Abstract
Stress is an important physiological regulator of brain function in young and adult mammals. The mechanisms underlying regulation of the consequences of stress, and in particular severe chronic stress, are thus important to investigate. These consequences most likely involve changes in synaptic function of brain areas being part of neural networks that regulate responses to stress. Cell adhesion molecules have been shown to regulate synaptic function in the adult and we were thus interested to investigate a regulatory mechanism that could influence expression of three adhesion molecules of the immunoglobulin superfamily (NCAM, L1 and CHL1) after exposure of early postnatal and adult mice to repeated stress. We hypothesized that reduction of adhesion molecule expression after chronic stress, as observed previously in vivo, could be due to gene silencing of the three molecules by DNA methylation. Although adhesion molecule expression was reduced after exposure of C57BL/6 mice to stress, thus validating our stress paradigm as imposing changes in adhesion molecule expression, we did not observe differences in methylation of CpG islands in the promoter regions of NCAM, L1 and CHL1, nor in the promoter region of the glucocorticoid receptor in the hippocampus, the expression of which at the protein level was also reduced after stress. We must therefore infer that severe stress in mice of the C57BL/6 strain downregulates adhesion molecule levels by mechanisms that do not relate to DNA methylation.
Key words: stress, immunoglobulin superfamily, adhesion molecules, promoter, DNA methylation, hippocampus, glucocorticoid receptor
Introduction
Stress is an epigenetic factor that can cause emotional perturbations that may trigger or aggravate mood disorders, among which depression, anxiety, aggression, addiction and schizophrenia are known to be caused by aversive experiences. Among the behavioural disorders resulting from stress, deficits in learning and memory have been reported.1,2 The cellular and molecular mechanisms underlying severe stress or a sometimes apparently only subtle exposure to a stressful experience have been studied extensively, but have remained incompletely understood.3 Among the consequences of chronic and overintense stress are morphological alterations such as, for instance, dendritic atrophy in the hippocampal CA3 subfield and inhibition of neurogenesis in the dentate gyrus.4 Abnormalities in dendritic structure in the CA3 subfield have been associated with abnormal arrangement of synaptic vesicles and mitochondria in mossy fiber terminals.5 Synaptic remodelling in the hippocampal CA1 subfield has also been reported after stress.6,7 Furthermore, stress compromises neuronal cell survival in the hippocampus affecting mainly inhibitory interneurons.8,9
The stress response is mediated by the hypothalamic-pituitary-adrenocortical axis that regulates the production and release of glucocorticoids by the adrenal cortex. Glucocorticoids have been implicated in affecting a broad range of molecular mechanisms underlying morphological and molecular remodelling of brain structures. The molecular mechanisms involved as downstream mediators of glucocorticoids are neurotrophins, especially the brain-derived neurotrophic factor BDNF,10 and chemokines, such as the vascular endothelial growth factor VEGF11 and fibroblast growth factor-2 (FGF2).12
In addition to neurotrophins and chemokines, neural recognition molecules have been implicated in stress and stress-related mood disorders. The neural cell adhesion molecules NCAM, its unusual α2,8 polysialic acid (PSA), and L1 are modulated in their expression as a result of severe stress. Exposure of rats to chronic stress resulted in reduced NCAM expression in the hippocampus.13–15 Chronic stress also led to reduced L1 expression in rats four months,16 but not one day after cessation of stress.15 Glucocorticoids have been identified to mediate these effects of stress, since chronic treatment with corticosteroids also resulted in decreased NCAM expression in the prefrontal cortex.17
Since an aversive experience at young ages is an important risk factor for the later occurrence of mood disorders, dysregulation of adhesion molecule expression early in development can predispose the organism to enhanced vulnerability in emotional and cognitive functions in later life. Because NCAM and L1 have been implicated in remodelling of synaptic structures, neurogenesis, neuronal survival and differentiation and synaptic plasticity in the context of learning and memory, it appeared worthwhile to investigate whether exposure to stress in early postnatal life or in the adult would reduce NCAM and L1 expression as a result of DNA methylation in the promoter region of the genes encoding the two molecules. This modification of the chromatin structure has been implicated in epigenetic programming by maternal behaviour and found to result in differential methylation of CpG islands in the promoter of the glucocorticoid receptor gene in rats,18 with the epigenomic state of this gene being established through behavioural programming.
