Abstract
Cell colonization is an important in a wide variety of biological processes and applications including vascularization, wound healing, tissue engineering, stem cell differentiation and biosensors. During colonization porous 3D structures are used to support and guide the ingrowth of cells into the matrix. In this review, we summarize our understanding of various factors affecting cell colonization in three-dimensional environment. The structural, biological and degradation properties of the matrix all play key roles during colonization. Further, specific scaffold properties such as porosity, pore size, fiber thickness, topography and scaffold stiffness as well as important cell material interactions such as cell adhesion and mechanotransduction also influence colonization.
Key words: colonization, pore size, porosity, topography, mechanotransduction, degradation, matrix turnover
Introduction
Advancements in tissue culture technique have allowed better understanding of cellular events and regulations in two-dimensional architecture. Interactions between the cells and the underlying matrix elements control cellular attachment, proliferation and activity. Dynamic cellmatrix interactions orchestrate the morphogenesis of cells. During the process, not only do cells undergo morphogenesis but they also remodel the matrix components. The general dogma is that a chemical1 or mechanical2 stimulus signaled through focal adhesion points3 changes the polymerization state of the cytoskeletal actin and subsequent cellular activity.4 A complex cascades of events follow including the activation of intracellular signaling pathways with tyrosine phosphorylation of focal adhesion kinases (FAKs) that change the differentiation, proliferation and migration of cells.5
Recent understanding of the components required to proliferate human embryonic stem cells in two-dimension, without lineage commitment, has opened a new window of opportunity to develop new cellular therapies and to generate functionally replaceable devices and/or tissue parts.6 Transforming these concepts into useful applications is currently limited due to the complexity of interactions that affect the differentiation and proliferation of stem cells. Despite much anecdotal evidence suggesting the plastic nature of mature cells, this possibility has not been explored. For example, chondrocytes (cells present in the cartilage) lose their round morphology in monolayer culture and assume a fibroblast-like phenotype. The phenotypic change subsequently leads to an altered biosynthesis of matrix proteins, increasing synthesis of collagen type I instead of collagen type II (a chondrogenic marker protein). After reseeding the differentiated chondrocytes on alginate microparticles (www.promocell.com), the cells redifferentiated into chondrocytes.
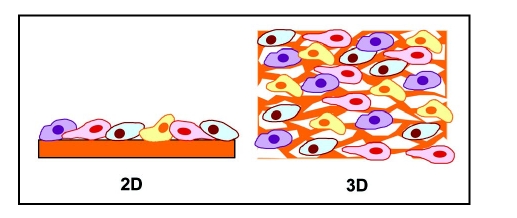
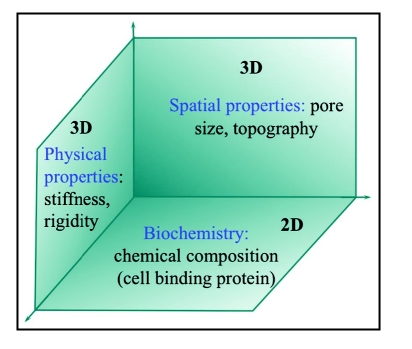
Much of the cell culture experiments performed in molecular biology are cultivated on two-dimensional (2D) tissue culture treated surfaces. Many in vitro experiments have shown that cells have different responses in colonization, proliferation and differentiation on 3D scaffolds than on traditional 2D-tissue culture. In 2D substrata, cultured cells are restricted to spread and attach to a flat rigid glass or tissue culture plastic surface (Fig. 1). Hence, effects of biophysical properties of the matrix that provide a spatio-temporal effect in the body are not part of the effect. However, biophysical properties significantly influence cell adhesion and functions in three-dimensional (3D) environment.7 3D matrices provide physical cues to guide cell colonization as well as chemical cues of cell-binding sites to support cell attachment and spreading (Fig. 2).
Figure 1.
2D and 3D cell culture.
Figure 2.
Factors influencing cell colonization in 3D.
To obtain useful functions and understand morphogenesis of tissues, one has to recreate the microenvironment that is conducive to drive the stem cells to required cell type. A number of investigators have focused on evaluating the role of various soluble factors on the regulation of lineage development in 2D culture systems. Although these results will help understand the interactions, evaluating 3D configurations are critical (1) to understand the spatio-temporal effects, (2) to evaluate the reorganization of various compartments and organ formation and (3) to develop devices that can be used in clinical applications. Three-dimensional porous matrices offer a spatio-temporal configuration similar to in vivo conditions. They have been generated and utilized in various tissue regeneration strategies8–10 or surrogate models for evaluating disease mechanisms. The purpose of this review is to summarize our understanding of factors affecting the cell colonization in 3-D porous structures.
Basics of Porous Structures
Tissue engineering has given promise for generating functionally replaceable 3D tissue parts, although currently the products obtainable are limited to avascular regions. Biodegradable scaffolds are used to support and guide the in-growth of cells i.e., they form the template for cell colonization. Scaffolding material eventually disappears leaving only the necessary healthy tissue in a topologically required form.8,9,11 The 3D matrix provides more space for cellular colonization and proliferation as well as provides a different set of physiological signals to the developing tissue. One option for creating tissue scaffolds is to use extracellular matrix (ECM) components derived from animal sources. For example porcine acellular dermis has been used for skin regeneration12 and control of hypertrophic scarring.13 Small intestinal submucosa (SIS) is another material that has shown significant success in various tissue engineering applications.14 SIS is a dense connective tissue harvested from the small intestine. SIS promotes cell migration of numerous cell types and has been tested for regeneration of diverse tissues including large vascular grafts,15 venous valves and leaflets,16–18 skin,19 tendons20 and wound dressing.21 For urinary tract reconstruction, SIS has been used for bladder augmentation,22–24 for ureter25 and urethra22,26 replacement and to promote regeneration of transitional epithelium, smooth muscle and peripheral nerves with no evidence of immunological rejection.27 Long-term studies show that SIS grafts can be remolded and replaced by the host and such regenerated tissues become histologically indistinguishable from native tissues.23 Large-scale preparation of SIS is hindered by various physiochemical properties which affect the quality and reliability of the tissue regeneration in clinical settings. The physical and mechanical characteristics of the matrix, such as permeability, thickness, tensile properties, fatigue properties and ultrastructural properties, vary depending on the age of the animal, the sterilization technique and the location within the small intestine it is harvested from.28
One way to overcome the heterogeneity in natural matrices uses polymers to fabricate degradable 3D porous matrices. Scaffolds generated from natural polymers such as alginates,29–31 chitosan,32–38 collagen,39 GAGs and elastin,40–43 gelatin44–46 and fibrin47–49 have also been used as scaffolding materials.50–52 A commonly used system is collagen/GAGs;53,54 collagen/GAG based skin equivalents are already in clinical use41,42 and under investigation for other applications such as heart valves, vascular grafts55–60 and vascular networks.61 However, weak mechanical strength, inadequate tailorability options in altering mechanical and degradation properties limit their usage. Synthetic polyesters such as poly (lactic acid) (PLA), poly (glycolic acid) (PGA), their copolymers (PLGA, PLLA, etc.),62–71 and poly (caprolactone) (PCL)72,73 have generated immense interest as tissue engineering materials.74 Further information on specific 3D matrices can be found in a variety of review articles.75–77 Various fabrication techniques such as free-form printing,78 controlled rate freezing and lyophilization,33 porogen-leaching,62 gas-foaming79 and microfabrication80 are available. Porous structures to be used as scaffolds should have the following basic properties: (1) biocompatible, bioresorbable and biodegradable during tissue regeneration process, (2) porous with an interconnected network to enable rapid tissue ingrowth through pores, and to allow unimpaired diffusion of nutrients, oxygen and wastes, (3) suitable surface properties (wettability, stiffness and compliance) to support cell attachment, proliferation and differentiation and (4) provide sufficient mechanical strength to withstand stresses at the site of implantation.
