Abstract
The neurotransmitter acetylcholine (ACh) is expressed in the developing telencephalon at the time when thalamic axons project to the cortex, long before synapses are being formed. Since previous studies demonstrated an influence of ACh on neurite extension we used different in vitro assays to examine possible effects of ACh on the growth of thalamic axons. In explant cultures, application of ACh reduced the length of thalamic axons in a dose dependent manner, an effect that could also be evoked by selective muscarinic and nicotinic agonists. Time-lapse imaging of thalamic axons exposed to microscopic gradients of ACh revealed that growth cones no longer advanced, but maintained high filopodial activity. This growth cone pausing was not accompanied by axon retraction or growth cone collapse. It could at least partially be blocked by muscarinic and nicotinic antagonists, indicating that both types of ACh receptors contribute to mediate these effects on thalamic axons. Finally, we also found that ACh changed the morphology of growth cones; they became larger and extended more filopodia. Since such changes in the structure and motility of growth cones are observed at decision regions along the path of many fiber populations including thalamic axons, we suggest that ACh plays a role during the elaboration of thalamocortical projections.
Key words: cortical development, thalamocortical projections, neurotransmitter, acetylcholine, growth cone, axonal guidance, wiring molecules
Introduction
During development of the nervous system neurotransmitters have been shown to be capable of playing roles entirely different from their classical function in synaptic transmission in the mature brain. Most released neurotransmitters and their receptors are expressed on neurons during early stages of the brain development, even before synaptic contacts are being formed.1 It is notably that specific neurotransmitter systems can have profound effects on histogenetic processes of the brain by influencing for example proliferation, differentiation, migration or survival of neurons.2–4 Neurotransmitters can also affect the behavior of neuronal growth cones, regulating their motility, rate of outgrowth and growth direction, and thereby contribute to the assembly of specific neuronal circuits.5–8 Disturbances of neurotransmitter systems can therefore lead to disrupted development in neurological and neuropsychiatric disorders.9–11
ACh acts as a neurotransmitter in the adult central and peripheral nervous system, which binds to two classes of receptors, ionotropic nicotinic and metabotropic muscarinic receptors. Since it is known, that the ACh synthesizing enzyme choline acetyltransferase (ChAT) and the ACh receptors are present during embryonal brain development, the role of ACh is investigated in early neural development.12,13 Several in vitro studies demonstrated that ACh influences neurite extension in different classes of neurons, in most cases by inhibiting neurite outgrowth. Moreover, inhibition of neurite outgrowth by ACh was mediated through muscarinic and/or nicotinic ACh receptors.6,14,15 ACh has been shown to influence also growth cone motility. For example, application of the neurotransmitter induces the formation of growth cone filopodia and lamellipodia.16 However, there are no data available concerning the effects of ACh on thalamic neurites. We therefore examined the effects of ACh on thalamic axons using different in vitro assays. Our results reveal a novel effect of ACh on growth cone behavior and morphology, suggesting that ACh may be one of the signals which control the development of thalamocortical projections.
Results
Effects of ACh on thalamic axons: explants studies.
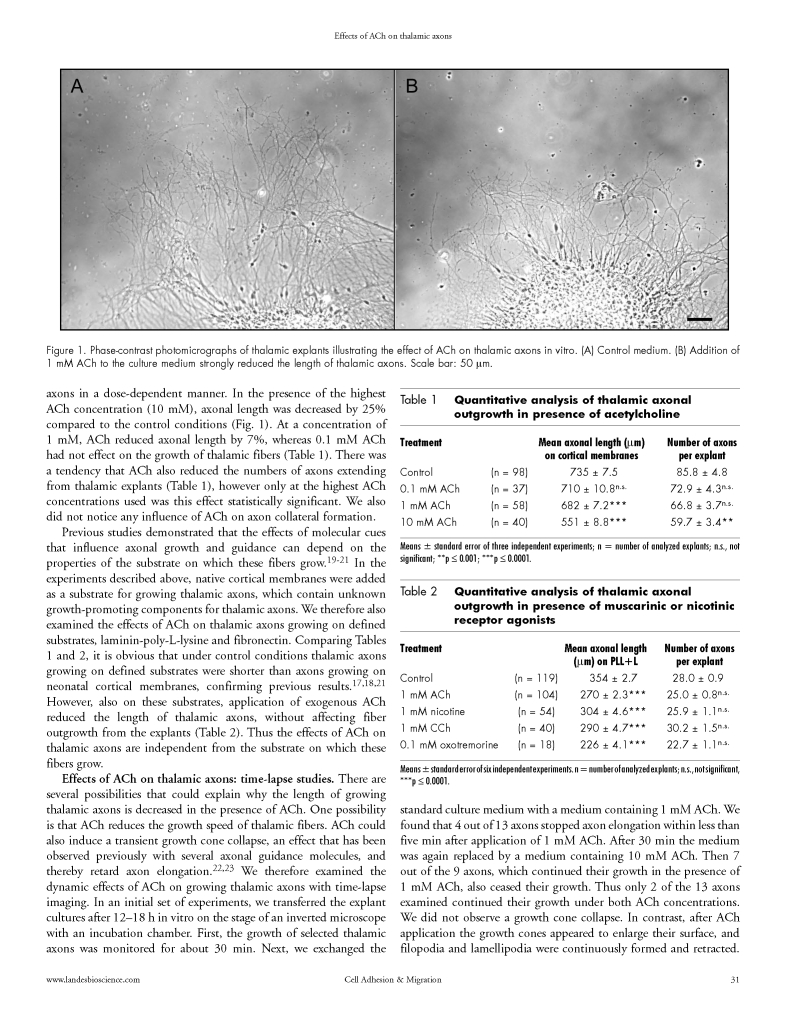
We first examined the possible effects of ACh on axonal growth using E14 mouse thalamic explant cultures. Thalamic explants were grown on a mixed substrate of laminin and poly-L-lysine enriched with membranes from neonatal cortex, a substrate which is known to strongly promote the growth of thalamic fibers.17,18 Explants were cultured in control medium and in the presence of different concentrations of ACh, ranging from 0.1 mM to 10 mM. After two days in vitro cultures were fixed and the number and length of axons extending from the explants were analyzed. Application of ACh to the culture medium significantly decreased the length of the thalamic axons in a dose-dependent manner. In the presence of the highest ACh concentration (10 mM), axonal length was decreased by 25% compared to the control conditions (Fig. 1). At a concentration of 1 mM, ACh reduced axonal length by 7%, whereas 0.1 mM ACh had not effect on the growth of thalamic fibers (Table 1). There was a tendency that ACh also reduced the numbers of axons extending from thalamic explants (Table 1), however only at the highest ACh concentrations used was this effect statistically significant. We also did not notice any influence of ACh on axon collateral formation.