Based on this observation and the finding that expression of L1 and NCAM is reduced as a consequence of stress, we investigated whether promoter silencing of the L1 and NCAM genes as a result of DNA methylation would underlie reduced expression of the two molecules following physiologically relevant exposure to stress. In addition to NCAM and L1, we also investigated stress-induced expression and DNA methylation of the promoter region of CHL1, the close homologue of L1, a cell adhesion molecule expressed in the nervous system that, similar to L1 and NCAM, has recently been associated with schizophrenia in a study on a Japanese cohort.19 Here we report that exposure to stress during early postnatal and adult ages does not lead to detectable methylation of CpG islands in the promoter regions of the three genes. However, expression of NCAM and CHL1 was reduced by stress in adulthood at the protein level, validating the stress paradigm used in this study as being in agreement with previous studies. Thus, reduction in the levels of NCAM and CHL1 but not L1 after exposure to stress in the present study is most likely caused by different regulatory mechanisms influenced by glucocorticoids.
Results
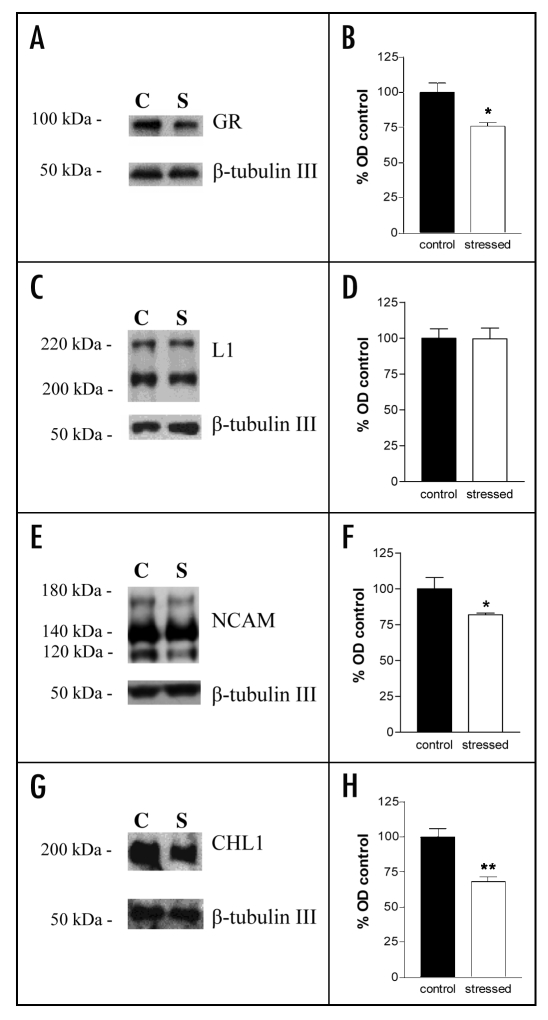
Analysis of the promoter regions of the neural cell adhesion molecules NCAM, L1 and CHL1 led to the identification of CpG islands (Fig. 1) that are putative targets for DNA methylation and thereby might contribute to a downregulation of adhesion molecule expression after exposure to stress. We have used ethologically relevant conditions to induce stress in early postnatal and adult mice to investigate the effects of these forms of stress on DNA methylation in the promoter regions of the three genes. We also evaluated DNA methylation in the promoter region of the glucocorticoid receptor, since it was found to be downregulated in its expression at the protein level coincident with increased methylation of its promoter region in the rat.18 As opposed to the findings in the rat, we did not find increased DNA methylation of this promoter region in the mouse (Table 1). However, we could show that, in agreement with previous studies, glucocorticoid receptor expression at the protein level was reduced in the hippocampus of adult stressed mice (Fig. 2A and B). This result validated the effectiveness of our stress paradigm in the adult.27 We also measured by quantitative Western blot analysis the expression levels of NCAM, L1 and CHL1 after stress in the adult mouse hippocampus and found that NCAM and CHL1 but not L1 were reduced in the hippocampus of stressed mice (Fig. 2C–H). The altered levels of expression of these molecules were not reflected in enhanced DNA methylation in the promoter regions of the genes for these molecules (Table 1). Universal methylated standard was used as a positive control to monitor the successful conversion of unmethylated cytosines into thymidine. The promoter region of the β-actin gene was used as a control for a promoter that does not contain CpG islands being the target for DNA methylation (Fig. 1). The combined observations show that altered levels of glucocorticoid receptor and adhesion molecule expression in the mouse are not due to a change in the methylation pattern of the promoter regions involved.