Importance of Spatial Architecture
The influence of spatial architecture of the porous matrix has been explored in various experiments. These studies8,9 have shown that besides chemical cues, 3D matrix physical properties such as stiffness,81 hydrophilicity, porosity,29 pore size and void fraction82,83 can affect cell morphology, attachment and function (Fig. 2). Especially the spatial structures or the topography of scaffolds influence cell alignment, orientation,84 multicellular organization,82 cell spreading85 and cell attachment.83 2D surface features such as edges, grooves, steps, roughness and pores of substratum significantly influence cell behavior.84,86
Important structural properties include both the mechanical properties inherent in the material, such as break stress, modulus of elasticity and stiffness, but also the properties of the scaffold's 3D architecture.87 Both the microscale properties experienced by the cells and the bulk material properties that provide physical support for both the scaffold and the surrounding tissue become important during tissue regeneration. The major architecture features discussed below include porosity, pore size, fiber orientation, pore interconnectivity, topography and scaffold stiffness.
Porosity.
A highly porous scaffold (>90% porosity) is desirable, since it can support the growth of tissue for the necessary nutrients transport.88,89 Porosity is a measure of the open pore volume within the matrix, often called the void fraction. Mathematically, porosity is defined as follows:
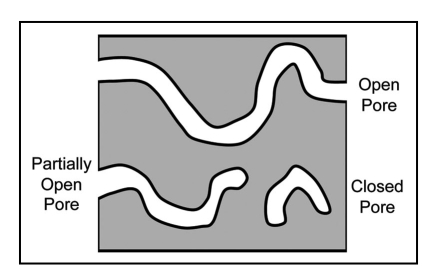
Several pore types are possible within a porous matrix (Fig. 3). Open pores have cellular access on both sides and allow for liquid flow and transport of nutrients through the porous matrix. Partially open pores are accessible on one side. They allow access for cell colonization, but mass transport of nutrients and waste products is limited to diffusion. Closed pores have no openings and are not accessible by cells. Other issues that complicate porosity are pore tortuosity and heterogeneous pore diameters. Consequently, materials for tissue engineering concentrate on creating an open pore architecture.
Figure 3.
Types of pores within the scaffold matrix.
Porosity also plays an important role in regulating cell adhesion and migration. High porosity provides a high surface area for cell-matrix interactions, sufficient space for ECM regeneration, uniform and efficient cell seeding.90 Higher porosity could also lead to increased cell adhesion.91 Pore interconnectivity increases the overall surface area for cell attachment and facilitates cell ingrowth in the scaffolds. Increased interconnectivity and porosity also affect the deposition of ECM elements.92
A functional approach to compare porous structures in a variety of materials is through their mass transport properties, such as permeability. The method of Raghavan et al., is one way to determine the permeability of the matrix,28 quasi steady-state transport between two sealed chambers is assumed and the following equation is used:
where C1 is the initial concentration of the compound of interest, C2 is the concentration of the compound of interest at time t. Am is the area of the matrix normal to the mass flux, V is the volume of the test chamber and P is the permeability. Permeability is related to the diffusion coefficient by:
where Dm is the diffusion coefficient, ϕ is the partition coefficient, L is the thickness of the membrane.
Pore size.
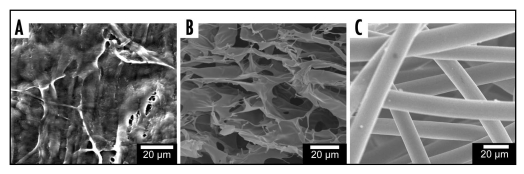
There are a variety of ways to fabricate porous materials, but there are three key methods. Porous matrices may be derived from heterogeneous natural matrices, by forming void spaces within a polymer, or by creating a layered bed of polymer fibers (Fig. 4). One method for generating scaffolds uses a two phase polymer/porogen system where the porogen is subsequently removed, leaving a system of interconnected pores. The pores can be aligned by controlling porogen formation or alignment. Example of this technique are controlled rate freezing and lyophilization (CRFLT)33 and salt leaching.93 In CRFLT the pore alignment is controlled using the rate and direction of heat transfer.33,94 Creating a uniform pore size and distribution is a common problem when working with porous 3D matrices. Firstly, the porous structure is usually formed in an asymmetric fashion. Non-spherical pores form where one axis is much longer than the other. The spatial arrangement of pores within the material may also be an issue. For example, in scaffolds frozen at constant temperature pores are formed in the direction of heat transfer. If a sample insulated on one side and placed in a freezer then areas near the surface of the scaffold (exposed to cold air) will freeze faster than areas deeper within the scaffold. The faster rate of freezing near the surface causes small ice crystals (and thus smaller pores) to be formed near the top edge of the scaffold material.
Figure 4.
Types of porous polymer structures. (A) Heterogeneous structures, porcine SIS. (B) Pore based structures, freeze dried chitosan scaffolds. (C) Fiber based structures, poly (glycolic acid) mesh.
Pore size refers to the distance between solid sections of the porous matrix. Pore size is typically reported as the diameter of circular pores or the major axis for noncircular pores. Pore size affects cell binding, migration, depth of cellular in-growth, cell morphology and phenotypic expression.95 Importantly, appropriate pore size provides structural advantages to allow cells to spread into the pores through “bridges” from adjacent cells. There is an “optimum size range” for supporting cell ingrowth. Outside this range, cells fail to spread and form networks. The optimal pore size range depends on the materials as well as cell types.96 Many mature cell types including endothelial cells (ECs) are unable to completely colonize scaffolds with the pore sizes >300 µm due to the difficulty in crossing large bridging distances.97–101 An “optimum pore size range” for supporting cell ingrowth for majority of the mature cell types (except osteoblasts and osteocytes) is in the range of 100–150 µm.97 Recently, we showed that gelatin-chitosan scaffolds (3:1) with 50–80 µm pore size diminished the viability of fibroblasts and ECs, relative to 100 to 150 µm pore size chitosan scaffolds.44 Pore sizes not only affect cell growth, but also affect scaffolds properties. For example, the elasticity of microporous scaffolds increases as the number of pores within the scaffold increases.102
Fiber thickness.
The matrix may also be characterized based on the microscale thickness of the individual material fibers. In some cases where the material is formed from a bed of stacked fibers fiber thickness is characterized as the diameter of the individual fibers. The fibers may be distributed randomly, as in electrospinning103–105 or form a highly organized system with regular repeating pore units, as in solid freeform fabrication.106 Thus the fiber thickness, length, width and shape (circular rectangular, etc.) must be evaluated. However, defining fiber thickness may not be suitable when pores are formed by CRFLT and salt leaching technique. Utilizing freeze dried scaffolds the material forms an interconnected series of planes that connect the pores.
Fiber orientation within a scaffold affect cell colonization. Scaffolds made of oriented polycaprolactone nanofibers (700 nm in diameter) were found to promote phenotypic differentiation of chondrocytes compared with 2D nonporous membranes.104 Cells seeded on oriented fibrous structures tended to maintain phenotypic shape and guided growth according to nanofiber orientation. Another study showed that significantly more collagen was synthesized by fibroblasts on aligned nanofibers than randomly orientated fibers despite similar proliferation.105 A hypothesis is that spindle-shaped and oriented fibroblasts in the direction of aligned fibers mimic in vivo condition better and thus produce more ECM. Further studies are necessary to understand the mechanisms involved in these cell-matrix and cell-cell interactions.