Figure 1.
Phase-contrast photomicrographs of thalamic explants illustrating the effect of ACh on thalamic axons in vitro. (A) Control medium. (B) Addition of 1 mM ACh to the culture medium strongly reduced the length of thalamic axons. Scale bar: 50 µm.
Table 1.
Quantitative analysis of thalamic axonal outgrowth in presence of acetylcholine
| Treatment | Mean axonal length (µm) on cortical membranes | Number of axons per explant | |
| Control | (n = 98) | 735 ± 7.5 | 85.8 ± 4.8 |
| 0.1 mM ACh | (n = 37) | 710 ± 10.8n.s. | 72.9 ± 4.3n.s. |
| 1 mM ACh | (n = 58) | 682 ± 7.2*** | 66.8 ± 3.7n.s. |
| 10 mM ACh | (n = 40) | 551 ± 8.8*** | 59.7 ± 3.4** |
Means ± standard error of three independent experiments; n = number of analyzed explants; n.s., not significant;
p ≤ 0.001;
p ≤ 0.0001.
Previous studies demonstrated that the effects of molecular cues that influence axonal growth and guidance can depend on the properties of the substrate on which these fibers grow.19–21 In the experiments described above, native cortical membranes were added as a substrate for growing thalamic axons, which contain unknown growth-promoting components for thalamic axons. We therefore also examined the effects of ACh on thalamic axons growing on defined substrates, laminin-poly-L-lysine and fibronectin. Comparing Tables 1 and 2, it is obvious that under control conditions thalamic axons growing on defined substrates were shorter than axons growing on neonatal cortical membranes, confirming previous results.17,18,21 However, also on these substrates, application of exogenous ACh reduced the length of thalamic axons, without affecting fiber outgrowth from the explants (Table 2). Thus the effects of ACh on thalamic axons are independent from the substrate on which these fibers grow.
Table 2.
Quantitative analysis of thalamic axonal outgrowth in presence of muscarinic or nicotinic receptor agonists
| Treatment | Mean axonal length (µm) on PLL+L | Number of axons per explant | |
| Control | (n = 119) | 354 ± 2.7 | 28.0 ± 0.9 |
| 1 mM ACh | (n = 104) | 270 ± 2.3*** | 25.0 ± 0.8n.s. |
| 1 mM nicotine | (n = 54) | 304 ± 4.6*** | 25.9 ± 1.1n.s. |
| 1 mM CCh | (n = 40) | 290 ± 4.7*** | 30.2 ± 1.5n.s. |
| 0.1 mM oxotremorine | (n = 18) | 226 ± 4.1*** | 22.7 ± 1.1n.s. |
Means ± standard error of six independent experiments. n = number of analyzed explants; n.s., not significant,
p ≤ 0.0001.
Effects of ACh on thalamic axons: time-lapse studies.
There are several possibilities that could explain why the length of growing thalamic axons is decreased in the presence of ACh. One possibility is that ACh reduces the growth speed of thalamic fibers. ACh could also induce a transient growth cone collapse, an effect that has been observed previously with several axonal guidance molecules, and thereby retard axon elongation.22,23 We therefore examined the dynamic effects of ACh on growing thalamic axons with time-lapse imaging. In an initial set of experiments, we transferred the explant cultures after 12–18 h in vitro on the stage of an inverted microscope with an incubation chamber. First, the growth of selected thalamic axons was monitored for about 30 min. Next, we exchanged the standard culture medium with a medium containing 1 mM ACh. We found that 4 out of 13 axons stopped axon elongation within less than five min after application of 1 mM ACh. After 30 min the medium was again replaced by a medium containing 10 mM ACh. Then 7 out of the 9 axons, which continued their growth in the presence of 1 mM ACh, also ceased their growth. Thus only 2 of the 13 axons examined continued their growth under both ACh concentrations. We did not observe a growth cone collapse. In contrast, after ACh application the growth cones appeared to enlarge their surface, and filopodia and lamellipodia were continuously formed and retracted. Occasionally, growth cones were bifurcating transiently or emitting small side branches that were rapidly retracted (Fig. 2). Growth cone pausing in the presence of ACh lasted as long as we monitored the axons in these experiments (up to 90 min).
