Abstract
The NFκB family of transcription factors, particularly the activated p50/p65 heterodimer, is expressed in vascular cells during intimal thickening formation when hemodynamic conditions are altered. Here, we report that p50, p65, IκBα and IKKα display different spatial and temporal patterns of expression and distribution during both chicken embryo aortic wall remodeling and intimal thickening development. Additionally, we show that both p50 and p65 were located in the nucleus of some mesenchymal cells expressing α-smooth muscle actin which are present in the spontaneous intimal thickening observed at embryonic days 12–14 of development. We also demonstrated that both NFκB subunits are present in monolayers of primary embryonic aortic endothelial cells attached to fibronectin and stimulated with complete medium. This study demonstrates for the first time the presence of activated NFκB during the remodeling of the embryonic aortic wall and the formation of intimal thickening, providing evidence that suggest a possible role for this transcription factor in the EndoMT process.
Key words: vascular remodeling, EndoMT, NF κB, IGFII, dynein, MMPs
Introduction
Vascular remodeling, more precisely arterial wall remodeling, is a dynamic process of structural and architectural alterations that occurs during embryonic and vascular development as well as in many vascular diseases.1,2 This process entails cell growth, cell migration, apoptosis and degradation and reorganization in the extracellular matrix (ECM) composition and is regulated by hemodynamic stimuli, as well as by environmental and genetic factors.1,3–6 Alterations in hemodynamic conditions have been widely known to cause intimal hyperplasia or intimal thickening.4,6,7 Intimal thickening is defined as an increase in cell numbers within the innermost region of the vessel wall (intima) which occurs concomitantly with the degradation and reorganization of ECM components.8 It may involve cytokines and growth factors, cell migration and proliferation and gene expression modulation.3 Currently, there is controversy regarding not only the origin or the source of vascular SMCs involved in development9 and the cells that conform the intimal thickening,10 but also regarding the mechanisms contributing to their formation. In this respect, recent studies have proposed different sources for intimal cells that include circulating hematopoietic stem cells, circulating smooth muscle progenitor cells of nonhematopoietic cells or local vascular smooth muscle cells.11 Other sources could be the endothelium by an EndoMT process12 or proepicardial/epicardial cells.9
EndoMT is a process through which certain endothelial cell subsets lose endothelial characteristics and transform into mesenchymal or SM-like cells. Emerging evidence suggests that this process is critical during embryonic and adult vascular development and during the formation of intimal thickening.12 It may involve several regulated steps: cell spreading, cell separation or loss of cell-cell contacts or adherence junctions, proteases, cytokine and growth factor synthesis and secretion, ECM remodeling, membrane receptor expression, cytoskeletal organization, cell separation from the substratum (cell detachment) and cell migration and differentiation.12 Of particular significance, intimal thickening formation involving EndoMT has been described in both aortic13 and pulmonary artery wall14 during advanced stages of chicken embryo development and correlated with an increase in blood pressure that occurs during these stages. Nevertheless, the mechanisms by which endothelial transformation occurs, as well as the signals controlling this process, are only beginning to be elucidated.
Several in vivo and in vitro studies have indicated that the regulation of transcription of a variety of genes is also an important event in the neointima formation, and that it may occur in response to hemodynamic influences.15–17
Members of the NFκB family play important roles controlling vascular gene expression.15–17 This family comprises five members that form either homodimers or heterodimers: RelA/p65, RelB and c-Rel, NFκB1 (p50 and its precursor p105) and NFκB2 (p52 and its precursor p100). The most widely expressed complex, often referred to as NFκB, is the 65 kDa RelA subunit and the 50 kDa p50 subunit (p65/p50). This dimer is present in virtually all cell types in a latent, inactive form in the cytoplasm where it is physically bound to IκBs conforming the NFκB: IκB complexes whose formation is normally subjected to a variety of extracellular stimuli.18 Several members of the IκB regulatory family have been characterized, including IκBα, IκBβ, IκBγ, IκBε and Bcl3. When a cell receives any stimulus, IκBs proteins are rapidly phosphorylated by IKK complexes and subsequently degraded by the proteasome machinery, resulting in the dissociation of the NFκB from its inhibitor IκB. This allows the active dimer to traslocate into the nucleus, binding specific DNA sites collectively called κB sites, and to activate the expression of specific target genes involved in cell adhesion, growth, differentiation, inflammatory responses and apoptosis.18,19
The NFκB family members, particularly the activated p65/p50 heterodimer, are expressed in vascular cells during neointima formation when hemodynamic conditions are altered.20,21 Moreover, increased nuclear localization of p65 and p50 is detected in the intimal thickening and medial cells of human atherosclerotic lesions, whereas little or no nuclear accumulation of both NFkB subunits is observed in the cells of healthy vessels.22–25
These findings prompted us to investigate the possible role of NFκB in the EndoMT process; for that, we examined the presence and localization of p50, p65, IκBα and IKKα in chicken embryo aorta wall during different stages of development, with particular emphasis in embryonic days 12–14 (days E12–E14) (stages 38 and 40), when intimal thickening and endothelial transformation are notorious. We also investigated the expression and activation of both NFκB subunits in monolayers of primary embryonic aortic endothelial cells attached to fibronectin and stimulated with complete medium.
As mentioned, arterial wall remodeling involves degradation and reorganization of ECM components with the participation of specialized proteases called MMPs, particularly MMP2 and MMP9.5 In this respect, studies related to hemodynamic alterations have demonstrated an increased expression of MMP-2 and MMP-9 during intimal thickening formation,5 some of them indicating that the translocation or activation of NFκB correlates with the expression and activation of certain MMPs in endothelial cells.26 We therefore evaluated the expression and localization of MMP-2 and MMP-9 in chicken embryo aortic wall during advanced stages of development and during EndoMT.
Results
In vivo p65, p50, IκBα and IKKα expression and immunolocalization.
In order to investigate the expression and localization of p65, p50 and its inhibitor, IκBα, in the aortic wall at days E12–E14 (stages 38 and 40) of development when intimal thickening is clearly evident and EndoMT occurs,13 serial paraffin sections were examined by immunoperoxidase staining. A specific polyclonal antibody that recognizes epitopes on the N-terminus of p65 subunit in the cytoplasm and in the nucleus and other that recognizes the NLS of p50 subunit and its precursor, p105, were used. Both p65 and p50 were selected because they are the NFκB subunits most frequently detected in human atherosclerotic lesions.22,24,25 We also studied the expression and localization of IKKa protein, being it considered as it is: a critical player in the regulation of NFκB activity.17,18
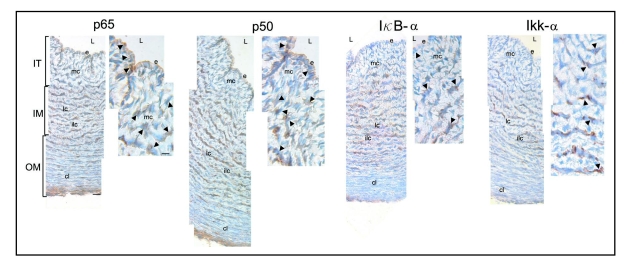
At days E12–E14 of development, the aortic wall is composed by the endothelium, which limits the vessel lumen, and radially oriented mesenchymal cells originating from the endothelium that constitute the intimal thickening. At these stages it is possible to distinguish organized cells in circular lamellar and interlamellar layers that form the inner and outer media where the cells appear as compact lamellae (Fig. 2). The immunoreactivity observed at these stages for p50 was more intense compared to p65. Even so, both immunoreactivities were detected in the nucleus and the cytoplasm of some endothelial and mesenchymal cells of the intimal thickening as well as in those cells arranged in lamellar layers that form the inner media (Fig. 2); the immunoreactivities being less prominent in interlamellar cell layers and in the compact lamellae (Fig. 2).
Figure 2.
Immunolocalization of p65, p50, IκBα and IKKα in serial paraffin cross-sections of chicken embryo aorta at day E13 of development. Immunoreactivity for p65 is observed in the nucleus (arrows) and the cytoplasm (arrowheads) of some endothelial cells (e) and mesenchymal cells (mc) constituting the intimal thickening (IT) and in cells of the lamellar layers (lc). Less prominent immunoreactivity is detected in the interlamellar cell layers (ilc) and compact lamellae (cl) of the outer media (OM). For p50, a more intense immunoreactivity is observed in the nucleus (arrows) and the cytoplasm (arrowheads) of some endothelial cells (e) and mesenchymal cells (mc) and cells of the lamellar layers (lc). Less immunoreactivity is observed in the interlamellar cell layers (ilc) and compact lamellae (cl). IκBα and IKKα immunoreactivities are detected in the cytoplasm (arrowheads) of some mesenchymal (mc) and lamellar cells (lc). L, lumen. IM, inner media. Scale bars = 100 and 15 µm.