Topography.
The surface characteristics of scaffold materials can be described by its topography, micro to nano-scale material surface features. Topography of scaffold surface influences spreading characteristics and activity of cells.107 The existence of grooves may inhibit cell movement to bend its cytoskeleton108 or reshape its actin filaments to adjust to the new topography.109 Curtis proposed a term “topographic reaction” to describe that cells react as a response to substratum in microscale through changes in cell orientation, motility and adhesion.86 Surface roughness can significantly increase cell migration area.110 Nanometer scale roughness has been shown to improve the adhesion and growth of both smooth muscle cells64 and chondrocytes111 on polymer scaffolds. However, the mechanisms for enhanced cell behavior are not completely understood. Additionally, the porous structure may be modified post fabrication by inclusion of nanoparticles112 or etching the surface of the matrix.64
Altered surface texture and charge could also affect cell spreading.7,64 In a separate study, we blended antibacterial chitosan with PCL and showed that blending compromises the antibacterial property of the material.113,114 Further, the blend membranes showed better support for fibroblast spreading and proliferation. Surface roughness analysis of blend membranes showed significant increase in roughness relative to chitosan membranes, and observed antibacterial activity could be partially attributed to changed topography. Nevertheless, decreased antibacterial activity could also be due to altered surface charge distribution. Additionally, colonization and proliferation of mammalian cells may be affected by altering the charge distribution within the porous structure.115
Cellular Interaction
Changes in cell-adhesion.
An important part of cell colonization is cell signaling. Cellular adhesion, proliferation and differentiation can be modified using specific signaling molecules, such as growth or differentiation factors. The presence of specific cellular binding sites greatly enhances cell adhesion. The proliferation and differentiation of various cell types may be controlled by incorporating signaling molecules into the tissue engineering matrix.
To incorporate bioregulation of matrix elements, grafting a small peptide Arginine-Glycine-Aspartic acid (RGD) onto polymers is an approach taken by many investigators.116 The use of RGD is based on the understanding that the majority of communication across the cell wall takes place via integrins, which communicate with many matrix elements through the RGD binding domain. Additionally, materials such as collagens, glycosaminoglycans (GAGs) and their analogues (e.g., Dextran sulfate) can be incorporated into the scaffold structure in order to direct cellular growth and provide binding cites.115,117 Our group has previously shown that the presence of binding cites improves cellular adhesion. Endothelial cells were grown on films of chitosan, gelatin or a blend of the two and then subjected to shear stresses similar to those present in arteries or veins. Cells remained attached to the blends containing gelatin, but no cells were found adhered to the pure chitosan film.7
Bioregulation of the porous matrix can also be achieved by the incorporation of growth factors. For example growth factors such as vascular endothelial growth factor (VEGF)116 and basic fibroblast growth factor (bFGF)118,119 as well as ECM components such as fibronectin120 are important in angiogenesis. Growth factors are typically proteins with short half-lives. Therefore, a controlled release system is needed in order to protect the growth factors and provide a sustained signal. Cells will also receive signals from other cells within their vicinity. Therefore, various co-culture techniques allow communication between multiple cell types.121
Many cell types such as fibroblasts, mesenchymal stem cells, epithelial cells and neural crest cells show different adhesions when grown on 3D matrices as opposed to 2D cell culture.122–124 A possible reason is that the 3D architecture could distribute binding sites in a variety of special locations rather than on only the single plane of rigid substrate as in traditional 2D architecture.124,125 Cells may have cytoskeletal adaptor proteins on 3D matrix in addition to proteins present in 2D focal adhesions.124,126 For example, focal adhesion kinase (FAK) in 3D matrix adhesion is poorly phosphorylated at its major tyrosine phosphorylation site for cell adhesion. Such differences in cell adhesion between 2D and 3D matrices lead to different signal transduction and subsequent alteration in cellular rearrangement.
Mechanotransduction.
The mechanical forces a scaffold is subjected to during tissue regeneration should also be accounted for during cell colonization. Studies have shown that both hydrodynamic stresses44,127 and mechanical stresses128 affect cell colonization. For example, endothelial cells44 and chondrocytes129 grown in a perfusion reactor align cells in the direction of flow. Beyond the structural modifications, shear stress initiates a number of signal transduction cascades leading to altered gene expression profiles and functional changes, particularly in endothelial cells.130 In vitro studies have shown that shear stress activates mitogen-activated protein (MAP) kinases (including extracellular signal regulated kinase and c-Jun N-terminal kinase),131–133 and kinases involved in focal adhesion such as FAKs, Src family kinases and phosphatidylinositol 3-kinase.134 Mechanical forces affect cells in a variety of ways including opening or closing ion channels (changing mass transport properties across the cell membrane) and unfolding selected protein domains (providing access to a different set of binding sites).135
Flow through the scaffold microarchitecture dictates the local shear stress rates experienced by the cells. Further the scaffold architecture controls the transport of nutrients within the samples. Channeling and other flow irregularities can result in local hypoxia or extracellular matrix washout.135 The presence of flow within a reactor also affects the production of ECM elements, for example rat bone marrow cells produce greater mineralization in scaffolds under direct perfusion.136
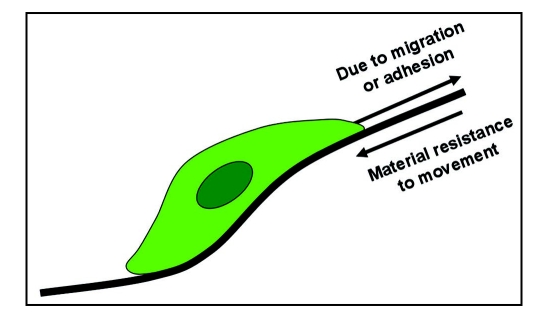
Once the scaffold is placed into a flow system (either implanted in vivo or grown in vitro in a bioreactor) the effect of loading from both external forces and fluid flow can affect cell colonization. While the scaffold itself will be subjected to the bulk forces supplied by the tissue and fluid flow, the cells will experience the micromechanical properties of the individual fibers and local shear stresses within the porous structure (Fig. 5). The cell senses both the porous structure and other cells near where it is attached. An important step in characterizing the porous structure is to examine microenvironment surrounding the cells.
Figure 5.
Micromechanical forces acting on a single cell within a porous structure.