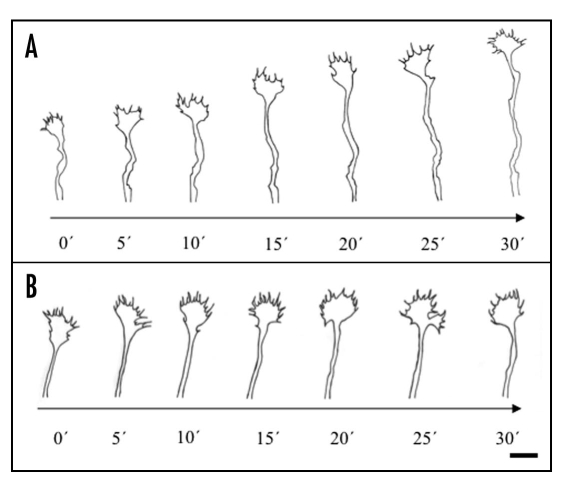
Figure 2.
Drawings of thalamic axons from time-lapse recordings under control conditions and in the presence of ACh. (A) In standard culture medium, the growth cone advanced constantly with a speed of about 40 µm/h. (B) After application of 10 mM ACh to the culture medium, the axon stopped its growth after less than 5 min. Growth cone filopodia, however, remained highly motile, but there was no forward movement of the growth cone. Twenty-five minutes after ACh was added to the medium, there was a transient bifurcation of the growth cone. Scale bar: 5 µm.
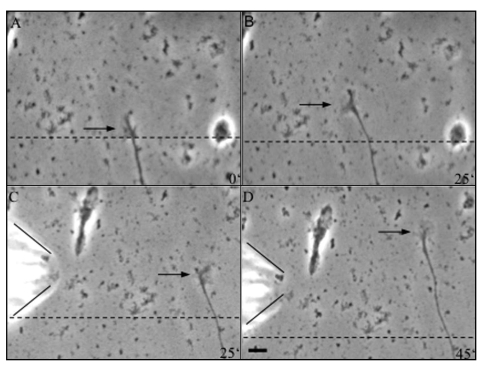
To examine more closely the response of thalamic axons to ACh, we exposed these fibers to ACh microgradients.24 Several ACh concentrations (0.1 mM, 1 mM, 10 mM and 100 mM ACh) were tested. For this we first imaged growing thalamic axons after one or two days in vitro for about 25 min and then positioned a micropipette containing ACh close to the fibers (distance about 100 µm, see Materials and Methods). ACh was ejected by pressure pulses from the micropipette for 15 min. A representative example of growth cone behavior in response to an ACh gradient is illustrated in Figure 3. It first shows the extension of a thalamic axon during 25 min under control conditions and then after pressure ejections of 10 mM ACh. As is evident from these recordings, this thalamic axon ceased its growth almost immediately after ACh was ejected from the pipette. As in the experiments described above, growth arrest was again not accompanied by a collapse or a retraction of the growth cone. In contrast, the growth cone was rather large with complex morphology. Growth cone pausing was observed for all fibers axons examined with 1 mM (13 axons), 10 mM (seven axons) and 100 mM (six axons) ACh in the micropipette. After removing the pipette, growth cone pausing lasted for at least one hour. Ejections of 0.1 mM ACh (12 axons) or control medium (eight axons) did not influence growth cone advance (see Suppl. data, movies 1 and 2).
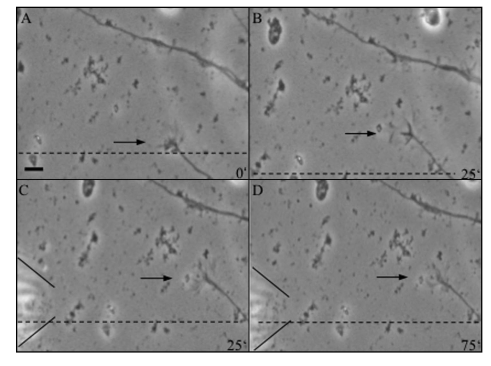
Figure 3.
Sequence of video-photomicrographs showing a thalamic axon under the influence of an ACh gradient. (A and B) Axon growing for 25 min under control conditions. In (B), the frame was shifted as indicated by the dashed line. (C and D) After application of an ACh (10 mM) gradient by a micropipette (indicated by the lines on the left), the axon stopped to advance for the next 50 min, but the growth cone remained highly motile. Scale bar: 5 µm.
Effects of different ACh agonists and antagonists.
To characterize the types of cholinergic receptors that mediate the actions of ACh on thalamic axons, we tested different cholinergic receptor agonists and antagonists. First, thalamic explants were cultured for two days in medium containing either 1 mM carbamylcholine chloride, a mixed muscarinic and nicotinic receptor agonist, or the selective agonists 1 mM nicotine or 0.1 mM oxotremorine sesquifumarate. Similar to the effects observed with ACh, all three agonists lead to a reduction in axon length (Table 2). The strongest effects on axon length were seen with the selective muscarinic agonist oxotremorine sesquifumarate, whereas nicotine was somewhat less potent. Carbamylcholine chloride reduced the length of thalamic fibers with the same potency as ACh.