Immunoperoxidase staining also revealed strong IκBα and IKKα immunoreactivities mostly cytoplasmic that were limited to some mesenchymal and lamellar cells of the inner media (Fig. 2). No immunoreactivity was observed when the primary antibody was omitted or replaced by specific IgG in control paraffin sections (not shown).
To ascertain whether p65, p50, IκBα, and IKKα contribute to the development of intimal thickening and to the remodeling of the embryonic aortic wall, the expression of these proteins was examined in serial paraffin sections from days E7, E14, and E21 (stages 31, 40 and 46) by immunoperoxidase staining.
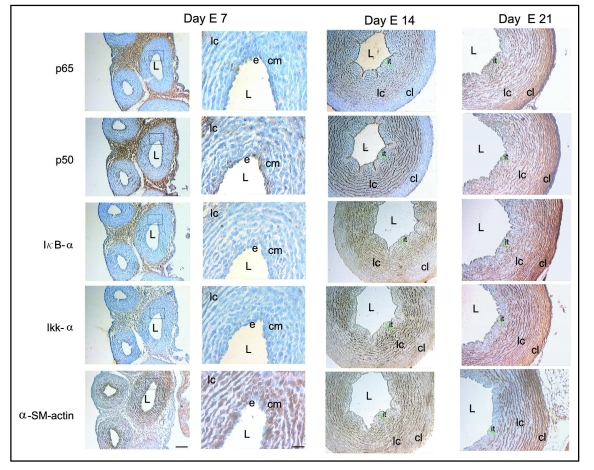
At day E7, intimal thickening is not evident and the aortic wall is composed of an endothelium and a condensed mesenchyme that is located closest to the endothelium, and few circular lamellar layers of cells, all of them displaying strong α-SM actin immunoreactivity, except the endothelium (Fig. 3). At this stage, immunoperoxidase revealed moderate expression of p50 in the endothelium, condensed mesenchyme and cellular layers (Fig. 3). Interestingly, little p65, IκBα and IKKα expression were observed throughout the vessel wall (Fig. 3). As described, at day E14, when intimal thickening is clearly evident and some α-SM actin positive mesenchymal cells are present (Fig. 3), p65, IκBα and IKKα were expressed, contrary to that observed at day E7. Specifically, expression of p65 was detected in the intimal thickening as well as in cells organized in lamellar layers, while no staining was detected in the outer media. In this stage, the expression of p50 was more intense when compared with its levels at day E7 and with the relative levels of p65. As mentioned, IκBα and IKKα expression appeared mainly to be limited to some mesenchymal cells and lamellar cells of the inner media (Fig. 3). At day E21, when intimal thickening appeared to be decreased, the p50, p65, IκBα and IKKα expression were similar to that observed at day E14, with the only difference that the compact lamellae that form the outer media now expressed these proteins (Fig. 3).
Figure 3.
Immunolocalization of p65, p50, IκBα, IKKα and α-SM actin in serial paraffin cross-sections of chicken embryo aorta at days E7, E14 and E21. At day E7, a moderate p50 expression is observed in the endothelium (e), condensed mesenchyme (cm) and in cells of the lamellar layers (lc). Little p65, IκBα and IKKα immunoreactivity is observed throughout the vessel wall. Strong α-SM actin immunoreactivity is detected in the condensed mesenchyme (cm) and in cells of the lamellar layers (lc) but not in the endothelium (e). At day E14, immunoreactivity for p65, p50, IκBα and IKKα was similar to that observed at day 13 (see Fig. 1). For α-SM actin, a strong immunoreactivity is observed in a few mesenchymal cells and in lamellar cell layers (lc). At day E21, the expression of p65, p50, IκBα and IKKα is detected throughout the vessel wall, including in the cells of the outer media where the cells appear as compact lamellae (cl). Strong α-SM actin expression is observed in a few mesenchymal cells adjacent to the endothelium and in the lamellar cell layers (lc) and compact lamellae (cl). L, lumen; it, intimal thickening. Scale bars = 100 and 50 µm.
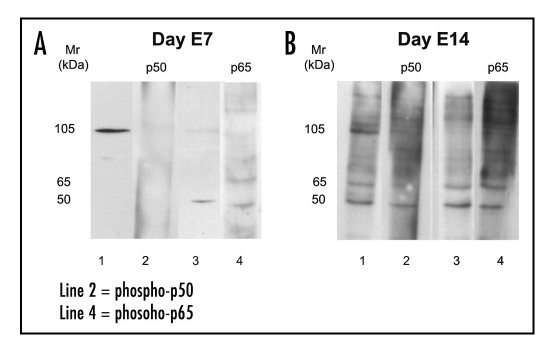
In order to confirm the presence of p50 and p65 in the aortic wall, Western blot analysis was performed on cytoplasmic and nuclear protein extracts from aortae at day E7 and E14. By using anti-p50 polyclonal antibody, at day E7 we detected in the cytoplasmic extract a strong band with a Mr of 105 kDa corresponding to the precursor of p50 (p105) (Fig. 4A, lane 1). When p50 expression was examined in the nuclear extract using anti-phospho-p50 polyclonal antibody, which recognizes the activated form of p50, no bands were detected (Fig. 4A, lane 2). At this stage, Western blotting of cytoplasmic extract with a polyclonal antibody against p65 showed that no band with a Mr of 65 kDa was present, instead a faint band with a Mr of 50 kDa, was observed (Fig. 4A, lane 3). When anti-phospho-p65, which recognizes the activated form of p65, was used, two faint bands, one of 65 kDa and other of 50 kDa were detected in the nuclear extract (Fig. 4A, lane 4). In contrast, at day E14 anti-p50 detected in the cytoplasmic extract a strong band with a Mr of 105 kDa and other less intense with a Mr of 50 kDa, corresponding to the precursor of p50 (p105) and to the homodimeric form of p50 (p50/p50), respectively (Fig. 4B, lane 1). Using anti-phospho-p50 we detected in the nuclear extract a band with a Mr of 50 kDa suggestive of p50 homodimer (p50/p50) (Fig. 4B, lane 2). Western blotting of cytoplasmic extract with anti-p65 revealed the presence of one band with a Mr of 65 kDa and another of 50 kDa (Fig. 4B, lane 3), the former corresponding to the inactivated form of p65 (IκB-α P65/p50) and the latter likely due to cross-reactivity. In nuclear extract, anti-phospho-p65 revealed a strong band corresponding to p65/p50 heterodimer (Fig. 4B, lane 4).
Figure 4.
Western blot analysis for p50 and p65 in aorta extracts from days E7 (A) and E14 (B). Cytoplasmic (lines 1 and 3) and nuclear (lines 2 and 4) extracts were obtained as described in experimental procedures. Protein (13 µg) of both extracts were separated by SDS-PAGE under reducing conditions and immunoblotted with the following polyclonal antibodies: anti-p50 (line 1), anti-p65 (line 3); anti-phospho-p50 (tracks 2 and 3) and anti-phospho-p65 (line 4).
As a whole, these findings indicate that p50, p65, IκBα and IKK-κ seem to have a dynamic pattern of expression and distribution during the remodeling of the embryonic aortic wall and the development of intimal thickening.
We previously reported that during intimal thickening formation certain subsets of endothelial cells lose endothelial characteristics and transform into mesenchymal cells, some of them becoming to express α-SM actin.13,14 Also, it has been proposed that the nuclear localization of both p50 and p65 subunits in intimal cells of atherosclerotic lesions is an indicator of NFκB activity.20–25 We therefore also examined by double immunoperoxidase staining whether p50, p65 or IKKα were present at the nucleus of those cells that expressed α-SM actin at days E12–E14 of development, when the intimal thickening is evident.
High magnification of serial paraffin sections showed that p50 and p65 were located in the nucleus of some mesenchymal cells that expressed α-SM actin as well as in some α-SM actin positive cells organized in lamellar cells (Fig. 5); whereas IKKα was found colocalizing with α-SM actin in the cytoplasm of some mesenchymal cells and some lamellar cells (Fig. 5).
Figure 5.