Cellular activity is influenced by the stiffness of the substrate.57,137,138 Stiffness is the resistance of the material to deformation, typically reported in force per distance. It is the slope of the load-extension curve. The dimensionality may be removed from the stiffness calculation by using the modulus of elasticity. The modulus of elasticity is the initial slope of the stress-strain curve. However, the thickness of the material must be measured to calculate the modulus of elasticity, and the thickness measurement becomes a major source of error when measuring very small thicknesses. The bulk stiffness controls the overall deformation of the matrix while each individual cell will encounter the stiffness of the individual fibers during colonization. Cells show reduced spreading and disassembly of actin even when soluble adhesive ligands are present in weak gels.139,140 This could be via the response of tractional forces between cells and materials; scaffold should be able to withstand cell contractile forces.138 Maximum tractional force generated by a cell could be as much as 10–15% of substrate modulus.139
In an effort to develop anti-scarring therapies in wound healing, understanding the process of increased collagen packing has been extensively investigated.141,142 A variety of cell- or matrix-based continuum modeling has also been attempted.143,144 It is very well established that fibroblasts hug the collagen fiber and induce tractional forces. The developed tractional forces lead to the generation of contractile forces which are essential for alignment of collagen fibers and tissue healing. Thus, one of the approaches to minimize scarring is to increase the tensile strength via wound dressing materials. 3D collagen sponges have been used alone145,146 or in conjunction with basic fibroblast growth factor,147 fibronectin or hyaluronic acid.148 Exogenous collagen increased wound tensile strength and increased degree of reepithelialization i.e., early dermal and epidermal wound healing. Further, hyaluronic acid and fibronectin may also be involved in faster wound healing via helping the migration of fibroblasts.149 However, it is not clear whether these treatments reduced scarring in the long term. Nevertheless, the rigidity of the scaffold affect the formation of extracellular matrix which affect cellular activity.123
Matrix Turnover
Cell colonization also involves the deposition of extracellular matrix elements. Cells can synthesize extracellular matrix components in response to different physical and chemical signals from surrounding 3D matrix. Unlike 2D architecture the degradation of a 3D matrix can create more space for cell expansion and migration. Scaffold degradation rate should be synchronized with the cell growth rate to ensure no space restriction due to slow degradation rate or the loss of structural support due to faster degradation. Key factors include the mode of degradation. For example, synthetic polyesters are hydrolytically degraded;75 therefore, they will begin to break down if they are not protected from moisture. Conversely, natural polymers, such as chitosan, are enzymatically degraded and can be stored in hydrated condition.33 Dynamic changes during the degradation process must also be accounted for. Some materials (hydrogels) can swell several times their dry weight.150 It has also been shown that cellular constructs grown in vitro will shrink, possibly as a result of cellular attachment and contraction or as a result of hydrodynamic forces compressing the scaffold. The molecular weight of a polymer will also change over the course of degradation. Amorphous 50:50 PLGA shows an 80% drop in molecular weight over the course of eight weeks which reduces the polymer's tensile break stress by 75%.151 Another positive aspect of degradable polymer systems is that they can also be used for the controlled release of bioactive molecules. Degradable polymers have been used for drug delivery for years. Recently they have been used to deliver growth and differentiation factors within tissue scaffolds.152
Matrix turnover significantly influences cellular phenotypic characters which in turn alters assembly of de novo synthesized matrix elements. Tissue remodeling in a variety of patho/physiological processes including embryogenesis,153 normal tissue development, cancer154 and wound healing155,156 has been evaluated. These studies implicate an array of molecules regulating the process which are regulated at transcriptional, translational and post-translation levels. Matrix metalloproteinases (MMP) form a degradative enzyme family with at least 20 members. MMPs mediate degradation of essentially all components of the ECM.157 Loss of GAGs in arthritic patients has been attributed to the increased production of stromelysin (MMP-3).158 Gelatin turnover is mediated either by MMP-2 (Gelatinase A), a constitutively produced homeostatic enzyme, or by MMP-9 (gelatinase B)159 and upregulated in acute and chronic inflammations. MMP expressions are regulated by soluble mediators, presence of substrates, matrix elements160 and adhesive interactions.161 In turn, MMPs influence rate of matrix synthesis. For example, cells exposed to hydrogels containing MMP-specific peptides show an increase in the transcriptional activity of collagen and proteoglycan synthesis.162 In addition, αVβ3 can bind to MMP-2,161 in an RGD-independent way, thereby localizing MMP-2 mediated matrix degradation to the endothelial cell surface.163
Summary
In summary understanding cell colonization phenomena in 3D porous matrices important in a wide variety of biological applications, including vascularization, wound healing, tissue engineering, stem cell differentiation and biosensors. Colonization is controlled by both the 3D architecture features of the porous structure, include porosity, pore size, fiber orientation, pore interconnectivity, topography and scaffold stiffness and cell—material interactions, such as cellular adhesion, mechanotransduction and matrix turnover. Further understanding of the colonization process should lead to more accurate pathological and physiological tissue models, as well as improved clinical outcomes.
Acknowledgements
Financial support was provided by the Oklahoma Center for Advancement of Science and Technology (HR05-075), and National Institutes of Health (1R21DK074858-01A2).
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/5884
References
- 1.Davies PF, Zilberberg J, Helmke BP. Spatial Microstimuli in Endothelial Mechanosignaling. Circ Res. 2003;92:359–370. doi: 10.1161/01.RES.0000060201.41923.88. [DOI] [PubMed] [Google Scholar]
- 2.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 3.Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 4.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112:3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 5.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 6.Guillot PV, Cui W, Fisk NM, Polak DJ. Stem cell differentiation and expansion for clinical applications of tissue engineering. Journal of Cellular and Molecular Medicine. 2007;11:935–944. doi: 10.1111/j.1582-4934.2007.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Siewe M, Madihally SV. Effect of spatial architecture on cellular colonization. Biotechnol Bioeng. 2006;93:64–75. doi: 10.1002/bit.20703. [DOI] [PubMed] [Google Scholar]
- 8.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 9.Tissue engineering. Nat Biotechnol. 2000;18:56–58. [Google Scholar]