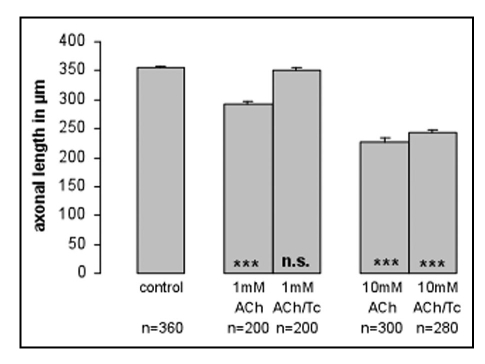
We also examined the ability of cholinergic receptor antagonists to block the effects of ACh on thalamic axons. The addition of the muscarinic receptor antagonist atropine (1 µM) or the nicotinic receptor antagonist d-tubocurarine (0.1 mM) could completely block the effects of 1 mM ACh, but only partially block the effects of 10 mM ACh (Fig. 4), suggesting that both muscarinic and nicotinic receptors contribute to the effects of ACh. Finally, we also tested the behavior of thalamic axons exposed to an ACh gradient in the presence of either atropine (1 µM) or d-tubocurarine (0.1 mM) in the culture medium. Figure 5 shows an example of a thalamic axon exposed to 10 mM ACh ejected from the pipette in a medium containing d-tubocurarine. In contrast to thalamic fibers in control medium, this axon continued its growth in a straight directory and did not pause in response to ACh. Altogether we examined 12 axons in the presence of d-tubocurarine and 16 axons in the presence of atropine, none of these axons were affected by ACh gradients.
Figure 4.
Effects of ACh on axonal length in the presence of d-tubocurarine. 1 mM and 10 mM ACh reduced the length of thalamic axons in a dose dependent manner. Addition of 0.1 mM d-tubocuraine (Tc), a selective nicotinic receptor antagonist, to the culture medium, completely blocked the effect of 1 mM ACh on thalamic axons. At 10 mM ACh d-tubocuraine blocked the effects only partially. n = number of analyzed thalamic axons; ***p ≤ 0.0001; n.s. = not significant. Asteriks in the bars refer always to control conditions.
Figure 5.
Sequence of video-photomicrographs showing a thalamic axon under the influence of an ACh gradient in the presence of a nicotinic receptor antagonist. (A and B) Thalamic axon under control conditions. (C and D) In the presence of 0.1 mM d-tubocurarine in the culture medium, ejections of ACh (10 mM) close to the growth cone had no effect on axon elongation. In (D), the frame was shifted as indicated by the dashed line. Scale bar: 5 µm.
Effects of ACh on thalamic growth cone size.
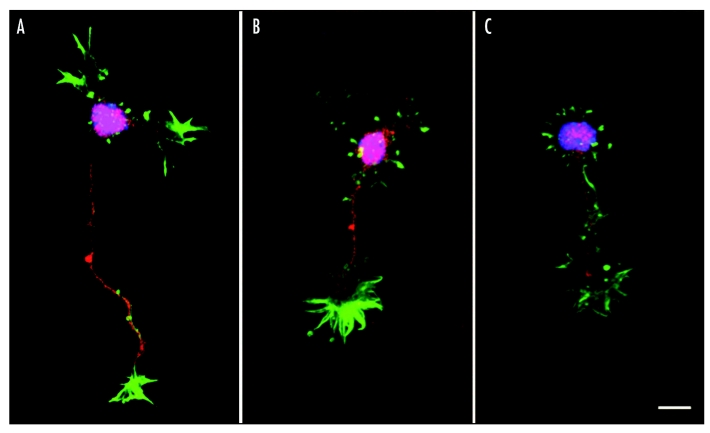
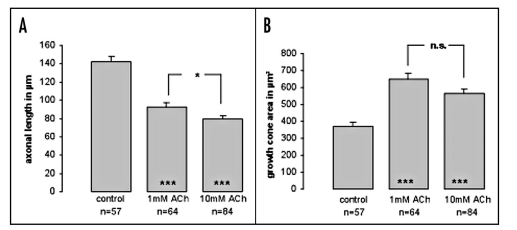
From the time-lapse imaging experiments we got the impression that the growth cones of pausing axons also became enlarged. To study this more systematically, we examined growth cone morphology of dissociated thalamic neurons exposed to different concentrations of ACh (1 mM and 10 mM). We used phalloidin staining to visualize growth cone morphology in more detail. Figure 6 illustrates the effects of ACh on single thalamic neurons after two days in vitro. As with thalamic explants, fibers of single thalamic neurons were reduced in length after ACh exposure and the growth cones exhibited a more complex morphology, characterized by multiple filopodia and side branches extending in several directions. A quantitative analysis of growth area revealed that growth cones increase by almost up to 50% in size in the presence of ACh in the culture medium. In these experiments we could not detect a significant difference in growth cone size between the two ACh concentrations (Fig. 7, Suppl. data movie 3).
Figure 6.
Effect of ACh on individual thalamic fibers and growth cones. (A–C) Photomicrographs of thalamic neurons stained with Alexa Fluor 488-phalloidin (green), anti SMI312 (red) and DAPI (blue). (A) Control medium; (B) medium containing 1 mM ACh; (C) medium containing 10 mM ACh. Addition of ACh decreased thalamic fiber length and at the same time increased growth cone size and number of filopodia. Scale bar: 15 µm.
Figure 7.
Quantitative analysis of ACh effects on individual thalamic fibers and growth cones. Application of 1 mM and 10 mM ACh to the culture medium decreased thalamic fiber length. At the same time growth cone size was increased almost up to 50% at both ACh concentrations used. n = number of thalamic axons/growth cones analyzed; ***p ≤ 0.0001; *p ≤ 0.01; n.s. = not significant. Asterisk in the bars refer to the control condition.