Double immunoperoxidase staining with antibodies to p65, p50, IKKα and α-SM actin in serial paraffin sections of chicken embryo aorta at day E14 of development. Nuclear immunoreactivity for p50 or p65 (brown) is observed in some mesenchymal and lamellar cells (arrows) that also exhibit cytoplasmic immunoreactivity for α-SM actin (red). For IKKα (brown), immunoreactivity is detected colocalizing with α-SM actin (red) in the cytoplasm of some mesenchymal and lamellar cells (arrows). L, lumen. e, endothelium. mc, mesenchymal cells. lc, lamellar cells. Scale bar = 20 µm.
These observations indicate that activation and translocation of NFκB subunits may be associated to the endothelial transformation.
In vitro p50, p65, IκBα and IKKα immunolocalization.
In the light of our findings suggesting that the expression and activation of p50 and p65 N-κB subunits may be associated to the endothelial transformation and that NFκB activation proceed rapidly by dissociation from its inhibitor, we also investigated whether both subunits and some of their regulators, particularly IκBα and IKKα, were expressed and activated when monolayers of embryonic endothelial cells obtained from aortic explants from days E11 and E12 (stages 37 and 38) that had been transiently mechanically altered during the explant removal, were stimulated for 1–2 hr and 48 hrs with complete medium (1% ITS or IGFII 500ηg/ml). Under these conditions the expression of both p50 and p65 was investigated in some of these cultures by immunoperoxidase staining using anti-phospho-p50 and anti-phospho-p65.
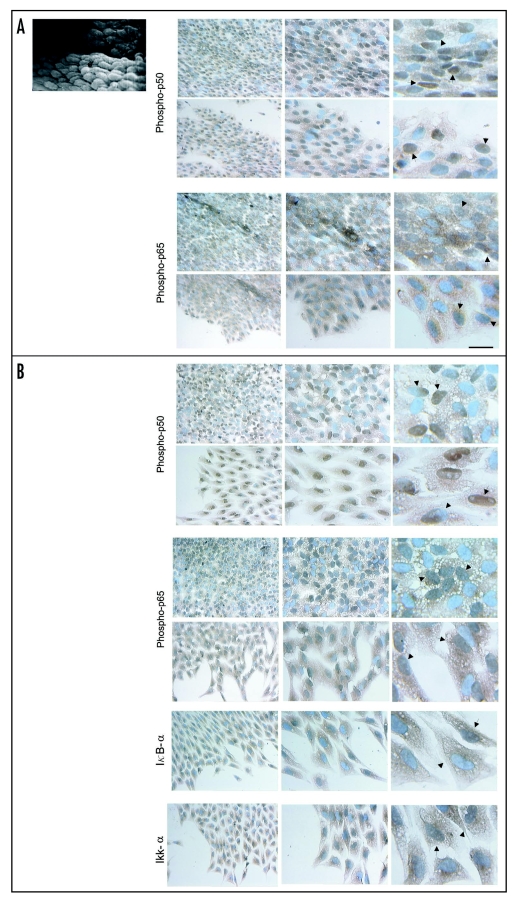
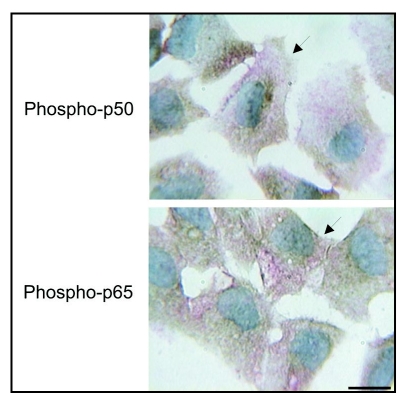
At 1–2 hr after stimulation, a strong nuclear staining for phospho-p50, suggestive of NFκB activation and translocation, was detected in the majority of endothelial cells of the monolayer which displayed an elongated form and ovoid nuclei, with an orientation that seems to reveal the direction of the blood flow observed in vivo (Fig. 6A). Strong staining was also observed in many of the cells located at the edges of the monolayer that appeared to be spreading and separating (Fig. 6A). High magnification of these cultures showed that in some cells the staining for phospho-p50 practically encompassed the complete nucleus, whereas in others, the staining encompassed the nucleus partially (Fig. 6A). For phospho-p65, a less intense nuclear staining was found in many endothelial cells of the monolayer and in spreading and separating cells with in some of these cells the staining encompassing the nucleus totally or partially (Fig. 6A).
Figure 6.
Scanning electron micrograph of an aortic explant showing the endothelial cells (e) oriented in the same direction of blood flow. Immunolocalization of phospho-p50, phospho-p65, IκBα and IKKα in monolayers of mechanically injured endothelial cells. At two hrs after stimulation (A), a strong nuclear staining for phospho-p50 is detected in the majority of cells of the monolayer that display an elongated form and ovoid nuclei and in many spreading and separating cells. At high magnification, the staining encompas the complete nucleus in some cells (arrows) and partially in others (arrowheads). For phospho-p65, nuclear staining is less intense. (B) After 48 hrs, a strong nuclear staining and conspicuous cytoplasmic staining for phospho-p50 is detected in many cells of the monolayer that now display a polygonal shape with a cobblestone appearance, and in some spreading, separating, detaching and migrating cells and some cells with mesenchymal characteristics. Similar to phospho-p50, but less intense, a nuclear and cytoplasmic staining for phospho-p65 is observed. At high magnification the nuclear staining distribution for both phospho-p50 and p65 is very similar to that observed at two hrs. For IκBα and IKKα, immunoreactivities are detected in separating, detaching, and migrating cells. Scale bars = 25 and 12.5 µm.
When similar endothelial cell monolayers were stimulated for 48 hrs, a strong nuclear staining and a conspicuous cytoplasmic staining for phospho-p50 was detected in many cells of the monolayer that now displayed a polygonal shape with a cobblestone appearance, as well as in some spreading, separating, detaching and migrating cells, and some of those that had acquired mesenchymal characteristics (Fig. 6B). Similar to phospho-p50, but less intense, the nuclear and cytoplasmic staining for phospho-p65 was also seen in most cells of the monolayer and in some spreading, detaching and migrating cells (Fig. 6B). High magnification showed that phospho-p50 and phospho-p65, had a nuclear distribution very similar to that observed in cultures maintained for 1–2 hr (Fig. 6B). The immunoreactivity observed in some of these cultures for IKKα was more intense as compared to IκBα. Both immunoreactivities were detected in the cytoplasm of many separating, detaching and migrating cells as well as in the nucleus of some of them (Fig. 6B).
At this time, the expression of phospho-p50 or -p65 and α-SM actin was also examined in some of these cultures using double immunoperoxidase staining. This technique revealed that some of the cells that had acquired mesenchymal characteristics, displayed immunoreactivity for both phospho-p50 or -p65 and a-SM actin (Fig. 7).
Figure 7.
Double immunolocalization of phospho-p50, phospho-p65 and α-SM actin during EndoMT after stimulation for 48 hrs complete medium. Nuclear immunoreactivity for phospho-p50 or phospho-p65 (brown) is observed in some separating, detaching and migrating cells and those cells that also display cytoplasmic immunoreactivity for α-SM actin (red) (arrows). Scale bar = 12.5 µm.
In conclusion, these observations give support in the importance of the role of the activated NFκB not only during endothelial cell spreading, detachment, and migration, but also during the transformation into mesenchymal or SM-like cells.
Effects of aspirin on embryonic endothelial cells.