- 10.Schoen FJ, Levy RJ. Founder's Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28–May 2, 1999. Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res. 1999;47:439–465. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Vyavahare N, Ogle M, Schoen FJ, Zand R, Gloeckner DC, Sacks M, Levy RJ. Mechanisms of bioprosthetic heart valve failure: fatigue causes collagen denaturation and glycosaminoglycan loss. J Biomed Mater Res. 1999;46:44–50. doi: 10.1002/(sici)1097-4636(199907)46:1<44::aid-jbm5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Medalie DA, Eming SA, Tompkins RG, Yarmush ML, Krueger GG, Morgan JR. Evaluation of human skin reconstituted from composite grafts of cultured keratinocytes and human acellular dermis transplanted to athymic mice. J Invest Dermatol. 1996;107:121–127. doi: 10.1111/1523-1747.ep12298363. [DOI] [PubMed] [Google Scholar]
- 13.Feng X, Tan J, Pan Y, Wu Q, Ruana S, Shena R, Chena X, Dua Y. Control of hypertrophic scar from inception by using xenogenic (porcine) acellular dermal matrix (ADM) to cover deep second degree burn. Burns. 2006;32:293–298. doi: 10.1016/j.burns.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Kropp BP. Small-intestinal submucosa for bladder augmentation: a review of preclinical studies. World J Urol. 1998;16:262–267. doi: 10.1007/s003450050064. [DOI] [PubMed] [Google Scholar]
- 15.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. The Journal Of Surgical Research. 1989;47:74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 16.Pavcnik D, Uchida BT, Timmermans HA, Corless CL, O'Hara M, Toyota N, Moneta GL, Keller FS, Rosch J. Percutaneous bioprosthetic venous valve: a long-term study in sheep. J Vasc Surg. 2002;35:598–602. doi: 10.1067/mva.2002.118825. [DOI] [PubMed] [Google Scholar]
- 17.Brountzos E, Pavcnik D, Timmermans HA, Corless C, Uchida BT, Nihsen ES, Nakata M, Schoder M, Kaufman JA, Keller FS, Rosch J. Remodeling of suspended small intestinal submucosa venous valve: an experimental study in sheep to assess the host cells' origin. J Vasc Interv Radiol. 2003;14:349–356. doi: 10.1097/01.rvi.0000058410.01661.62. [DOI] [PubMed] [Google Scholar]
- 18.Matheny RG, Hutchison ML, Dryden PE, Hiles MD, Shaar CJ. Porcine small intestine submucosa as a pulmonary valve leaflet substitute. J Heart Valve Dis. 2000;9:769–774. discussion 74–5. [PubMed] [Google Scholar]
- 19.Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2:305–313. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 20.Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, Kraine MR, Simmons C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. Journal Of Biomedical Materials Research. 1995;29:977–985. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 21.Prevel CD, Eppley BL, Summerlin DJ, Sidner R, Jackson JR, McCarty M, Badylak SF. Small intestinal submucosa: utilization as a wound dressing in full-thickness rodent wounds. Ann Plast Surg. 1995;35:381–388. [PubMed] [Google Scholar]
- 22.Nuininga JE, Moerkerk H, Hanssen A, Hulsbergen CA, Oosterwijk Wakka J, Oosterwijk E, de Gier RP, Schalken JA, Kuppevelt TH, Feitz WF. A rabbit model to tissue engineer the bladder. Biomaterials. 2004;25:1657–1661. doi: 10.1016/s0142-9612(03)00519-2. [DOI] [PubMed] [Google Scholar]
- 23.Cheng EY, Kropp BP. Urologic tissue engineering with small-intestinal submucosa: potential clinical applications. World J Urol. 2000;18:26–30. doi: 10.1007/PL00007071. [DOI] [PubMed] [Google Scholar]
- 24.Colvert JR, 3rd, Kropp BP, Cheng EY, Pope JCt, Brock JW, 3rd, Adams MC, Austin P, Furness PD, 3rd, Koyle MA. The use of small intestinal submucosa as an off-the-shelf urethral sling material for pediatric urinary incontinence. J Urol. 2002;168:1872–1875. doi: 10.1097/01.ju.0000027285.05828.e6. [DOI] [PubMed] [Google Scholar]
- 25.Smith TG, 3rd, Gettman M, Lindberg G, Napper C, Pearle MS, Cadeddu JA. Ureteral replacement using porcine small intestine submucosa in a porcine model. Urology. 2002;60:931–934. doi: 10.1016/s0090-4295(02)01890-3. [DOI] [PubMed] [Google Scholar]
- 26.El Assmy A, El Hamid MA, Hafez AT. Urethral replacement: a comparison between small intestinal submucosa grafts and spontaneous regeneration. BJU Int. 2004;94:113–125. doi: 10.1111/j.1464-410X.2004.05115.x. [DOI] [PubMed] [Google Scholar]
- 27.Kropp BP, Cheng EY. Bioengineering organs using small intestinal submucosa scaffolds: in vivo tissue-engineering technology. J Endourol. 2000;14:59–62. doi: 10.1089/end.2000.14.59. [DOI] [PubMed] [Google Scholar]
- 28.Raghavan D, Kropp BP, Lin HK, Zhang Y, Cowan R, Madihally SV. Physical characteristics of small intestinal submucosa scaffolds are location-dependent. J Biomed Mater Res A. 2005;73:90–96. doi: 10.1002/jbm.a.30268. [DOI] [PubMed] [Google Scholar]
- 29.Dar A, Shachar M, Leor J, Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80:305–312. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Goto M, Ise H, Cho CS, Akaike T. Galactosylated alginate as a scaffold for hepatocytes entrapment. Biomaterials. 2002;23:471–479. doi: 10.1016/s0142-9612(01)00129-6. [DOI] [PubMed] [Google Scholar]
- 31.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 32.Tan W, Krishnaraj R, Desai TA. Evaluation of nanostructured composite collagen—chitosan matrices for tissue engineering. Tissue Eng. 2001;7:203–210. doi: 10.1089/107632701300062831. [DOI] [PubMed] [Google Scholar]
- 33.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–1142. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno K, Yamamura K, Yano K, Osada T, Saeki S, Takimoto N, Sakurai T, Nimura Y. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res. 2003;64:177–181. doi: 10.1002/jbm.a.10396. [DOI] [PubMed] [Google Scholar]
- 35.Zhu H, Ji J, Lin R, Gao C, Feng L, Shen J. Surface engineering of poly(D,L-lactic acid) by entrapment of chitosan-based derivatives for the promotion of chondrogenesis. J Biomed Mater Res. 2002;62:532–539. doi: 10.1002/jbm.10313. [DOI] [PubMed] [Google Scholar]
- 36.Chung TW, Yang J, Akaike T, Cho KY, Nah JW, Kim SI, Cho CS. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials. 2002;23:2827–2834. doi: 10.1016/s0142-9612(01)00399-4. [DOI] [PubMed] [Google Scholar]
- 37.Cai K, Yao K, Cui Y, Lin S, Yang Z, Li X, Xie H, Qing T, Luo J. Surface modification of poly (D,L-lactic acid) with chitosan and its effects on the culture of osteoblasts in vitro. J Biomed Mater Res. 2002;60:398–404. doi: 10.1002/jbm.10008. [DOI] [PubMed] [Google Scholar]
- 38.Lahiji A, Sohrabi A, Hungerford DS, Frondoza CG. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000;51:586–595. doi: 10.1002/1097-4636(20000915)51:4<586::aid-jbm6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 39.Chvapil M. Collagen sponge: theory and practice of medical applications. Journal of Biomedical Materials Research. 1977;11:721–741. doi: 10.1002/jbm.820110508. [DOI] [PubMed] [Google Scholar]
- 40.Singla A, Lee CH. Effect of elastin on the calcification rate of collagene-lastin matrix systems. J Biomed Mater Res. 2002;60:368–374. doi: 10.1002/jbm.10077. [DOI] [PubMed] [Google Scholar]
- 41.Dantzer E, Braye FM. Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts. Br J Plast Surg. 2001;54:659–664. doi: 10.1054/bjps.2001.3684. [DOI] [PubMed] [Google Scholar]
- 42.Orgill DP, Straus FH, 2nd, Lee RC. The use of collagen-GAG membranes in reconstructive surgery. Ann N Y Acad Sci. 1999;888:233–248. doi: 10.1111/j.1749-6632.1999.tb07959.x. [DOI] [PubMed] [Google Scholar]
- 43.Xiao Y, Qian H, Young WG, Bartold PM. Tissue engineering for bone regeneration using differentiated alveolar bone cells in collagen scaffolds. Tissue Eng. 2003;9:1167–1177. doi: 10.1089/10763270360728071. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials. 2005;26:7616–7627. doi: 10.1016/j.biomaterials.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 45.Mao JS, Liu HF, Yin YJ, Yao KD. The properties of chitosan-gelatin membranes and scaffolds modified with hyaluronic acid by different methods. Biomaterials. 2003;24:1621–1629. doi: 10.1016/s0142-9612(02)00549-5. [DOI] [PubMed] [Google Scholar]
- 46.Santhosh Kumar TR, Krishnan LK. Endothelial cell growth factor (ECGF) enmeshed with fibrin matrix enhances proliferation of EC in vitro. Biomaterials. 2001;22:2769–2776. doi: 10.1016/s0142-9612(01)00020-5. [DOI] [PubMed] [Google Scholar]