Discussion
In the adult nervous system, ACh acts as neurotransmitter that mediates neuronal signaling in the central and peripheral nervous system. Because ACh, its receptors and synthesizing enzymes are expressed already during early embryonic stages in the brain, ACh has also been implicated to play regulatory roles in the development of the nervous system.13,25,26 Our data demonstrate that ACh acts as a regulator of neurite outgrowth and motility in thalamic axons. Furthermore, application of ACh affects growth cone morphology. First, addition of ACh to the culture medium of thalamic explants reduced axonal length in a dose dependent manner. Second, after acute exposure to microscopic gradients of ACh, thalamic fibers completely stopped elongation but maintained high filopodial activity. These effects of ACh were mediated both by muscarinic and nicotinic ACh receptors, since they could be mimicked by specific receptor agonists and at least partially blocked by muscarinic as well as nicotinic antagonists. Finally, we also observed that exposure of ACh changed growth cone morphology. During the pauses in axon elongation growth cones increased in size and they displayed a more complex morphology.
Many previous studies reported that chronic exposure to ACh can influence neurite growth of different types of neurons in vertebrate and invertebrate species.6,14,15,27 In most cases, ACh inhibited neurite outgrowth and the effects were mediated by muscarinic and/or nicotinic ACh receptors. However, there are also reports showing that ACh can promote neurite outgrowth28 and induce the formation of growth cone filopodia and lamellipodia.16,24,29 An effect on axon guidance of ACh has been described for neurites from Xenopus spinal neurons.24 Application of an ACh gradient near the growth cone by a micropipette induced filopodia formation on the side facing the pipette and neurites turned towards the ACh gradient. However, when cAMP levels of the spinal neurons were reduced by pharmacological reagents, growth cones turned away from ACh gradients.30 Thus, depending on the level of cyclic nucleotides, ACh can act as an attractive or repulsive cue for neurites from spinal neurons. In our experiments with thalamic axons we observed a novel effect of ACh on growth cones. Axon trajectories were not biased toward or away from the ACh delivery pipette, rather ACh triggered growth cones pauses, during which they maintained filopodial activity but ceased forward elongation.
The mechanism how activation of cholinergic receptors regulates neurite outgrowth and growth cone motility are beginning to be understood. There is compelling evidence that intracellular calcium levels modulate growth cone behavior in different species.31–35 It was also shown that muscarinic36 and nicotinic ACh receptors37,38 are able to mediate calcium influx into neurons. Zheng et al., (1994) provided direct evidence that calcium is needed for the response of neuronal growth cones to ACh. As described above, spinal growth cones perform an attractive turning response towards an ACh gradient. However, in the presence of a calcium-free medium the turning response was abolished, indicating that extracellular calcium is essential for the chemoattractive effect of ACh on spinal growth cones.24 It would therefore be interesting to investigate whether growth cone pausing of thalamic axons in response to ACh also requires calcium influx via ACh receptors.
In addition to trigger growth cone pausing, ACh had also a strong effect on growth cone morphology of thalamic axons; they almost doubled in size, displayed many filopodia and sometimes they transiently bifurcated. These characteristic changes in growth cone behavior and morphology are characteritics of developing axons reaching so-called decision points in their paths. During the formation of the precise and stereotyped axon pathways that connect different structures of the brain, elongating fibers often reach regions were they have to change their initial trajectories. In these regions there is a transition of growth cone behavior, from elongation to an “exploration” mode. During elongation axons advance rapidly and their growth cones are small and streamlined, whereas during exploration they often stop growing for a prolonged time and their growth cones become large and more complex. It is thought that these alterations in growth cone morphology correlate with interactions with guidance cues in the extracellular environment, often leading to changes in growth directions.39–41 For instance, as axons from retinal ganglion cells reach the optic chiasm, they go through protracted pauses and their growth cones change to a complex morphology.42–46 Such behaviors have also been described in many other transitions points.47–50
Anatomical tracing studies during the development of thalamocortical projections revealed that thalamic axons first grow ventrally out of the thalamus and then turn dorsolaterally at the boundary between the diencephalon and telencephalon, where they enter the internal capsule by E13 in the mouse. They advance rapidly through this region, but as thalamic axons reach the pallial-subpallial boundary they pause at around E14 for a protracted period of time before they change their growth direction and enter the cerebral cortex.51–54 Time-lapse imaging of thalamic axons in a slice preparation that contained the entire pathway between cortex and thalamus directly revealed that thalamic growth cones, as they reached the pallial-subpallial boundary, change from an elongation to an exploration mode. These axons stopped their growth for up to six hours, before they resumed advance into a different direction.40 Thus, the growth cone behavior of thalamic axons in the pallial-subpallial boundary is very similar to what we described here for growth cones exposed to ACh. A waiting period for thalamic axons has also been described for thalamic axons in the subplate zone, before these fibers enter their appropriate cortical target area.55
Different families of signaling molecules contribute to the guidance of the precise projections of thalamocortical axons, however very little is known about the molecular cues that trigger growth cone pauses at specific regions along the thalamocortical pathway (reviewed in refs. 54 and 56). Immunostaining with antibodies directed against choline acetyltransferase (ChAT), the synthesizing enzyme of ACh, a specific marker for cholinergic neurons, revealed that there is a transient expression of ChAT during early embryonic development in the cortex. ChAT-positive cells are present in the cortical plate and subplate, as well as in the ventricular and subventricular zone, and these cells disappear shortly after birth.12 There is also a cholinergic projection of neurons from the basal forebrain to the cortex57,58 and a more recent study using sensitive ChAT antibodies has indicated an early cholinergic innervation of the cerebral cortex, comparable to some of the other cortical afferents.59 Finally, there is also evidence that ACh receptors are expressed in the embryonic thalamus.13,60,61 Taken together with the results presented here, we suggest that ACh released from cortical cells and/or cholinergic cortical projections participates to the formation of thalamocortical projections by regulating growth cone pauses at decision regions of thalamic axons.
Materials and Methods
Tissue culture.