Figure 1.
Sequences of CpG islands of the mouse promoters of the glucocorticoid receptor (A), L1 (B), CHL1 (C) and NCAM (D) genes. CpG islands are indicated in bold and numbered. Non-coding sequences are indicated in small letters and coding sequences in capital letters. NCAM has a promoter ending in the coding sequence. Note that the beta-actin promoter (E) does not contain CpG islands and was therefore used in sodium bisulfite mapping experiments as negative control. Universal methylated standard (F) is used as positive control to monitor the conversion of non-methylated cytosine into thymidine and the non-conversion of methylated cytosine. GenBank accession numbers of the different genes are indicated in the Materials and Methods section.
Table 1.
Adult or early postnatal stress does not affect cytosine methylation of the promoters of β-actin, glucocorticoid receptor, L1, CHL1 or NCAM genes
| % CpG dinucleotide methylation | ||||
| Adult | Pup | |||
| CpG island/promoter | Control (n = 5) | Stress (n = 5) | Handling (n = 5) | Maternal separation (n = 5) |
| GR | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | 0.3 ± 0.2 |
| L1 | 0.3 ± 0.22 | 0.0 ± 0 | 1 ± 0.87 | 3.95 ± 1.62 |
| CHL1 | 2.44 ± 0.63 | 2.7 ± 0.67 | 0.62 ± 0.35 | 0.91 ± 0.39 |
| NCAM | 0.0 ± 0 | 0.0 ± 0 | 0.12 ± 0.1 | 0.18 ± 0.12 |
| Beta-actin | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 |
| UMS | 99.02 ± 0.47 | 99.02 ± 0.47 | 98.23 ± 0.78 | 98.23 ± 0.78 |
Adult mice or pups were placed under stress or control conditions as described in Materials and Methods. Hippocampal genomic DNA from each animal was prepared and CpG dinucleotide methylation was analyzed by sodium bisulfite mapping as described in Material and Methods: for each promoter, Ten clones were sequenced per hippocampus (N = 5 mice per group). Universal methylated standard (UMS) was used as positive control to check completion of the sodium bisulfite reaction. The β-actin promoter does not contain CpG islands and was used as negative control. The glucocorticoid receptor (GR) promoter CpG islands known to be methylated by maternal separation in the rat was used as positive control. Valus of the β-actin promoter were set to 0.0. Results are expressed as mean percentage of cytosine methylation over the total amount of CpG dinucleotides ± SEM.
Figure 2.
Levels of glucocorticoid receptor, L1, NCAM and CHL1 in the hippocampus of stressed and control adult mice measured by quantitative Western blot analysis. (A, C, E and G) Representative Western blots using antibodies against the glucocorticoid receptor (GR) (A), L1 (C), NCAM (E) and CHL1 (G). β-tubulin III was used as loading control. In all blots, the lane on the left side is representative of the control unstressed (C) group and the lane on the right side is representative of the stressed (S) group. (B, D, F and H) Relative protein levels were quantified for the glucocorticoid receptor (B), L1 (D), NCAM (F) and CHL1 (G). Values are indicated as percentage of the control unstressed group. N = 5 mice per group. *p < 0.05 as compared to the control group (Mann-Whitney test).
Discussion
Cell adhesion molecules of the immunoglobulin superfamily play important roles in structural re-organization and signal transduction mechanisms in different paradigms assessing learning and memory (reviewed in refs. 28 and 29). Glucocorticoids, the levels of which are regulated by the hypothalamic-pituitary-adrenal axis, have also been implicated in structural modifications of nervous system connections leading to transient, but also irreversible changes in behavioural and, more specifically, cognitive functions.30–33 Stress and glucocorticoids also modify expression of cell adhesion molecules, among which NCAM and L1 are the most studied ones (reviewed in ref. 34).