- 47.Bensai d W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–2502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 48.Jockenhoevel S, Chalabi K, Sachweh JS, Groesdonk HV, Demircan L, Grossmann M, Zund G, Messmer BJ. Tissue engineering: complete autologous valve conduit—a new moulding technique. Thorac Cardiovasc Surg. 2001;49:287–290. doi: 10.1055/s-2001-17807. [DOI] [PubMed] [Google Scholar]
- 49.Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. Journal Of Cellular Physiology. 1992;151:497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 50.Lindahl U, Kusche Gullberg M, Kjellen L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- 51.Babu M, Diegelmann R, Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989;9:1642–1650. doi: 10.1128/mcb.9.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denuziere A, Ferrier D, Damour O, Domard A. Chitosan-chondroitin sulfate and chitosan-hyaluronate polyelectrolyte complexes: biological properties. Biomaterials. 1998;19:1275–1285. doi: 10.1016/s0142-9612(98)00036-2. [DOI] [PubMed] [Google Scholar]
- 53.Lamme EN, de Vries HJ, van Veen H, Gabbiani G, Westerhof W, Middelkoop E. Extracellular matrix characterization during healing of full-thickness wounds treated with a collagen/elastin dermal substitute shows improved skin regeneration in pigs. J Histochem Cytochem. 1996;44:1311–1322. doi: 10.1177/44.11.8918906. [DOI] [PubMed] [Google Scholar]
- 54.Garg HG, Lippay EW, Carter EA, Donelan MB, Remensnyder JP. Proteoglycan synthesis in human skin and burn scar explant cultures. Burns. 1991;17:452–457. doi: 10.1016/0305-4179(91)90070-w. [DOI] [PubMed] [Google Scholar]
- 55.Rothenburger M, Vischer P, Volker W, Glasmacher B, Berendes E, Scheld HH, Deiwick M. In vitro modelling of tissue using isolated vascular cells on a synthetic collagen matrix as a substitute for heart valves. Thorac Cardiovasc Surg. 2001;49:204–209. doi: 10.1055/s-2001-16108. [DOI] [PubMed] [Google Scholar]
- 56.Yannas IV. Models of organ regeneration processes induced by templates. Ann N Y Acad Sci. 1997;831:280–293. doi: 10.1111/j.1749-6632.1997.tb52203.x. [DOI] [PubMed] [Google Scholar]
- 57.Zaleskas JM, Kinner B, Freyman TM, Yannas IV, Gibson LJ, Spector M. Growth factor regulation of smooth muscle actin expression and contraction of human articular chondrocytes and meniscal cells in a collagen-GAG matrix. Exp Cell Res. 2001;270:21–31. doi: 10.1006/excr.2001.5325. [DOI] [PubMed] [Google Scholar]
- 58.Spilker MH, Asano K, Yannas IV, Spector M. Contraction of collagen-glycosaminoglycan matrices by peripheral nerve cells in vitro. Biomaterials. 2001;22:1085–1093. doi: 10.1016/s0142-9612(00)00345-8. [DOI] [PubMed] [Google Scholar]
- 59.Kessler PD, Byrne BJ. Myoblast cell grafting into heart muscle: cellular biology and potential applications. Annu Rev Physiol. 1999;61:219–242. doi: 10.1146/annurev.physiol.61.1.219. [DOI] [PubMed] [Google Scholar]
- 60.Taylor PM, Allen SP, Dreger SA, Yacoub MH. Human cardiac valve interstitial cells in collagen sponge: a biological three-dimensional matrix for tissue engineering. J Heart Valve Dis. 2002;11:298–306. [PubMed] [Google Scholar]
- 61.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJ, Brouwer LA, Veerkamp JH, van Kuppevelt TH. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;62:185–194. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 62.Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, Vacanti JP. Preparation and characterization of poly(L-lactic acid) foams. Polymer. 1994;35:1068–1077. [Google Scholar]
- 63.Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999;17:149–155. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 64.Pattison MA, Wurster S, Webster TJ, Haberstroh KM. Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials. 2005;26:2491–2500. doi: 10.1016/j.biomaterials.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 65.Lavik E, Teng YD, Snyder E, Langer R. Seeding neural stem cells on scaffolds of PGA, PLA, and their copolymers. Methods Mol Biol. 2002;198:89–97. doi: 10.1385/1-59259-186-8:89. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura T, Hitomi S, Watanabe S, Shimizu Y, Jamshidi K, Hyon SH, Ikada Y. Bioabsorption of polylactides with different molecular properties. J Biomed Mater Res. 1989;23:1115–1130. doi: 10.1002/jbm.820231003. [DOI] [PubMed] [Google Scholar]
- 67.Mooney DJ, Mazzoni CL, Breuer C, McNamara K, Hern D, Vacanti JP, Langer R. Stabilized polyglycolic acid fibrebased tubes for tissue engineering. Biomaterials. 1996;17:115–124. doi: 10.1016/0142-9612(96)85756-5. [DOI] [PubMed] [Google Scholar]
- 68.Gao J, Niklason L, Langer R. Surface hydrolysis of poly(glycolic acid) meshes increases the seeding density of vascular smooth muscle cells. J Biomed Mater Res. 1998;42:417–424. doi: 10.1002/(sici)1097-4636(19981205)42:3<417::aid-jbm11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 69.Marra KG, Szem JW, Kumta PN, DiMilla PA, Weiss LE. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. J Biomed Mater Res. 1999;47:324–335. doi: 10.1002/(sici)1097-4636(19991205)47:3<324::aid-jbm6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 70.Engelberg I, Kohn J. Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials. 1991;12:292–304. doi: 10.1016/0142-9612(91)90037-b. [DOI] [PubMed] [Google Scholar]
- 71.Ozawa T, Mickle DA, Weisel RD, Koyama N, Wong H, Ozawa S, Li RK. Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats. J Thorac Cardiovasc Surg. 2002;124:1157–1164. doi: 10.1067/mtc.2002.127449. [DOI] [PubMed] [Google Scholar]
- 72.Hutmacher DW, Schantz T, Zein I, Ng KW, Teoh SH, Tan KC. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res. 2001;55:203–216. doi: 10.1002/1097-4636(200105)55:2<203::aid-jbm1007>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 73.Lowry KJ, Hamson KR, Bear L, Peng YB, Calaluce R, Evans ML, Anglen JO, Allen WC. Polycaprolactone/glass bioabsorbable implant in a rabbit humerus fracture model. J Biomed Mater Res. 1997;36:536–541. doi: 10.1002/(sici)1097-4636(19970915)36:4<536::aid-jbm12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 74.Kulkarni RK, Moore EG, Hegyeli AF, Leonard F. Biodegradable poly(lactic acid) polymers. J Biomed Mater Res. 1971;5:169–181. doi: 10.1002/jbm.820050305. [DOI] [PubMed] [Google Scholar]
- 75.Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. discussion. [DOI] [PubMed] [Google Scholar]
- 76.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Advanced Drug Delivery Reviews. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 77.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 78.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 79.Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 80.Wang YC, Ho CC. Micropatterning of proteins and mammalian cells on biomaterials. Faseb J. 2004;8:8. doi: 10.1096/fj.03-0490fje. [DOI] [PubMed] [Google Scholar]
- 81.Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–1084. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 82.Ranucci CS, Moghe PV. Substrate microtopography can enhance cell adhesive and migratory responsiveness to matrix ligand density. J Biomed Mater Res. 2001;54:149–161. doi: 10.1002/1097-4636(200102)54:2<149::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 83.Zeltinger J, Sherwood JK, Graham DA, Mueller R, Griffith LG. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557–572. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 84.Rajnicek A, Britland S, McCaig C. Contact guidance of CNS neurites on grooved quartz: influence of groove dimensions, neuronal age and cell type. J Cell Sci. 1997;110:2905–2913. doi: 10.1242/jcs.110.23.2905. [DOI] [PubMed] [Google Scholar]
- 85.Chung TW, Lu YF, Wang SS, Lin YS, Chu SH. Growth of human endothelial cells on photochemically grafted Gly-Arg-Gly-Asp (GRGD) chitosans. Biomaterials. 2002;23:4803–4809. doi: 10.1016/s0142-9612(02)00231-4. [DOI] [PubMed] [Google Scholar]