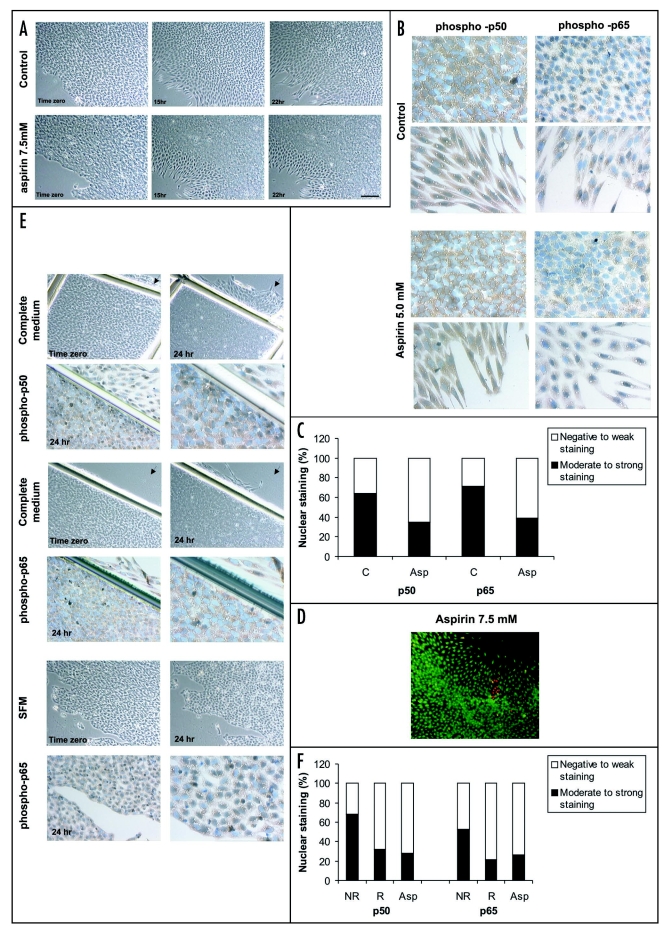
Based on the fact that aspirin, at concentrations of 5–10 mM, inhibits the migration and the activation and translocation of NFκB in normal and cancer cells,28,29 we decided to investigate whether it affects the migration and the NFκB activation and translocation in embryonic endothelial cells. For this, monolayers, after removal of the explants were pretreated for two hrs with SFM or SFM containing aspirin (5–7.5 mM) and then stimulated for 24 hrs with complete medium or complete medium containing aspirin (5–7.5 mM). Both control and aspirin-treated cells were then analyzed for cell migration and expression of both p50 and p65 subunits. Compared with complete medium-treated cultures, aspirin-treated monolayers showed, after 24 hrs of stimulation, a decreased cell migration where some cells appeared to be overlapped with their neighbors (Fig. 8A). In this condition, immunoperoxidase staining showed that a decreased number of cells of the monolayer displayed nuclear staining for phospho-p50 and phospho-p65 as compared to stimulated control-cultures (Fig. 8B). Significant differences of nuclear staining of p50 and p65 were observed between control-cultures and aspirin-treated cultures when the percentage of cells displaying nuclear staining was determined (p < .005, χ2 test; Fig. 8C).
Figure 8.
Effects of aspirin on monolayers of endothelial cells (A–F). (A) Phase-contrast micrographs of the monolayers pretreated for two hrs with SFM containing aspirin (7.5 mM) and stimulated for 24 hrs with complete medium (control) using DMSO as a vehicle or complete medium containing aspirin (7.5 mM). Monolayers are shown at 0, 15 and 22 hrs. Note that in aspirin condition, endothelial cell migration appears decreased and some cells overlap with their neighbors. Scale bar = 150 µm. (B) Immunoperoxidase revealed that in aspirin (5 mM) condition a decreased number of cells of the monolayer displayed nuclear staining for phospho-p50 and -p65, compared with control cultures. Scale bar = 30 µm. (C) Percentage of cells displaying nuclear staining for phospho-p50 and phospho-p65 in monolayers stimulated for 24 hrs with complete medium (control cultures, C) and complete medium containing aspirin 5 mM (Asp). Significant differences of nuclear staining of p50 and p65 were observed between C and Asp conditions (p < .005, χ2 test). (D) Cell viability examination using ethidium bromide and acridine orange showed very few cells labeled with ethidium bromide (dead cells, orange nucleus). (E) Monolayers restrained by scratching and not restrained and maintained for 24 hrs in complete medium and SFM respectively, using DMSO as a vehicle. Note that in the restrained, no cell migration is observed and the number of cells displaying nuclear staining appears decreased. Also note that, in the same monolayers, some of the remaining nonrestrained areas (arrows) show cells that appear separating and migrating and displaying nuclear staining, whereas in the nonrestrained and maintained in SFM, not migrating cells are observed and a significant number of immunoreactive cells is detected. (F) Percentage of cells displaying nuclear staining for phospho-p50 and -p65 in monolayers that were migration restrained (R) and non restrained (NR) and stimulated for 24 hrs with complete medium and monolayers that were not restrained and stimulated with complete medium containing aspirin 7.5 mM (Asp). Significant differences of nuclear staining of p50 and p65 were observed between NR and R (p < .005, χ2 test), and NR and Asp (p < .005, χ2 test).
To determine whether the observed decreased migration by aspirin was caused by cytotoxicity, cell viability was evaluated using both ethidium bromide and acridine orange. As shown in Figure 8D, very few cells fluorescing with ethidium bromide (dead cells, orange nucleus) were observed when monolayer cells were pretreated for two hrs with aspirin 7.5 mM and stimulated for 24 hrs with complete medium containing aspirin 7.5 mM.
For the purpose of demonstrating that the in vitro effects of aspirin upon stimulated monolayer of endothelial cells are related with the diminishing cell migration rather than the inhibition of NF-κB activation and translocation, some monolayers were migration restrained by scratching their borders and stimulated with complete medium for 24 hrs. As controls, other monolayers were not restrained but stimulated with either complete medium or complete medium containing aspirin, or nonstimulated (that is, arrested in SFM). Under these conditions, the expression of both phospho-p50 and phospho-p65 was examined by immunoperoxidase staining.
When the monolayers that had been restrained were stimulated for 24 hrs with complete medium, no cell separation, spreading, detachment and migration was observed and many cells appeared to be overlapped with their neighbors (Fig. 8E). As expected, in monolayers that were not restrained and pretreated for two hrs with SFM contain-ing aspirin (5–7.5 mM) and stimulated with complete medium containing aspirin and maintained in culture for 24 hrs, these cellular events appeared to be decreased, with some cells overlapped as well. In contrast, cell spreading, detachment and migration occurred when monolayers that had not been restrained were cultured in complete medium (not shown). Examination by immunoperoxidase of the monolayers that had been restrained and cultured in complete medium as well as of the aspirin-treated monolayers, revealed nuclear phospho-p65 and -p50 staining in less cells as compared with nonrestrained and stimulated monolayers (Fig 8E). Significant differences of nuclear staining of p50 and p65 were observed between nonrestrained and restrained monolayers (p < .005, χ2 test; Fig. 8F), and nonrestrained and aspirin-treated monolayers (p < .005, .2 test; Fig. 8F) when the percentage of cells displaying nuclear staining was determined. Interestingly, in the SFM condition in which neither detaching nor migrating cells were observed (Fig. 8E), a strong nuclear phospho-p65 and -p50 staining was detected in a significant number of cells (Fig. 8E). This nuclear localization, in this particular condition, would be associated to the activation of anti-apoptotic Bcl2 family members to protect cells from the apoptosis induced by serum deprivation.30
In vitro tubulin and cytoplasmic dynein immunolocalization.
In this study, we have demonstrated the activation and translocation in vitro of NFκB, a nuclear factor known to be inhibited by IκBα. It is also known that the stability of this NFκB activation inhibitor depends on its physical interaction with tubulin31 and cytoplasmic dynein32 which on their turn may also interact between themselves. Interestingly, alterations in these interactions have been associated with NFκB activation by tubulin depolymerization and IκBα degradation.31 –33 We therefore investigated if the interactions between tubulin and cytoplasmic dynein are affected when cell separation, detachment and migration occur; that is, when NFκB activation and translocation takes place.
Double immunofluorescence analyzed by confocal microscopy determined that under these culture conditions, in which separating, detaching and migrating cells are observed, some cells displayed immunoreactivity for both vWf and α-SM actin (Fig. 9A) and the localization of tubulin and dynein appeared to be altered. Specifically, both proteins in addition to being located at the plasma membrane of endothelial cells which displayed a cobblestone appearance, were also located in a punctate and linear arrays at the leading edge as well as in the periphery and in the filopodia extended between neighboring cells that were separating, detaching, and migrating towards cell-free areas (Fig. 9B).
Figure 9.
CLSM fluorescence images of vWf, α-SM actin, tubulin and cytoplasmic dynein localization during EndoMT in vitro after 48 hrs in culture in complete medium. (A) Strong immunoreactivity for vWf (red) in punctated and granular pattern typical of endothelial cells and for α-SM actin (green) delineating cellular margin are observed. Double immunofluorescence of the same region shows some of the cells displaying immunoreactivity for both vWf and α-SM actin (arrows, merged). Scale bar = 25 µm. (B) Strong immunoreactivity for both tubulin (red) and dynein (green) at the plasma membrane of the endothelial cells that display a cobblestone appearance (arrowhead) as well as in the periphery and the leading edge and filopodia (arrows) of separating, detaching and migrating cells is observed. Colocalization of both proteins is detected by the yellow fluorescence generated after simultaneous excitation in both fluorescein (green) and rhodamine (red) wavelengths. Scale bar = 20 µm.