A number of molecular mechanisms can be envisaged that change the stress-induced expression of adhesion molecules, resulting from elevation of glucocorticoid levels and altered glucocorticoid receptor expression. An intriguing one is the silencing of genes involved in structural and functional re-organization of learning and memory processes in the context of cell adhesion molecules, since maternal separation can cause irreversible changes in cognitive functions in the offspring. The finding that stress early in life causes delayed impairments of synaptic and cognitive measures of hippocampal function35,36 requires investigation of permanent and irreversible changes in gene expression, often caused by silencing of genes via methylation of their promoter regions. In the present study we did not detect any increased DNA methylation in the hippocampus of adult mice of the promoters of all genes investigated. Failure to detect changes in DNA methylation in the hippocampus may be due to the fact that other stress-relevant brain regions may be implicated. Thus, not only for the hippocampus, but also for the amygdala37 and the prefrontal cortex38 structural changes have been reported, relating to emotional and cognitive functions, with the amygdala being the target of several emotional disturbances and the prefrontal cortex being implicated not only in cognitive, but also in a wide range of neuropsychiatric disorders.39–41
Despite a stress-induced downregulation of the glucocorticoid receptor protein, no enhanced methylation of the promoter region was found, in contrast to what has been observed in the rat in which decreased glucocorticoid receptor expression in the hippocampus correlated with enhanced methylation of the promoter region.18 This difference can be explained by species-specific differences between rat and mouse. Recent observations indicate that epigenetic effects known to lead to drastic behavioural alterations in the rat, such as maternal separation and postnatal handling, have no or little effect on several mouse strains, including the inbred C57BL/6 mouse,42 suggesting higher resistance to stress-induced epigenetic alterations in the mouse as compared to the rat. One possible ecological explanation of these species-specific differences could be that the mouse is under a remarkable predatory pressure, much more than the rat. Thus, it can be speculated that it would be maladaptive for a species under constant threat of potential predators to have stable and long-lasting stress-induced alterations in gene expression as those mediated by DNA methylation.
In the present study, we observed a downregulation of NCAM and CHL1 protein in the hippocampus of adult stressed mice, confirming several observations on NCAM expression resulting from chronic and thus severe stress in the rat.34 We thus infer that dowregulation of expression of NCAM and CHL1 proteins induced by severe chronic stress must be regulated by other molecular mechanisms independent of gene silencing. Several of these molecular mechanisms can be envisaged: transcriptional regulation of adhesion molecule expression, altered membrane turnover resulting from cell activation, altered protein degradation by post-transcriptional events, such as ubiquitinylation and sumoylation, and inhibition of translational events by micro RNA.
Materials and Methods
Animals.
C57BL/6J mice (Charles River, Hannover, Germany and bred at the mouse facility of the University Hospital Hamburg-Eppendorf ) were kept in an animal facility with an inverted 12:12 h light:dark cycle (light off at 7:00 am) under standard housing conditions (23 ± 1°C; 50% humidity; food and water ad libitum). All experiments were performed during the dark cycle in an experimental room adjacent to the animal facility and illuminated with red dim light.
Postnatal stress paradigms: maternal separation and postnatal handling.
In the rat, maternal separation and postnatal handling differentially affect the activity of the hypothalamic-pituitary-adrenal axis and expression of the glucocorticoid receptor in the hippocampus of adult offspring (reviewed in ref. 20). On the basis of these observations, we designed the experimental paradigms in the mouse. Breeding cages composed of one male and one female mouse were maintained for one week after which the male mouse was removed. At postnatal day (PD) 0 the offspring of each dam was randomly assigned to one of the two treatments: (1) maternal separation and (2) postnatal handling. The sizes of the litters were between eight and nine pups. Pups underwent maternal separation or handling daily from PD1 to PD15. All pups of one mother were either separated for 180 min from the mother (maternal separation) or handled for 5 min daily (handling). Pups of the maternal separation group were taken out of the cage and placed into plastic cylinders with clean nesting material on a heating plate to prevent hypothermia. The mother was left in the home cage during separation. Pups of the handling group were removed from the mother, handled for 5 min by an experimenter wearing gloves and then returned to the mother. The experiments were always performed between 9 am to 12 pm. All pups were left undisturbed with the mother from PD16 onward and sacrificed at PD25.