- 86.Curtis A, Wilkinson C. New depths in cell behaviour: reactions of cells to nanotopography. Biochem Soc Symp. 1999;65:15–26. [PubMed] [Google Scholar]
- 87.Huang Y. Influence of Scaffold Properties on Cellular Colonization for Tissue Engineering. 2005. PhD Dissertation. [Google Scholar]
- 88.Freed LE, Vunjak Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology. 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 89.Ishaug Riley SL, Crane Kruger GM, Yaszemski MJ, Mikos AG. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials. 1998;19:1405–1412. doi: 10.1016/s0142-9612(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 90.Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141–150. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 91.Marois Y, Sigot Luizard MF, Guidoin R. Endothelial cell behavior on vascular prosthetic grafts: effect of polymer chemistry, surface structure, and surface treatment. Asaio J. 1999;45:272–280. [PubMed] [Google Scholar]
- 92.Miot S, Woodfield T, Daniels AU, Suetterlin R, Peterschmitt I, Heberer M, van Blitterswijk CA, Riesle J, Martin I. Effects of scaffold composition and architecture on human nasal chondrocyte redifferentiation and cartilaginous matrix deposition. Biomaterials. 2005;26:2479–2489. doi: 10.1016/j.biomaterials.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 93.Sodian R, Hoerstrup SP, Sperling JS, Martin DP, Daebritz S, Mayer JE, Jr, Vacanti JP. Evaluation of biodegradable, three-dimensional matrices for tissue engineering of heart valves. Asaio J. 2000;46:107–110. doi: 10.1097/00002480-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 94.Zhang H, Cooper AI. Aligned Porous Structures by Directional Freezing. Advanced Materials. 2007;19:1529–1533. [Google Scholar]
- 95.O'Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–441. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 96.Teebken OE, Haverich A. Tissue engineering of small diameter vascular grafts. Eur J Vasc Endovasc Surg. 2002;23:475–485. doi: 10.1053/ejvs.2002.1654. [DOI] [PubMed] [Google Scholar]
- 97.Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeltinger J, Sherwood JK, Graham DA, Mueller R, Griffith LG. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Engineering. 2001;7:557–572. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 99.Salem AK, Stevens R, Pearson RG, Davies MC, Tendler SJ, Roberts CJ, Williams PM, Shakesheff KM. Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. J Biomed Mater Res. 2002;61:212–217. doi: 10.1002/jbm.10195. [DOI] [PubMed] [Google Scholar]
- 100.Ng KW, Khor HL, Hutmacher DW. In vitro characterization of natural and synthetic dermal matrices cultured with human dermal fibroblasts. Biomaterials. 2004;25:2807–2818. doi: 10.1016/j.biomaterials.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 101.Wang YC, Ho CC. Micropatterning of proteins and mammalian cells on biomaterials. Faseb J. 2004;18:525–527. doi: 10.1096/fj.03-0490fje. [DOI] [PubMed] [Google Scholar]
- 102.Doi K, Nakayama Y, Matsuda T. Novel compliant and tissue-permeable microporous polyurethane vascular prosthesis fabricated using an excimer laser ablation technique. J Biomed Mater Res. 1996;31:27–33. doi: 10.1002/(SICI)1097-4636(199605)31:1<27::AID-JBM4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 103.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Engineering. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 104.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 105.Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26:1261–1270. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 106.Sachlos E, Czernuszka JT. Making Tissue Engineering Scaffolds Work. Review: The application of solid freeform fabrication technology to the production of tissue engineering scaffolds. European Cells and Materials Journal. 2003;5:29–40. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- 107.Ranucci CS, Kumar A, Batra SP, Moghe PV. Control of hepatocyte function on collagen foams: sizing matrix pores toward selective induction of 2-D and 3-D cellular morphogenesis. Biomaterials. 2000;21:783–793. doi: 10.1016/s0142-9612(99)00238-0. [DOI] [PubMed] [Google Scholar]
- 108.Clarke KM, Lantz GC, Salisbury SK, Badylak SF, Hiles MC, Voytik SL. Intestine Submucosa and Polypropylene Mesh for Abdominal Wall Repair in Dogs. Journal of Surgical Research. 1996;60:107–114. doi: 10.1006/jsre.1996.0018. [DOI] [PubMed] [Google Scholar]
- 109.Walboomers XF, Croes HJ, Ginsel LA, Jansen JA. Contact guidance of rat fibroblasts on various implant materials. J Biomed Mater Res. 1999;47:204–212. doi: 10.1002/(sici)1097-4636(199911)47:2<204::aid-jbm10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 110.Lampin M, Warocquier C, Legris C, Degrange M, Sigot Luizard MF. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J Biomed Mater Res. 1997;36:99–108. doi: 10.1002/(sici)1097-4636(199707)36:1<99::aid-jbm12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 111.Park GE, Pattison MA, Park K, Webster TJ. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials. 2005;26:3075–3082. doi: 10.1016/j.biomaterials.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 112.Mondalek FG, Lawrence BJ, Kropp BP, Grady BP, Fung KM, Madihally SV, Lin HK. The incorporation of poly(lactic-co-glycolic) acid nanoparticles into porcine small intestinal submucosa biomaterials. Biomaterials. 2008;29:1159–1166. doi: 10.1016/j.biomaterials.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sarasam A, Madihally SV. Characterization of chitosan-polycaprolactone blends for tissue engineering applications. Biomaterials. 2005;26:5500–5508. doi: 10.1016/j.biomaterials.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 114.Sarasam AR, Krishnaswamy RK, Madihally SV. Blending Chitosan with Polycaprolactone: Effects on Physicochemical and Antibacterial Properties. Biomacromolecules. 2006;7:1131–1138. doi: 10.1021/bm050935d. [DOI] [PubMed] [Google Scholar]
- 115.Tillman J, Ullm A, Madihally SV. Three-dimensional cell colonization in a sulfate rich environment. Biomaterials. 2006;27:5618–5626. doi: 10.1016/j.biomaterials.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 116.VandeVondele S, Vörös J, Hubbell JA. RGD-grafted poly-l-lysine-graft(polyethylene glycol) copolymers block non-specific protein adsorption while promoting cell adhesion. Biotechnology and Bioengineering. 2003;82:784–790. doi: 10.1002/bit.10625. [DOI] [PubMed] [Google Scholar]
- 117.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Current Opinion in Biotechnology. 2003;14:551–558. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 118.Nomi M, Atala A, Coppi PD, Soker S. Principals of neovascularization for tissue engineering. Molecular Aspects of Medicine. 2002;23:463–483. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 119.Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio Padron N, Schramm R, Rucker M, Junker D, Haufel JM, Carvalho C, Heberer M, Germann G, Vollmar B, Menger MD. Angiogenesis in Tissue Engineering: Breathing Life into Constructed Tissue Substitutes. Tissue Engineering. 2006;12:2093–2104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 120.Vogel V, Baneyx G. The Tissue Engineering Puzzle: A Molecular Perspective. Annual Review of Biomedical Engineering. 2003;5:441–463. doi: 10.1146/annurev.bioeng.5.040202.121615. [DOI] [PubMed] [Google Scholar]
- 121.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Seminars in Cancer Biology. 2005;15:405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 122.Yamada KM, Pankov R, Cukierman E. Dimensions and dynamics in integrin function. Braz J Med Biol Res. 2003;36:959–966. doi: 10.1590/s0100-879x2003000800001. [DOI] [PubMed] [Google Scholar]