In vivo and in vitro MMP-2, MMP-9 and MMP-14 expression and immunolocalization.
As the activation and translocation of NFκB has been correlated with the expression and activation of some MMPs in endothelial cells,26 we also evaluated the expression and localization of MMP-2 and MMP-9 by immunoperoxidase staining at days E7, E14 and E21 of development. Since MT1-MMP is considered as a membrane-spanning MMP able to activate MMP-2 and that expression of this cell surface protein also correlates with translocation of NFκB,34 we further studied the expression and localization of MMP-14.
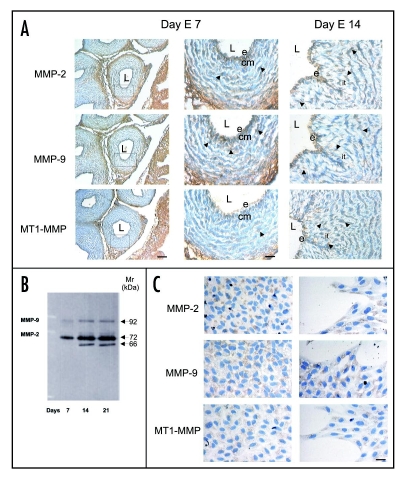
At day E7, a moderate immunoreactivity for MMP-2 and MMP-9 was detected in the endothelium, condensed mesenchyme and cellular layers (Fig. 10A). In contrast, a less intense and more restricted immunoreactivity for MT1-MMP was observed at this stage (Fig. 10A). At days E14 and E21, the immunoreactivities for these MMPs appeared to be increased (Fig. 10A).
Figure 10.
(A) Immunlocalization of MMP-2, MMP-9 and MT1-MMP in serial paraffin cross-sections of chicken embryo aorta at days E7 and E14 at day 7, a moderate immunoreactivity for MMP-2 and MMP-9 is detected in the endothelium (e), condensed mesenchyme (cm) and lamellar cell layers (lc). Less intense and more restricted immunoreactivity for MT1-MMP is observed. At day 14, an increased immuoreactivity for MMP-2, MMP-9 and MT1-MMP is detected in the endothelium and mesenchymal cells (arrows). L, lumen; it, intimal thickening. Scale bars = 100 and 50 µm. (B) Western blot analysis for MMP-2 and MMP-9 in aorta extracts from days E7, E14 and E21. Extracts (50 µg of total protein) were separated by 10% SDS-PAGE under reducing conditions and immunoblotted with polyclonal antibodies against MMP-2 and MMP-9. (C) Immunolocalization of MMP-2, MMP-9 and MT1-MMP in monolayers of endothelial cells. Strong cytoplasmic reactivity for MMP-2 and MMP-9 is observed in many of the endothelial cells of the monolayer and in most of the separating, detaching, and migrating cells. Less intense staining for MT1-MMP is detected. Scale bar = 25 µm.
To corroborate MMP-2 and MMP-9 presence in the aortic wall, we examined aorta extracts from day E7, E14 and E21 by Western blotting. In aorta extracts at days E7, E14 and E21, anti-MMP-2 detected a band with a Mr of 72 kDa corresponding to pro-MMP-2 being more intense at days E14 and E21 (Fig. 10B). Of interest, in extracts at days E14 and E21, the same antibody detected another intense band with a Mr of 66 kDa corresponding to the active form of MMP-2 (Fig. 10B). These observations suggest that the active form of MMP-2 is decreased or absent at day E7 of development. In contrast, anti-MMP-9 detected a faint band at day E7 and a moderate band at days E14 and E21, these bands with a Mr of 92 kDa, corresponding to the monomeric form of MMP-9 (Fig. 10B).
Based on our findings in vivo, we also investigated whether MMP-2, -9 and MT1-MMP were present in monolayers of stimulated endothelial cells when they were maintained in culture for 48 hrs. At this time, when separating, detaching, and migrating cells are observed, we attained a strong cytoplasmic immunoreactivity for MMP-2 and MMP-9 in most of the separating, detaching, and migrating cells as well as in many of endothelial cells of the monolayer (Fig. 10C). Less intense cytoplasmic staining for MT1-MMP was seen in the monolayer as well as in the migrating cells (Fig. 10C).
No immunolabelling was observed when a nonimmune serum was used as negative control (not shown).
Discussion
The current study demonstrates for the first time the presence of activated NFκB during the remodeling of the embryonic aortic wall and the formation of intimal thickening. Our study also provides evidence that allows us to suggest a possible role for this transcription factor in the EndoMT process.
With the use of specific antibodies that recognize p50, p105 and p65-NFκB subunits in the cytoplasm as well as in the nucleus, and others that recognize IκBα and IKKα, we not only demonstrated in vivo the presence and activity of these proteins but also that they change as the vessel maturation progresses. Nuclear p65 or p50 immunoreactivity is considered as an indicator that NFκB has been activated and that the p50/65 or p50/p50 dimers have been formed, whereas p65 or p50 cytoplasmic immunolocalization is thought to correspond to either the inactive p50/p65 form which is complexed with the inhibitory protein IκBα, or to the inactive p50 form complexed with its precursor, p105. In support of these findings, our Western blot analysis of cytoplasmic and nuclear extracts revealed the presence of both the inactivated and activated forms of NFκB, suggesting that heterodimeric and homodimeric forms of NFκB, particularly p50/p65 and p50/p50, may be expressed when the remodeling of the aortic wall and intimal thickening formation occur. The presence of these forms of NFκB is congruent with studies related to neointima formation suggesting that this transcriptional factor is involved in the development of atherosclerosis. Of significance, in vivo nuclear localization of p65 and p50 in the intimal thickening and medial cells has been described in human atherosclerotic lesions, whereas little or no nuclear localization of these NFκB subunits is observed in healthy vessels.22–25 Other studies in atherosclerotic lesions related to p50/p65 have suggested that homodimers of p50 may have opposite role to that of p50/p65 operating as repressors of the transcriptional activity.35 All these studies suggest an important contribution for NFκB in the initiation of atherosclerosis by regulation of different genes that are involved in inflammatory process.
The importance of the role of p50 and p65 subunits and IKKα during the EndoMT process manifests itself when double immunolabelling revealed that both subunits were present at the nucleus of some the cells expressing α-SM actin constituting the intimal thickening and some cells of the lamellar layers, while IKKα was colocalizing with α-SM actin in the cytoplasm of some of these cells. In relation to the EndoMT process, our previous studies have emphasized the possible correlation between the events observed in this process and certain hemodynamic forces which in form of shear and pressure stretch generate a range of electrophysiological, biochemical, and gene regulatory responses, some of them being extremely rapid.13,14 Therefore, the changes observed in vivo in p50, p65, IκBα, and IKKα expression would be related to blood pressure alterations that occur during chicken embryo development.36 Nonetheless, we cannot exclude the possibility that mechanical forces affecting the myocardial function during early cardiogenesis, might also contribute to these changes; moreover, considering that mechanical perturbation may regulate NFκB activation and translocation and therefore the gene expression modulation.37,38
In view of the above assertions and as the activation of NFκB depends on the type and intensity of the stimulus and that activation proceeds rapidly, we also investigated the presence of the activated forms of p50 and p65-subunits in monolayer of endothelial cells that had been mechanically altered during the explant removal and stimulated for 1–2 hr with complete medium. Using antibodies that specifically recognize the activated form of p50 and p65, we demonstrated total or partial nuclear staining in the majority of the endothelial cells of the monolayer that displayed an elongated form and ovoid nuclei as well as in many of the cells that appeared spreading and separating. These observations suggest that under the conditions assessed the NFκB activation and translocation seems to occur. Evidence of activated NFκB have been provided by others, when endothelial cells were subjected to hemodynamic forces that mimic or closely reflect the mechanical environment to which endothelial cells are exposed in vivo.15–17 Also, elevated nuclear translocation of NFκB has been demonstrated in aortic endothelial cells of regions prone for the development of atherosclerotic lesions.24
To gain an insight into the possible role of activated NFκB in the EndoMT process, the presence of the activated form of p50 and p65-subunits as well as the IκBα and IKKα expression were examined in some monolayers that were stimulated for 48 hrs with complete medium, because it is as of this time and under these conditions that cell migration and transformation occur.39 Immunolocalization evidenced not only the presence of activated p50 and p65-subunits in some spreading, separating, detaching and migrating cells and some of those that had acquired mesenchymal characteristics, but also in those cells that expressed α-SM actin (as demonstrated by double immunoperoxidase). Immunolabelling also revealed that IKKα was located in separating, detaching and migrating cells, implying its activation and participation in the translocation of the active dimers of NFκB, as it has been described. Of note, IKKα activation followed by IκBα degradation and NFκB translocation has been described in endothelial cells exposed to shear stress, suggesting that this process is at least mediated by integrins such as αvβ3 integrin.40 To this respect, the presence of αvβ3 and αvβ5 integrins in vivo in the intimal thickening and in vitro in separating, detaching and migrating cells has been reported by Arciniegas et al.,39 suggesting a key role for these molecules during the EndoMT process. Consistent with these findings, our observations support importance of the role for the activated NFκB during the EndoMT process, where IGFII fulfil a critical function, as we have recently demonstrated.39 It is noteworthy that the presence of high levels of IGFII in the plasma41 as well as in the spontaneous intimal thickening,39 have been demonstrated during chicken embryo development. These findings are interesting if we consider that from day E3 to day E10 of development, the arterial pressure rises rapid and exponentially and that the cardiac function may be altered36 and that it is in those stages where changes in the expression and activation of p65 and p50-subunits were observed. In this context, recent in vitro studies related to myogenesis have shown the expression of p65 and IκB induced by IGFII during the skeletal muscle cell differentiation.42 Of note, IκBα degradation might be required for NFκB activation by IGFII in human keratinocytes cultures.43 Thus, it results tempting to speculate that mechanical forces could generate biochemical responses, including releasing of IGFII from the SMCs and/or endothelium, which in turn could promote NFκB expression and activation that would be accompanied by the modulation of specific genes to control not only the organization and functionallity of the SMCs, but also the eventual transformation of the endothelial cells.
