migration when a large number of cells continued their outward movement and ended up in extra-embryonic regions or lateral mesoderm. This resulted in cardia bifida. We speculate that this behaviour may be due to the fact that RhoA-V14 sensitises the cells and causes a more pronounced outward migration in response to Wnt3a. It is also possible that cells are moving faster and/or that RhoA-V14 interferes with factor(s) involved in guiding cells back towards the midline.
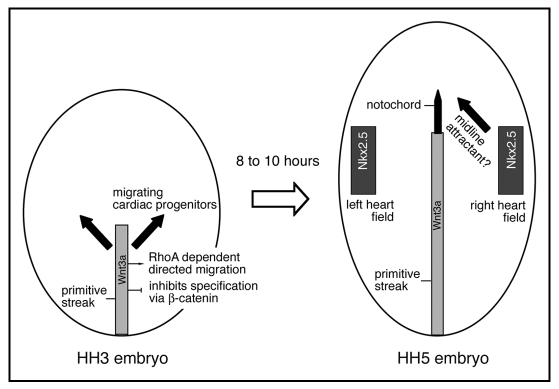
Taken together our data supports a model whereby Wnt3a acts as a chemo-repellent signal to guide the movement of cardiac progenitor cells away from the streak, resulting in lateral migration (Fig. 2). Our evidence suggests that directed migration is mediated by a pathway, which involves RhoA activity. Interestingly, Wnt3a signalling through β-catenin inhibits cardiac cell fate specification.18,19 We propose that these different activities are integrated by Wnt3a through cross-talk between multiple downstream effector pathways.20,21
Figure 2.
Schematic representation of cardiac progenitor cell migration in response to Wnt3a. At Hamburger Hamilton (HH) stage 3 cardiac progenitors (black arrows) migrate away from the primitive streak (grey) in an anterior-lateral direction. Wnt3a is expressed in the primitive streak and is involved in the directed migration of cardiac progenitors through a RhoA dependent mechanism. It is likely that Wnt3a simultaneously inhibits cardiac fate specification via β-catenin. By HH stage 5 cardiac progenitors have arrived in the bilateral heart fields and in response to inductive signals22,23 some cells begin to express the cardiac transcription factor Nkx2.5. The signals that guide cardiac progenitor cells back to the midline (black arrow) are currently unknown.
Acknowledgements
We would like to thank Grant Wheeler for discussions. Research on cardiogenesis is funded by grants from the British Heart Foundation to A.M., references: PG/03/041, PG/06/136.
Abbreviations
- Wnt
a family of secreted glycoproteins involved in cell-cell signalling
- RhoA
member of the Rho family of small GTPases also including Rac and Cdc42
- HH
stages of chicken embryo development as defined by Hamburger and Hamilton2
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6295
References
- 1.Hatada Y, Stern CD. A fate map of the epiblast of the early chick embryo. Development. 1994;120:2879–2889. doi: 10.1242/dev.120.10.2879. [DOI] [PubMed] [Google Scholar]
- 2.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- 3.Rosenquist GC. Location and movements of cardiogenic cells in the chick embryo: the heart-forming portion of the primitive streak. Dev Biol. 1970;22:461–475. doi: 10.1016/0012-1606(70)90163-6. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Martinez V, Schoenwolf GC. Primitive-streak origin of the cardiovascular system in avian embryos. Dev Biol. 1993;159:706–719. doi: 10.1006/dbio.1993.1276. [DOI] [PubMed] [Google Scholar]
- 5.Yutzey KE, Kirby ML. Wherefore heart thou? Embryonic origins of cardiogenic mesoderm. Dev Dyn. 2002;223:307–320. doi: 10.1002/dvdy.10068. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Dormann D, Münsterberg AE, Weijer CJ. Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev Cell. 2002;3:425–437. doi: 10.1016/s1534-5807(02)00256-3. [DOI] [PubMed] [Google Scholar]
- 7.Zamir EA, Czirok A, Cui C, Little CD, Rongish BJ. Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proc Natl Acad Sci USA. 2006;103:19806–19811. doi: 10.1073/pnas.0606100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leslie NR, Yang X, Downes CP, Weijer CJ. PtdIns(3,4,5)P(3)-Dependent and -Independent Roles for PTEN in the Control of Cell Migration. Curr Biol. 2007;17:115–125. doi: 10.1016/j.cub.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature. 2007;449:1049–1052. doi: 10.1038/nature06211. [DOI] [PubMed] [Google Scholar]
- 10.Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 12.Yue Q, Wagstaff L, Yang X, Weijer C, Münsterberg A. Wnt3a-mediated chemorepulsion controls movement patterns of cardiac progenitors and requires RhoA function. Development. 2008;135:1029–1037. doi: 10.1242/dev.015321. [DOI] [PubMed] [Google Scholar]
- 13.Hoppler S, Brown JD, Moon RT. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- 14.Endo Y, Wolf V, Muraiso K, Kamijo K, Soon L, Uren A, Barshishat-Kupper M, Rubin JS. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J Biol Chem. 2005;280:777–786. doi: 10.1074/jbc.M406391200. [DOI] [PubMed] [Google Scholar]
- 15.Kaarbo M, Crane DI, Murrell WG. RhoA is highly upregulated in the process of early heart development of the chick and important for normal embryogenesis. Dev Dyn. 2003;227:35–47. doi: 10.1002/dvdy.10283. [DOI] [PubMed] [Google Scholar]
- 16.Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci. 2001;114:3795–3803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- 17.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 18.Marvin MJ, Di-Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bejsovec A. Wnt pathway activation: new relations and locations. Cell. 2005;120:11–14. doi: 10.1016/j.cell.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Veeman MT, Axelrod JD, Moon RT. A second canon, Functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 22.Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 23.Alsan BH, Schultheiss TM. Regulation of avian cardiogenesis by Fgf8 signaling. Development. 2002;129:1935–1943. doi: 10.1242/dev.129.8.1935. [DOI] [PubMed] [Google Scholar]