Thalami from embryonic day 14 (E14) mice were dissected under a microscope and cut into 200 µm3 cubes with a McIlwain tissue chopper (The Mickle Laboratory Engineering, England). After three hours of incubation at 37°C in culture medium containing 50% basal medium Eagle (BME), 25% horse serum, 25% Hanks' balanced salt solution (HBSS; Gibco) supplemented with 4 mg/ml methylcellulose, 1 mM glutamine, 200 mg/ml glucose, 100 units/ml penicillin and 100 µg/ml streptomycin (all from Gibco), explants were transferred to coverslips. Different substrates were used: laminin in combination with poly-L-lysine- (laminin: 19 µg/ml and poly-L-lysine: 10 µg/ml; Sigma-Aldrich), fibronectin (10 µg/ml; Sigma-Aldrich) and homogeneous carpets of cortical postnatal membranes. Membranes were prepared as described previously by Götz et al., 1992.17 Thalamic explants were grown for maximum 48 hours. Various in vitro outgrowth assays were used to analyse axonal growth after cholinergic stimulation; explants grew in presence of several substances: 0.1–100 mM ACh; 0.1 mM d-tubocuraine chloride; 1 µM atropine; 1–10 mM nicotine; 0,1 mM oxotremorine sesquifumarate; 1 mM carbamylcholine chloride (all from Sigma-Aldrich). To confirm neuronal origin of the fibers extending from the explants, immunostaining was performed after fixation in 4% paraformaldehyde using the mouse monoclonal antibody SMI312 (1:1000, Sternberger Monoclonals Inc.) and Cy3-conjugated donkey anti-mouse (1:400, Jackson ImmunoResearch Laboratories). To analyse axonal outgrowth, ten explants per coverslip were randomly selected using a 10x phase-contrast objective of an inverted microscope (Axiovert S 100, Zeiss). The number of fibers extending from the explants was counted, and the length of the ten longest axons in each explant was measured. Statistic comparisons were done using Student's T-test applying Statview software.
To prepare dissociated thalamic neurons, thalamic tissue from E14 mice was collected in HBSS containing 6.5 mg/ml glucose. Then 0.05% Trypsin (Sigma-Aldrich) was added and the thalamic tissue was incubated for 17 minutes at 37°C. After dissociation, thalamic neurons were passed through a nylon mesh. Cell density was adjusted to 100.000 cells per coverslip. Laminin-poly-L-lysine-coated coverslips were used as described before. Thalamic neurons were cultured for 48 hours in DMEM/F12 (Sigma-Aldrich) supplemented with 10% fetal bovine serum, 2 mM glutamine, 9.75 mg/ml glucose, 100 units/ml penicillin and 100 µg/ml streptomycin (all from Gibco). ACh was added to the culture medium at 1 mM and 10 mM. Staining of the F-actin of the thalamic cells with Alexa Fluor 488-phalloidin (1:200; Molecular Probes) was performed to visualize the detailed morphology of growth cones after fixation with 4% paraformaldehyde. Thalamic neurons were also stained with anti SMI312 and and DAPI (50 ng/ml). Images of growth cones were taken with a SPOT camera (Diagnostic instruments). Growth cone size and axonal length were analyzed with ImageJ software.
Time-lapse video microscopy.
Time-lapse studies were performed with an inverted microscope (Axiovert S 100, Zeiss) equipped with a cell culture chamber, where temperature (37°C) and CO2 (5°C) were kept constant. For time-lapse imaging a coverslip with a thalamic explant culture was transferred to a Petriperm dish (Vivascience). Microscopic gradients of ACh molecules were established by pulsed pressure ejections of ACh from a micropipette (Picospritzer II, Parker Instrumentation). The micropipette was positioned through a micromanipulator (Leitz) at a distance of about 100 µm away from the growth cone. An Anapulse Stimulator (301-T, WPI) triggered the ACh ejections at a pressure of 5 psi with a frequency of 2 Hz and a duration of 10 ms. With an ACh concentration of 0.1 mM to 100 mM, the behavior of the growth cone was observed within the gradient for 25 min and after removing the pipette for 1 hour. Video images were taken every 60 s with a CCD camera (Sony). In some gradient assays atropine (1 µM) or tubocurarine (0.1 mM) were applied to the culture medium.
Acknowledgements
We thank Christine Raue for the excellent technical assistance. This work was supported by the IZKF Jena and the DAAD.