In this study, we also established that aspirin affects the migration and eventually the NFκB activation and translocation in monolayer of stimulated endothelial cells, and that this inhibitory effect of aspirin on NFκB activation and translocation is secondary to the diminishing cell separation, detachment and migration, considering that a decreased expression and activation of this transcription factor was also observed in monolayers that had been migration restrained and cultured in the absence of aspirin. These assertions indicate that cell separation, cell detachment and migration, important steps for the EndoMT, may be accompanied by NFκB activation and translocation. Evidence of an essential role for NFκB in epithelial-mesenchymal transition during the breast cancer progression has been provided by Huber et al.44 Interestingly, most recent reports have indicated that β-catenin can form a complex with NFκB, inhibiting its activity and that the loss of E-cadherin and cytoplasmic β-catenin leads to upregulation of NFκB activity in epithelial cells.45,46 Similarly, another recent study demonstrated that overexpression of the p65 subunit in epithelial cells results in the loss of E-cadherin and desmoplakin, suggesting that activation of NFκB in nontransformed mammary epithelial cells leads to conversion into a mesenchymal phenotype characterized by vimentin expression.47 Recently, we have shown that both IGFII and vitronectin may induce endothelial cell separation, detachment and migration involving relocation of VE-cadherin and β-catenin in vivo and in vitro, suggesting a fundamental role for these molecules during the EndoMT process.39 Based on these asseverations and on the results derived from this study, we propose that stimulation of endothelial cells may lead to the loss of cell-cell contacts by removal of VE-cadherin and redistribution of β-catenin and to the activation and translocation of NFκB, and subsequent transformation into mesenchymal cells and regulation of α-SM actin expression.
In this study, we also found alterations in tubulin and cytoplasmic dynein distribution when monolayers of endothelial cells were stimulated with complete medium. We suggest that the activation of NFκB would be associated with these alterations considering that the degradation of IκBα, important step for the activation of NFκB, seems to occur during depolymerization of tubulin to which IκBα, via dynein, is anchored31–33 and that dynein also localizes with β-catenin at sites of cell-cell contact and filopodial extensions contributing with the organization of microtubules at adherents junctions.48 Therefore, a connection between tubulin dynein, β-catenin and NFκB during EndoMT is proposed.
In addition to these findings, we also demonstrated in vivo the expression of both active and pro- MMP-2, pro-MMP-9 and MT1-MMP in those stages of development where the intimal thickening is clearly evident; that is, at days E14 and E21. These findings are interesting if we consider that in vivo altered expression of MMP-2, MMP-9 and MT1-MMP have been described in human and rat aortic remodeling and intimal thickening formation during aging49 and that it is precisely in these stages where we detected the activated form of NFκB. In accordance with these findings, our results in vitro established that these MMPs were located in separating, detaching and migrating cells, suggesting an important role for these molecules during the transformation of the endothelial cells. In this context, recent studies have suggested that exposure of epithelial cells to specific MMPs results in cleavage of E-cadherin and redistribution of β-catenin facilitating cell separation, detachment and invasion.50 These regulated steps are consistently observed during the epithelial-mesnchymal transition.50 As mentioned, the loss of VE-cadherin and redistribution of β-catenin may result in the activation of NF-κB and subsequent expression of α-SM actin. Thus, it is possible to assume that a link exists between the activation of NF-κB and the expression of some MMPs. This is important because the modulation of the expression of MMP-2 and -9 by activated NFκB have been demonstrated in endothelial cells exposed to shear stress26 and in melanoma cells treated with osteopontin.34 Moreover, an important link between the activated NFκB and the expression of both MMP-2 and -9 has also been proposed during the progression of carcinoma oral in vivo, suggesting a regulatory role for this transcription factor upon activation of these MMPs.51 Thus, our in vivo as well as in vitro data are compatible with the hypothesis that the production of MMP-2, -9 and MT1-MMP production and activation in stimulated endothelial cells requires the activation of NFκB.
Consistent with our observations and the studies mentioned here, we hypothesize that stimulation of endothelial cells (by mechanical injury, shear stress, growth factors, cytokines) may cause alterations in the cell-cell contacts and cytoskeleton leading to a rapid activation and translocation of NFκB. Active NFκB may aid in the modulation of the expression of some MMPs, and cell transformation by inducing gene expression (α-SM actin).
Material and Methods
Tissue extraction.
Fertilized chicken eggs (White leghorn) were obtained from a local hatchery (Granja Avícola Agropollito C.A., Paracotos, Estado Miranda) and incubated at 37°C and 60% humidity. The aortae were dissected from 7-, 12-, 14- and 21-day-old embryos (stages 31, 38, 40 and 46 of development, respectively). Embryos were staged according to Hamburger and Hamilton.27 The excised aortae were placed in Hank's balanced salt solution (HBSS) (GIBCO, Invitrogen, Carlsbad, CA), and fixed for 20 min at room temperature with 4% formaldehyde prepared from paraformaldehyde in phosphate-buffered saline (PBS). Figure 1 shows the studied region. The aortae were dehydrated in graded ethanol and embeded in paraffin. Paraffin sections (5µm thick) were mounted on silanized slides (Dako North America, Inc., Carpinteria, CA). A total of six aortae for each stage obtained from three different lots of fertilized chicken eggs, were processed.
Figure 1.
Photograph of chicken embryo heart on day E12 showing the region studied (Ao, arrow).
Immunoperoxidase.
Immunoperoxidase staining was performed in paraffin sections as described previously14 using the following antibodies: a rabbit polyclonal antibody anti-p65 raised against a peptide mapping within the N-terminus of NFκB p65 of human origin, a rabbit polyclonal antibody anti-p50 raised against a peptide mapping within the nuclear localization sequence (NLS) region of NFκB p50 of human origin, a mouse monoclonal antibody anti-IκBα raised against amino acids 1–317 representing full length IκBα of human origin, a rabbit polyclonal antibody anti-IKKα raised against amino acids 1–745 representing full length IKKα of human origin, a rabbit polyclonal anti-phospho-p65 (Ser 536) raised against a short amino acid sequence containing phosphorylated Ser 536 of NFκB p65 of human origin and a rabbit polyclonal antibody anti-phospho-p50 (Ser 337) raised against a short amino acid sequence containing Ser 337 of NFκB p50 of human origin (Santa Cruz Biotechnology Inc., Santa Cruz, CA), a mouse ascites fluid monoclonal antibody anti-α-SM actin (clone 1A4) (Sigma-Aldrich, Saint Louis, MO), a goat polyclonal antibody anti-human MMP-2, a goat polyclonal antibody anti-human MMP-9 and a goat polyclonal antibody anti-MT1-MMP (Santa Cruz Biotechnology Inc.). For double antibody staining for α-SM actin and p65, p50 or IKKα, the EnVision G/2 Doublestain System (Dako) was used.