- 123.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 124.Sato H, Takahashi M, Ise H, Yamada A, Hirose S, Tagawa Y, Morimoto H, Izawa A, Ikeda U. Collagen synthesis is required for ascorbic acid-enhanced differentiation of mouse embryonic stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2006;342:107–112. doi: 10.1016/j.bbrc.2006.01.116. [DOI] [PubMed] [Google Scholar]
- 125.Chang Z, Meyer K, Rapraeger AC, Friedl A. Differential ability of heparan sulfate proteoglycans to assemble the fibroblast growth factor receptor complex in situ. Faseb J. 2000;14:137–144. doi: 10.1096/fasebj.14.1.137. [DOI] [PubMed] [Google Scholar]
- 126.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 127.Chen HC, Hu YC. Bioreactors for tissue engineering. Biotechnology Letters. 2006;28:1415–1423. doi: 10.1007/s10529-006-9111-x. [DOI] [PubMed] [Google Scholar]
- 128.Grashow J, Yoganathan A, Sacks M. Biaixal Stress-Stretch Behavior of the Mitral Valve Anterior Leaflet at Physiologic Strain Rates. Annals of Biomedical Engineering. 2006;34:315–325. doi: 10.1007/s10439-005-9027-y. [DOI] [PubMed] [Google Scholar]
- 129.Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physicochemical determinants of the chondrocyte biosynthetic response. Journal of Orthopaedic Research. 1988;6:777–792. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 130.Papadaki M, Eskin SG, Ruef J, Runge MS, McIntire LV. Fluid shear stress as a regulator of gene expression in vascular cells: possible correlations with diabetic abnormalities. Diabetes Research and Clinical Practice. 1999;45:89–99. doi: 10.1016/s0168-8227(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 131.Takahashi M, Berk BC. Mitogen-activated Protein Kinase (ERK1/2) Activation by Shear Stress and Adhesion in Endothelial Cells . Essential Role for a Herbimycin-sensitive Kinase. J Clin Invest. 1996;98:2623–2631. doi: 10.1172/JCI119083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JYJ. Mechanotransduction in Response to Shear Stress. Roles of Receptor Tyrosine Kinases, Integrins, and Sh. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 133.Burridge K, Chrzanowska Wodnicka M. Focal adhesions, contractility, and signaling. Annual Review Of Cell And Developmental Biology. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 134.Gu J, Tamura M, Pankov R, Danen EHJ, Takino T, Matsumoto K, Yamada KM. Shc and FAK Differentially Regulate Cell Motility and Directionality Modulated by PTEN. J Cell Biol. 1999;146:389–404. doi: 10.1083/jcb.146.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 136.Gomes ME, Sikavitsas VI, Behravesh E, Reis RL, Mikos AG. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. Journal of Biomedical Materials Research Part A. 2003;67:87–95. doi: 10.1002/jbm.a.10075. [DOI] [PubMed] [Google Scholar]
- 137.Lee CR, Grodzinsky AJ, Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials. 2001;22:3145–3154. doi: 10.1016/s0142-9612(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 138.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574–584. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 139.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Trelstad RL, Hayashi K. Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Dev Biol. 1979;71:228–242. doi: 10.1016/0012-1606(79)90166-0. [DOI] [PubMed] [Google Scholar]
- 142.Hsieh P, Chen LB. Behavior of cells seeded in isolated fibronectin matrices. J Cell Biol. 1983;96:1208–1217. doi: 10.1083/jcb.96.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tranquillo RT, Murray JD. Continuum model of fibroblast-driven wound contraction: inflammation-mediation. Journal Of Theoretical Biology. 1992;158:135–172. doi: 10.1016/s0022-5193(05)80715-5. [DOI] [PubMed] [Google Scholar]
- 144.Dallon JC, Sherratt JA, Maini PK. Mathematical Modelling of Extracellular Matrix Dynamics using Discrete Cells: Fiber Orientation and Tissue Regeneration. Journal of Theoretical Biology. 1999;199:449–471. doi: 10.1006/jtbi.1999.0971. [DOI] [PubMed] [Google Scholar]
- 145.Dunn MG, Doillon CJ, Berg RA, Olson RM, Silver FH. Wound healing using a collagen matrix: effect of DC electrical stimulation. J Biomed Mater Res. 1988;22:191–206. doi: 10.1002/jbm.820221310. [DOI] [PubMed] [Google Scholar]
- 146.Inoue M, Ono I, Tateshita T, Kuroyanagi Y, Shioya N. Effect of a collagen matrix containing epidermal growth factor on wound contraction. Wound Repair Regen. 1998;6:213–222. doi: 10.1046/j.1524-475x.1998.60307.x. [DOI] [PubMed] [Google Scholar]
- 147.Ono I, Tateshita T, Inoue M. Effects of a collagen matrix containing basic fibroblast growth factor on wound contraction. J Biomed Mater Res. 1999;48:621–630. doi: 10.1002/(sici)1097-4636(1999)48:5<621::aid-jbm5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 148.Doillon CJ, Silver FH, Olson RM, Kamath CY, Berg RA. Fibroblast and epidermal cell-type I collagen interactions: cell culture and human studies. Scanning Microsc. 1988;2:985–992. [PubMed] [Google Scholar]
- 149.Doillon CJ, Silver FH. Collagen-based wound dressing: effects of hyaluronic acid and fibronectin on wound healing. Biomaterials. 1986;7:3–8. doi: 10.1016/0142-9612(86)90080-3. [DOI] [PubMed] [Google Scholar]
- 150.Smeds KA, Grinstaff MW. Photocrosslinkable polysaccharides for in situ hydrogel formation. Journal of Biomedical Materials Research. 2001;54:115–121. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 151.Lawrence B, Maase E, Lin HK, Madihally S. Multilayer. Composite Scaffolds with Properties Similar to Small Intestinal Submucosa. Journal of Biomedical Materials Research, Part A. 2007 doi: 10.1002/jbm.a.31903. [in press] [DOI] [PubMed] [Google Scholar]
- 152.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. The FASEB Journal. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 153.D'Amore PA, Thompson RW. Mechanisms of angiogenes. Annu Rev Physiol. 1987;49:453–464. doi: 10.1146/annurev.ph.49.030187.002321. [DOI] [PubMed] [Google Scholar]
- 154.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 155.Galeano M, Deodato B, Altavilla D, Squadrito G, Seminara P, Marini H, Stagno d'Alcontres F, Colonna M, Calo M, Lo Cascio P, Torre V, Giacca M, Venuti FS, Squadrito F. Effect of recombinant adeno-associated virus vector-mediated vascular endothelial growth factor gene transfer on wound healing after burn injury. Crit Care Med. 2003;31:1017–1025. doi: 10.1097/01.CCM.0000059435.88283.C2. [DOI] [PubMed] [Google Scholar]
- 156.Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 157.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix Metalloproteinases: Biologic Activity and Clinical Implications. J Clin Oncol. 2000;18:1135. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 158.Fosang AJ, Last K, Maciewicz RA. Aggrecan Is Degraded by Matrix Metalloproteinases in Human Arthritis . Evidence That Matrix Metalloproteinase and Aggrecanase Activities Can Be Independent. J Clin Invest. 1996;98:2292–2299. doi: 10.1172/JCI119040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Makowski GS, Ramsby ML. Identification and partial characterization of three calcium- and zinc-independent gelatinases constitutively present in human circulation. Biochem Mol Biol Int. 1998;46:1043–1053. doi: 10.1080/15216549800204592. [DOI] [PubMed] [Google Scholar]
- 160.Schonherr E, Schaefer L, O'Connell BC, Kresse H. Matrix metalloproteinase expression by endothelial cells in collagen lattices changes during co-culture with fibroblasts and upon induction of decorin expression. J Cell Physiol. 2001;187:37–47. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 161.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 162.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10:515–522. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 163.Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO, Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. PNAS. 2003;100:13350–13355. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]