Abbreviations
- ACh
acetylcholine
- E14
embryonic day 14
- ChAT
choline acetyltransferase
Note
Supplementary material can be found at:
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/5909
References
- 1.Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, et al. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 2.Behar T N, Li YX, Tran HT, Ma W, Dunlap V, Scott C, et al. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 4.Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCobb DP, Haydon PG, Kater SB. Dopamine and serotonin inhibition of neurite elongation of different identified neurons. J Neurosci Res. 1988;19:19–26. doi: 10.1002/jnr.490190104. [DOI] [PubMed] [Google Scholar]
- 6.Owen A, Bird M. Acetylcholine as a regulator of neurite outgrowth and motility in cultured embryonic mouse spinal cord. Neuroreport. 1995;6:2269–2272. doi: 10.1097/00001756-199511270-00001. [DOI] [PubMed] [Google Scholar]
- 7.Wolff JR, Joo F, Dames W. Plasticity in dendrites shown by continuous GABA administration in superior cervical ganglion of adult rat. Nature. 1978;274:72–74. doi: 10.1038/274072a0. [DOI] [PubMed] [Google Scholar]
- 8.Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A, Potter A, Newhouse P. Nicotinic acetylcholine receptor system and neuropsychiatric disorders. IDrugs. 2004;7:1096–1103. [PubMed] [Google Scholar]
- 10.Garcia Alloza M, Tsang SW, Gil Bea FJ, Francis PT, Lai MK, Marcos B, et al. Involvement of the GABAergic system in depressive symptoms of Alzheimer's disease. Neurobiol Aging. 2006;27:1110–1117. doi: 10.1016/j.neurobiolaging.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Garcia Alloza M, Hirst WD, Chen CP, Lasheras B, Francis PT, Ramirez MJ. Differential involvement of 5-HT(1B/1D) and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer's disease. Neuropsychopharmacology. 2004;29:410–416. doi: 10.1038/sj.npp.1300330. [DOI] [PubMed] [Google Scholar]
- 12.Dori I, Parnavelas JG. The cholinergic innervation of the rat cerebral cortex shows two distinct phases in development. Exp Brain Res. 1989;76:417–423. doi: 10.1007/BF00247899. [DOI] [PubMed] [Google Scholar]
- 13.Zoli M, Le Novere N, Hill JA, Jr, Changeux JP. Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J Neurosci. 1995;15:1912–1939. doi: 10.1523/JNEUROSCI.15-03-01912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Small DH, Reed G, Whitefield B, Nurcombe V. Cholinergic regulation of neurite outgrowth from isolated chick sympathetic neurons in culture. J Neurosci. 1995;15:144–151. doi: 10.1523/JNEUROSCI.15-01-00144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipton SA, Frosch MP, Phillips MD, Tauck DL, Aizenman E. Nicotinic antagonists enhance process outgrowth by rat retinal ganglion cells in culture. Science. 1988;239:1293–1296. doi: 10.1126/science.3344435. [DOI] [PubMed] [Google Scholar]
- 16.Rosner H, Fischer H. In growth cones of rat cerebral neurons and human neuroblastoma cells, activation of protein kinase C causes a shift from filopodial to lamellipodial actin dynamics. Neurosci Lett. 1996;219:175–178. doi: 10.1016/s0304-3940(96)13201-8. [DOI] [PubMed] [Google Scholar]
- 17.Götz M, Novak N, Bastmeyer M, Bolz J. Membrane-bound molecules in rat cerebral cortex regulate thalamic innervation. Development. 1992;116:507–519. [Google Scholar]
- 18.Hübener M, Götz M, Klostermann S, Bolz J. Guidance of thalamocortical axons by growth-promoting molecules in developing rat cerebral cortex. European Journal of Neuroscience. 1995;7:1963–1972. doi: 10.1111/j.1460-9568.1995.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 19.Suh LH, Oster SF, Soehrman SS, Grenningloh G, Sretavan DW. L1/Laminin modulation of growth cone response to EphB triggers growth pauses and regulates the microtubule destabilizing protein SCG10. J Neurosci. 2004;24:1976–1986. doi: 10.1523/JNEUROSCI.1670-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Höpker VH, Shewan D, Tessier Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin- 1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- 21.Bagnard D, Chounlamountri N, Puschel AW, Bolz J. Axonal surface molecules act in combination with semaphorin 3a during the establishment of corticothalamic projections. Cereb Cortex. 2001;11:278–285. doi: 10.1093/cercor/11.3.278. [DOI] [PubMed] [Google Scholar]
- 22.Shi L, Fu WY, Hung KW, Porchetta C, Hall C, Fu AK, et al. Alpha2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc Natl Acad Sci USA. 2007;104:16347–16352. doi: 10.1073/pnas.0706626104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niclou SP, Jia L, Raper JA. Slit2 is a repellent for retinal ganglion cell axons. J Neurosci. 2000;20:4962–4974. doi: 10.1523/JNEUROSCI.20-13-04962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 25.Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 26.Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environ Health Perspect. 1999;107:65–69. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- 28.Coronas V, Durand M, Chabot JG, Jourdan F, Quirion R. Acetylcholine induces neuritic outgrowth in rat primary olfactory bulb cultures. Neuroscience. 2000;98:213–219. doi: 10.1016/s0306-4522(00)00143-3. [DOI] [PubMed] [Google Scholar]
- 29.Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 31.Davenport RW, Kater SB. Local increases in intracellular calcium elicit local filopodial responses in Helisoma neuronal growth cones. Neuron. 1992;9:405–416. doi: 10.1016/0896-6273(92)90179-h. [DOI] [PubMed] [Google Scholar]
- 32.Davenport RW, Dou P, Mills LR, Kater SB. Distinct calcium signaling within neuronal growth cones and filopodia. J Neurobiol. 1996;31:1–15. doi: 10.1002/(SICI)1097-4695(199609)31:1<1::AID-NEU1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Cheng S, Geddis MS, Rehder V. Local calcium changes regulate the length of growth cone filopodia. J Neurobiol. 2002;50:263–275. doi: 10.1002/neu.10027. [DOI] [PubMed] [Google Scholar]