Adult stress paradigm.
Social defeat and exposure to a rat are commonly used models for chronic stress in adult mice that cause stress-related diseases and affect gene expression in the hippocampus (reviewed in ref. 21).22,23 Mice were isolated one week before the experiments started and randomly assigned to the control (left undisturbed in the home cage) or stressed (undergoing the stress protocol) groups. The stress protocol was performed over four consecutive days and comprised social defeat and exposure to a rat. The social defeat protocol was designed such that mice experienced social submissiveness towards an unfamiliar male in their home cage, a condition that has been shown to robustly induce social stress in mice.24 On the first day, a C57BL/6J male mouse previously selected for elevated aggressive behavior (i.e., a mouse that within 5 min had attacked and bitten an unfamiliar male both as resident and intruder in all out of 6 confrontations performed over three days) was introduced for 20 min into the home cage of the mouse of the stressed group. Then, a mouse of the stressed group was exposed for 12 h of the light period to an adult male Wistar rat (Charles River), while the aggressive mouse was left in the home cage of the stressed mouse. The exposure to a rat was exerted in a Plexiglas cage (42 x 26 cm and 16 cm high) subdivided by a vertical metal grid into two equally sized compartments, and closed by a horizontal metal grid on the top. Each mouse of the stressed group was placed for 12 h into one compartment having a rat in the adjacent compartment. On the second day the mice were returned individually to their home cage, in which the aggressive mouse had remained present, and exposed to a 20 min session of social defeat. After this session, the aggressive mouse was removed. On the third day the mice underwent two sessions per day of social defeat with an unfamiliar aggressive male mouse introduced into the home cage. In all sessions, the mice of the stressed group were promptly attacked by the aggressive mice that continued to chase and bite throughout the 20 min duration of each session. All mice were left undisturbed for four days before being sacrificed. Mice of the control group were always left undisturbed in their home cages before being sacrificed.
Tissue preparation.
Mice were anesthetized with carbon dioxide and sacrificed by decapitation. Brains were removed, and the hippocampi were dissected and snap-frozen in liquid nitrogen.
Promoter and CpG island analysis.
Promoter regions of mouse L1, CHL1, NCAM, glucocorticoid receptor and β-actin genes were defined and analyzed using Genomatix software (http://www.genomatix.de). These promoter regions were also analyzed for CpG patterns using EMBOSS (www.ebi.ac.uk/emboss/cpgplot) and the method described by Gardiner-Garden and Frommer (1987).
Preparation of mouse genomic hippocampal DNA.
Genomic DNA from snap-frozen hippocampi was prepared using the DNeasy tissue kit (Qiagen, Valencia, CA, USA). Before treatment with sodium bisulfite, genomic DNA was concentrated by glycogen precipitation (Invitrogen, Carlsbad, CA, USA). 1–1.5 µg of genomic DNA, 60 µg of glycogen and 2.4 volumes of cold ethanol were mixed and stored for 3 h at -20°C. The precipitation mixture was collected in a microcentrifuge (Eppendorf, Hamburg, Germany) at 4°C and maximum speed for 10 minutes. The supernatant was discarded and the pellet dried for 10 min at room temperature. The pellet was then resuspended in 45 µl of nuclease-free water (Ambion, Austin, TX, USA).
Sodium bisulfite mapping.
Sodium bisulfite mapping was performed using the EZ DNA Methylation kit protocol (Zymo Research, Orange, CA, USA). 1–1.5 µg of genomic DNA and 1 µg of Universal Methylated Standard (Zymo Research) were used in each bisulfite conversion experiment. Universal Methylated Standard was used to check completion of the sodium bisulfite conversion reaction carried out for 16 h at 50°C in the dark. DNA was recovered in 20 µl of 10 mM Tris-EDTA, pH 8.0.