Western blot analysis.
For Western blot analysis aortae from days E7 and E14 (stages 31 and 38) were pooled and homogenized in buffer Tris-HCl 10 mM, pH: 7.2, NaCl 150 mM, EDTA 1 mM, phenylmethylsulfonylfluoride 0.5 mM, sodium orthovanadate 10 mM, sodium fluoride 10 mM, nonidet P-40 (NP-40) 0.1%, and protease inhibitor cocktail (Sigma-Aldrich). Homogenates were centrifuged at 13,000 rpm for 10 min, and supernatants were collected (cytoplasmic extracts). The resulting pellets were rinsed with the above buffer without NP-40 and incubated for 30min on ice. The nuclear suspension were centrifuged at 13,000 rpm for 10 min. The resulting supernatant (nuclear extracts) were collected. The total protein concentration of cytoplasmic and nuclear extracts were determined by a BCA assay (Pierce Biotechnology Co, Rockford, IL). Protein (13 µg) of both extracts were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and immunoblotted with polyclonal antibodies against p65, p50, phospho-p65 and phospho-p50 (Santa Cruz Biotechnology Inc.).
For MMP-2 and MMP-9 detection, aorta extracts from days E7, E14 and E21 were used. Extracts (50 µg of total proteins) were separated by 10% SDS-PAGE under reducing conditions and immunoblotted with polyclonal antibodies against MMP-2 and MMP-9 (Santa Cruz Biotechnology Inc.).
Cell cultures.
Aortae from days E11–E12 (stages 37 and 38) were dissected in HBSS (GIBCO) at 37°C. Segments, approximately 8mm2 in surface area, were isolated (distal to the aortic arches) and opened along longitudinal axis. Explants were rinsed in PBS without Ca++ and Mg++ (GIBCO) and placed in medium 199 with Earle's salts with L-Glutamine (GIBCO) supplemented with 2% chicken serum (ChS) (Sigma-Aldrich), 1% insulin-transferrin-selenium (ITS) (GIBCO), 100 εg/ml streptomycin, and 100U/ml penicillin (GIBCO). Aortic explants were placed, with the endothelial side down, on fibronectin-coated 35 mm Petri dishes (Nunclon, Delta, IL) containing 300 µl of medium 199 supplemented with 2% ChS and 1% ITS. They were incubated at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air for four hrs. After this interval, 1 ml of medium supplemented with 2% ChS and 1% ITS was gently added to each dish. Two hours later, the adhered explants were removed with the aid of a thin needle, leaving a monolayer of retracted endothelial cells that exhibited zones denuded of cells. These monolayers were rinsed five times with serum-free medium (SFM) before stimulation with Medium 199 containing 1% ITS or recombinant human IGFII (500 ηg/ml) (R&D Systems, Minneapolis, MN) and 2% ChS, both called complete medium. Similar effects in terms of cell spreading, separation, detachment, and migration were observed when ITS or IGFII were added in the presence of ChS. Cultures were then incubated for periods of 1, 2 and 48 hrs and examined with an inverted microscope (IX-70 Olympus, Olympus America Inc., Melville, NY). During these periods, images were captured using an image editing capture and processing software program (Image Pro Plus, Media Cybernetic, Silver Spring, MD). Each set of experiments included at least 20 dishes.
Immunoperoxidase.
The dishes were rinsed twice with PBS (GIBCO) and the cells fixed in 2% formaldehyde, 1% sucrose in PBS for 20 min. Fixed cells were processed for immunostaining as described previously14 using anti-phospho-p65 and antiphospho-p50 (Santa Cruz Biotechnology Inc.). Negative controls were produced by the use of purified normal serum (Sigma-Aldrich) or PBS in place of primary antibody. The images were captured using the Image Pro Plus software program. For double antibody staining for α-SM actin and p65 or p50, the EnVision G/2 Doublestain System (Dako) was used.
Effects of aspirin on embryonic endothelial cells.
To investigate whether aspirin affects the migration and the NF-κB activation and translocation in embryonic endothelial cells, monolayers attached to fibronectin were pretreated for two hrs with SFM or SFM containing acetylsalicylic acid (aspirin) (5–7.5 mM) (Sigma-Aldrich), using DMSO (Sigma-Aldrich) as a vehicle, and then stimulated for 24 hrs with complete medium or complete medium containing aspirin (5–7.5 mM), also using DMSO as a vehicle. The final concentration of DMSO in the medium was 1/1000 v/v. During this period the images were captured every three hrs using the Image Pro Plus software program. Five independent experiments were performed with consistent results. The dishes were processed for immunostaining using anti-phospho-p50 and anti-phospho-p65 (Santa Cruz Biotechnology Inc.) All cells in the monolayers were counted in each microscope field (one per dish) and the stained nuclei were scored. Counting was performed from the images captured using the Image Pro Plus software program. The percentage of p50 and p65 nuclei was expressed as the number of positive nuclei per total number of cells. The χ2 test was used to compare the quantitative differences of p50 and p65 staining between the different experimental conditions. A p < .005 was considered significant.
To investigate whether the in vitro effects of aspirin were related with the diminishing cell migration rather than the inhibition of NF-κB activation and translocation, some monolayers were migration restrained by scratching their borders with a sterile surgical scalpel blade (Lance Blades LTD, Sheffield, UK), rinsed five times with SFM and stimulated for 24 hrs with complete medium in the absence of aspirin, using DMSO as vehicle. At this time, images were captured using the Image Pro Plus software program. Two independent experiments were performed with consistent results. The percentage of p50 and p65 stained nuclei was determined as described above.
Cell viability.
To determine whether the effects of aspirin were caused by cytotoxicity, cell viability was evaluated using ethidium bromide and acridine orange solution (BD Biosciences, ON, Canada). Live cells green fluorescent (with acridine orange) and dead cells orange fluorescent (with ethidium bromide) were viewed and photographed with a camera (Olympus SC35) using an IX-70 Olympus microscope.
Immunofluorescence.
Fixed and permeabilized cells were processed for double antibody staining as described previously13 using the following antibodies: a rabbit antibody anti-human vWf (Dako), a mouse monoclonal antibody anti-a-SM actin (clone 1A4) (Sigma-Aldrich), a mouse ascites monoclonal antibody anti-chicken dynein (heavy chain), and a rabbit polyclonal antibody anti-chicken tubulin (Sigma-Aldrich). Cell cultures were examined with a confocal laser scanning microscope (CLSM) (Eclipse TE-300 Nikon inverted microscope) (Nikon Instruments Inc., Melville, NY) equipped with a Nikon 100/1.30 oil Ph4L immersion objective coupled to a C1-LU2 unit Neon (543 ηm) and Argon cooled air (488 ηm) lasers. These laser units were controlled by a D-eclipse C1 interface.
Acknowledgements
The authors thank Wilman Clark for the photographic contribution (Servicio de Informática, SAIB). The authors also thank Héctor Rojas for his help with the confocal laser scanning microscope (Laboratorio de Fisiología Celular, IVIC). This work was supported by Consejo de Desarrollo Cientifico y Humanistico, UCV grant PG 09 00 6087 2005 and FONACIT grant G2005000405.