- 34.Rehder V, Kater SB. Regulation of neuronal growth cone filopodia by intracellular calcium. J Neurosci. 1992;12:3175–3186. doi: 10.1523/JNEUROSCI.12-08-03175.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau PM, Zucker RS, Bentley D. Induction of filopodia by direct local elevation of intracellular calcium ion concentration. J Cell Biol. 1999;145:1265–1275. doi: 10.1083/jcb.145.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel FJ, Fortin GD, Martel P, Yeomans J, Trudeau LE. M3-like muscarinic receptors mediate Ca2+ influx in rat mesencephalic GABAergic neurones through a protein kinase C-dependent mechanism. Neuropharmacology. 2005;48:796–809. doi: 10.1016/j.neuropharm.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 38.Vijayaraghavan S, Pugh PC, Zhang ZW, Rathouz MM, Berg DK. Nicotinic receptors that bind alpha-bungarotoxin on neurons raise intracellular free Ca2+ Neuron. 1992;8:353–362. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- 39.Bovolenta P, Dodd J. Guidance of commissural growth cones at the floor plate in embryonic rat spinal cord. Development. 1990;109:435–447. doi: 10.1242/dev.109.2.435. [DOI] [PubMed] [Google Scholar]
- 40.Skaliora I, Adams R, Blakemore C. Morphology and growth patterns of developing thalamocortical axons. J Neurosci. 2000;20:3650–3662. doi: 10.1523/JNEUROSCI.20-10-03650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godement P. Specific guidance and modulation of growth cone motility during in vivo development. J Physiol Paris. 1994;88:259–264. doi: 10.1016/0928-4257(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 42.Chan SO, Wong KF, Chung KY, Yung WH. Changes in morphology and behaviour of retinal growth cones before and after crossing the midline of the mouse chiasm - a confocal microscopy study. Eur J Neurosci. 1998;10:2511–2522. doi: 10.1046/j.1460-9568.1998.00257.x. [DOI] [PubMed] [Google Scholar]
- 43.Mason CA, Wang LC. Growth cone form is behavior-specific and, consequently, position-specific along the retinal axon pathway. J Neurosci. 1997;17:1086–1100. doi: 10.1523/JNEUROSCI.17-03-01086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bovolenta P, Mason C. Growth cone morphology varies with position in the developing mouse visual pathway from retina to first targets. J Neurosci. 1987;7:1447–1460. doi: 10.1523/JNEUROSCI.07-05-01447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sretavan DW, Reichardt LF. Time-lapse video analysis of retinal ganglion cell axon pathfinding at the mammalian optic chiasm: growth cone guidance using intrinsic chiasm cues. Neuron. 1993;10:761–777. doi: 10.1016/0896-6273(93)90176-r. [DOI] [PubMed] [Google Scholar]
- 46.Godement P, Wang LC, Mason CA. Retinal axon divergence in the optic chiasm: dynamics of growth cone behavior at the midline. J Neurosci. 1994;14:7024–7039. doi: 10.1523/JNEUROSCI.14-11-07024.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaethner RJ, Stuermer CA. Dynamics of terminal arbor formation and target approach of retinotectal axons in living zebrafish embryos: a time-lapse study of single axons. J Neurosci. 1992;12:3257–3271. doi: 10.1523/JNEUROSCI.12-08-03257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halloran MC, Kalil K. Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J Neurosci. 1994;14:2161–2177. doi: 10.1523/JNEUROSCI.14-04-02161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris WA, Holt CE, Bonhoeffer F. Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: a time-lapse video study of single fibres in vivo. Development. 1987;101:123–133. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto N, Higashi S, Toyama K. Stop and branch behaviors of geniculocortical axons: a time-lapse study in organotypic cocultures. J Neurosci. 1997;17:3653–3663. doi: 10.1523/JNEUROSCI.17-10-03653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catalano SM, Robertson RT, Killackey HP. Individual axon morphology and thalamocortical topography in developing rat somatosensory cortex. J Comp Neurol. 1996;367:36–53. doi: 10.1002/(SICI)1096-9861(19960325)367:1<36::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 52.Auladell C, Perez Sust P, Super H, Soriano E. The early development of thalamocortical and corticothalamic projections in the mouse. Anat Embryol (Berl) 2000;201:169–179. doi: 10.1007/pl00008238. [DOI] [PubMed] [Google Scholar]
- 53.Molnar Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18:389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- 54.Molnar Z, Higashi S, Lopez Bendito G. Choreography of early thalamocortical development. Cereb Cortex. 2003;13:661–669. doi: 10.1093/cercor/13.6.661. [DOI] [PubMed] [Google Scholar]
- 55.Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- 56.Bolz J, Uziel D, Muhlfriedel S, Gullmar A, Peuckert C, Zarbalis K, et al. Multiple roles of ephrins during the formation of thalamocortical projections: maps and more. J Neurobiol. 2004;59:82–94. doi: 10.1002/neu.10346. [DOI] [PubMed] [Google Scholar]
- 57.Divac I. Magnocellular nuclei of the basal forebrain project to neocortex, brain stem, and olfactory bulb. Review of some functional correlates. Brain Res. 1975;93:385–398. doi: 10.1016/0006-8993(75)90178-x. [DOI] [PubMed] [Google Scholar]
- 58.Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull. 1982;8:727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- 59.Mechawar N, Descarries L. The cholinergic innervation develops early and rapidly in the rat cerebral cortex: a quantitative immunocytochemical study. Neuroscience. 2001;108:555–567. doi: 10.1016/s0306-4522(01)00389-x. [DOI] [PubMed] [Google Scholar]
- 60.Bina KG, Guzman P, Broide RS, Leslie FM, Smith MA, O'Dowd DK. Localization of alpha 7 nicotinic receptor subunit mRNA and alpha-bungarotoxin binding sites in developing mouse somatosensory thalamocortical system. J Comp Neurol. 1995;363:321–332. doi: 10.1002/cne.903630212. [DOI] [PubMed] [Google Scholar]
- 61.Broide RS, O'Connor LT, Smith MA, Smith JA, Leslie FM. Developmental expression of alpha 7 neuronal nicotinic receptor messenger RNA in rat sensory cortex and thalamus. Neuroscience. 1995;67:83–94. doi: 10.1016/0306-4522(94)00623-d. [DOI] [PubMed] [Google Scholar]