Abbreviations
- SMCs
smooth muscle cells
- EndoMT
endothelial mesenchymal transition
- NFκB
nuclear factor of κ light chain gene enhancer in B cells
- IκBs
inhibitors κB
- IKK
IκB kinase
- MMPs
metalloproteinases
- α-SM actin
α-smooth muscle actin
- ITS
insulin transferrin selenium
- IGFII
insulin growth factor II
- aspirin
acetylsalicylic acid
- Mr
relative molecular mass
- Ao
aorta
- B
brachiocephalic arteries
- V
ventricles
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: www.landesbioscience.com/journals/celladhesion/article/5789
References
- 1.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 2.Stehbens WE. Structural and architectural changes during arterial development and the role of hemodynamics. Acta Anat. 1996;157:261–274. doi: 10.1159/000147889. [DOI] [PubMed] [Google Scholar]
- 3.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Korshunov VA, Schwartz SM, Berck BC. Vascular remodeling: Hemodynamic and biochemical mechanism underlying Glagov's phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 5.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 6.Wentzel JJ, Gijsen FJ, Stergiopulos N, Serruys PW, Slager CJ, Krams R. Shear stress, vascular remodeling and neointimal formation. J Biomech. 2003;36:681–688. doi: 10.1016/s0021-9290(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 7.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima Y, Xiang Chen Y, Kinukawa N, Sueishi K. Distributions of diffuse intimal thickening in human arteries: Preferential expression in atherosclerosis-prone arteries from an early age. Virchows Arch. 2002;441:279–288. doi: 10.1007/s00428-002-0605-1. [DOI] [PubMed] [Google Scholar]
- 9.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Throm Vasc Biol. 2007;27:1–11. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 10.Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK. Origin of neointimal smooth muscle: We've come full circle. Arterioscler Thromb Vasc Biol. 2006;26:2579–2581. doi: 10.1161/01.ATV.0000249623.79871.bc. [DOI] [PubMed] [Google Scholar]
- 11.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 12.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: Potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 13.Arciniegas E, Ponce L, Hartt Y, Graterol A, Carlini RG. Intimal thickening involves transdifferentiation of embryonic endothelial cells. Anat Record. 2000;258:47–57. doi: 10.1002/(SICI)1097-0185(20000101)258:1<47::AID-AR6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Arciniegas E, Neves CY, Carrillo LM, Zambrano EA. Endothelial-mesenchymal transition occurs during embryonic pulmonary artery development. Endothelium. 2005;12:193–200. doi: 10.1080/10623320500227283. [DOI] [PubMed] [Google Scholar]
- 15.Lan Q, Kwesi O, Mercurius K, Davies PF. Stimulation of transcription factors NF-κB and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun. 1994;201:950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- 16.Nagel T, Resnick N, Dewey CF, Jr, Gimbrone MA., Jr Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler Thromb Vasc Biol. 1999;19:1825–1834. doi: 10.1161/01.atv.19.8.1825. [DOI] [PubMed] [Google Scholar]
- 17.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 18.Gilmore TD. Introduction to NF-κB: Players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 19.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 20.Squadrito F, Deodato B, Bova A, Marini H, Saporito F, Cal M, Giacca M, Minutoli L, Venuti FS, Caputi AP, Altavilla D. Crucial role of nuclear factor-κB in neointimal hyperplasia of the mouse carotid artery after interruption of blood flow. Atherosclerosis. 2003;166:233–242. doi: 10.1016/s0021-9150(02)00336-2. [DOI] [PubMed] [Google Scholar]
- 21.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor κB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 22.Brand K, Page S, Rogler G, Bartsch A, Brand R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson SH, Best PJ, Edwards WD, Holmes DR, Carlson PJ, Jr, Celermajer DS, Lerman A. Nuclear factor- κB immunoreactivity is present in human coronary plaque and enhanced in patients with unstable angina pectoris. Atherosclerosis. 2002;160:147–153. doi: 10.1016/s0021-9150(01)00546-9. [DOI] [PubMed] [Google Scholar]
- 24.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-κB signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;99:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins T, Cybulsky MI. NF-κB: Pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun HW, Li CJ, Chen HQ, Lin HL, Lv HX, Zhang Y, Zhang M. Involvement of integrins, MAPK, and NF-κB in regulation of the shear stress-induced MMP-9 expression in endothelial cells. Biochem Biophys Res Commun. 2007;353:152–158. doi: 10.1016/j.bbrc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 28.Koop E, Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd FP, Jr, Slivova V, Valachovicova T, Sliva D. Aspirin inhibits highly invasive prostate cancer cells. Int J Onc. 2003;23:1277–1283. [PubMed] [Google Scholar]
- 30.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-κB mediates αvβ3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosette C, Karin C. Cytoskeletal control of gene expression: Depolymerization of microtubules activates NF-κB. J Cell Biochem. 1995;128:1111–1119. doi: 10.1083/jcb.128.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crépieux P, Kwon H, Leclerc N, Spencer W, Richard S, Lin R, Hiscott J. IκB-α physically interacts with a cytoskeleton-associated protein through its signal response domain. Mol Cell Biol. 1997;17:7375–7385. doi: 10.1128/mcb.17.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honore S, Pasquier E, Braguer D. Understanding microtubule dynamics for improved cancer therapy. Cell Mol Life Sci. 2005;62:3039–3056. doi: 10.1007/s00018-005-5330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philip S, Bulbule A, Kundu GC. Matrix metalloproteinase-2: Mechanism and regulation of NF-κB- mediated activation and its role in cell motility and ECM-invasion. Glycoconjugate J. 2004;21:429–441. doi: 10.1007/s10719-004-5533-7. [DOI] [PubMed] [Google Scholar]
- 35.Kanters E, Gijbels MJ, Van der Made I, Vergouwe MN, Heeringa P, Kraal G, Hofker MH, Winther MP. Hematopoietic NF-κB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood. 2004;103:934–940. doi: 10.1182/blood-2003-05-1450. [DOI] [PubMed] [Google Scholar]
- 36.Girard H. Arterial pressure in the chick embryo. Am J Physiol. 1973;224:454–460. doi: 10.1152/ajplegacy.1973.224.2.454. [DOI] [PubMed] [Google Scholar]
- 37.Bartman T, Hove J. Mechanics and function in heart morphogenesis. Dev Dyn. 2005;233:373–381. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- 38.Mironov V, Visconti RP, Markwald RR. On the role of shear stress in cardiogenesis. Endothelium. 2005;12:259–261. doi: 10.1080/10623320500476708. [DOI] [PubMed] [Google Scholar]
- 39.Arciniegas E, Neves YC, Carrillo LM. Potential role for insulin-like growth factor II and vitronectin in the endothelial-mesenchymal transition process. Differentiation. 2006;74:277–292. doi: 10.1111/j.1432-0436.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 40.Bhullar IS, Li YS, Miao H, Zandi E, Kim M, Shyy YJ, Chien S. Fluid shear stress activation of IκB kinase is integrin-dependent. J Biol Chem. 1998;273:30544–30549. doi: 10.1074/jbc.273.46.30544. [DOI] [PubMed] [Google Scholar]
- 41.McMurtry JP, Rosebrough RW, Brocht DM, Francis GL, Upton Z, Phelps P. Assessment of developmental changes in chicken and turkey insulin-like growth factor-II by homologous radioimmunoassay. J Endocrin. 1998;157:463–473. doi: 10.1677/joe.0.1570463. [DOI] [PubMed] [Google Scholar]
- 42.Kaliman P, Canicio J, Testar J, Palacin M, Zorzano A. Insulin-like growth factor II, phosphatidylinositol 3-kinase, nuclear factor -κB and inducible nitric-oxide synthase define a common myogenic signaling pathway. J Biol Chem. 1999;274:17437–17444. doi: 10.1074/jbc.274.25.17437. [DOI] [PubMed] [Google Scholar]
- 43.Kwon YW, Jang ER, Lee YM, Kim YS, Kwon KS, Jang HS, Oh CK, Kim KW. Insulin-like growth factor-II induces interleukin-6 expression via NF-κB activation in psoriasis. Biochem Biophys Res Commun. 2000;278:312–317. doi: 10.1006/bbrc.2000.3806. [DOI] [PubMed] [Google Scholar]
- 44.Huber MA, Azoitel N, Baumann B, Grunert S, Sommer A, Pehemberger H, Kraut N, Beug H, Wirth T. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, Lin SY, Hung MC. β-catenin interacts with and inhibits NF-κB in human colon and breast cancer. Cancer Cell. 2002;4:323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- 46.Kuphal S, Poser I, Jobin C, Hellerbrand C, Bosserhoff AK. Loss of E-cadherin leads to upregulation of NF-κB activity in malignant melanoma. Oncogene. 2004;23:8509–8519. doi: 10.1038/sj.onc.1207831. [DOI] [PubMed] [Google Scholar]
- 47.Chua HL, Nakshatri PB, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 48.Ligon LA, Karki S, Tokito M, Holzbaur LF. Dynein binds to β-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 50.Stallings-Mann M, Radisky D. Matrix metalloproteinase-induced malignancy in mammary epithelial cells. Cells Tissues Organs. 2007;185:104–110. doi: 10.1159/000101310. [DOI] [PubMed] [Google Scholar]
- 51.Bindhu OS, Ramadas K, Sebastian P, Pillai MR. High expression levels of nuclear factor kappa B and gelatinases in the tumorigenesis of oral squamous cell carcinoma. Head and Neck. 2006;28:916–925. doi: 10.1002/hed.20437. [DOI] [PubMed] [Google Scholar]