The CpG islands of the glucocorticoid receptor promoter region (GenBank accession number: X66367) of the sodium bisulfitetreated DNA (2 µl) were subjected to touch-down PCR amplification (forward primer: 1387-GGGTTTATAGTATGTATGTGTTGA-1410; reverse primer: 1712-CTCTTCTCCCTAACTCCTTC-1693). The thermocycler protocol involved an initial denaturation cycle (2 min, 94°C), 20 cycles of denaturation (30 sec, 94°C), annealing (30 sec, 57°C initial temperature, -0.5°C per cycle) and extension (1 min, 68°C) followed by 20 cycles of denaturation (30 sec, 94°C), annealing (30 sec, 47°C) and extension (1 min, 68°C), followed by a final extension cycle (7 min, 68°C) and terminating at 4°C. CpG islands of the L1 promoter region (GenBank accession number: U91929) of the sodium bisulfite-treated DNA (2 µl) were subjected to touch-down PCR amplification (forward primer: 2845-ATGAGGGTGTGGTGAGGT-2862; reverse primer: 3150-AACCCACTCCCTCTAAACCTAAA-3128). The thermocycler protocol was as described for the glucocorticoid receptor promoter region except for the annealing steps (30 sec, 62°C initial temperature, -0.5°C per cycle for the 20 first cycles and 30 sec, 52°C for the following 20 cyles). CpG islands of the CHL1 promoter region (GenBank accession number: NT039353) of the sodium bisulfite-treated DNA (2 µl) were subjected to touch-down PCR amplification (forward primer: 17758223-AAGGGAGTGTGTGTAAGAGGGA-17758246; reverse primer: 17758363-ACCAACGAAATCCAACGCCT-17758340). The thermocycler protocol was as described for the glucocorticoid receptor promoter region except for the annealing steps (30 sec, 64°C initial temperature, -0.5°C per cycle for the 20 first cycles and 30 sec, 54°C for the following 20 cyles). CpG islands of the NCAM promoter region (GenBank accession number: NT039473) of the sodium bisulfite-treated DNA (2 µl) were subjected to touch-down PCR amplification (forward primer: 9270475-TGGATGTTAGGAATTATTTGTGGT-9270452; reverse primer: 9270011-ACAAACAAACAATTAACAAACCCA-9270034). The thermocycler protocol was as described for the glucocorticoid receptor promoter region except for the annealing steps (30 sec, 59°C initial temperature, -0.5°C per cycle for the 20 first cycles and 30 sec, 49°C for the following 20 cyles). CpG islands of the β-actin promoter region (GenBank accession number: NT081055) of the sodium bisulfite-treated DNA (2 µl) were subjected to touch-down PCR amplification (forward primer: 161991-AGGATGATTTTTTTTTTTTTTTTGAGG-1620611; reverse primer: 162338-AATATCTAATAAAAAAACTACAAACCCT-162311). The thermocycler protocol was as described for the glucocorticoid receptor promoter region except for the annealing steps (30 sec, 58°C initial temperature, -0.5°C per cycle for the 20 first cycles and 30 sec, 48°C for the following 20 cycles).
The PCR products were separated on a 2% agarose gel. The band corresponding to the expected DNA fragment was extracted and purified using MinElute DNA extraction kit (Qiagen). The PCR products (4 µl) were then subcloned (Original TA cloning kit; Invitrogen) and transformed into chemically competent bacteria (Top10; Invitrogen) and grown on Luria broth/agar-kanamycin treated plates (50 µg/ml; Invitrogen). Mini-preparations of ten different clones per plate were obtained and grown (14 h, 37°C) in 2 ml of Luria broth-treated with kanamycin (50 µg/ml). Recombinant plasmids were purified using PureLink HQ mini plasmid purification kit (Invitrogen) and 250–300 ng of plasmid DNA were automatically sequenced using an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Quantitative Western blot analysis.
Hippocampi were homogenized in 200 µl phosphate buffered saline, pH 7.5 (PBS) containing 1.6 mg/ml protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). All samples were incubated for 30 min at room temperature (20–25°C) with 40 µl lysis buffer (Promega, Mannheim, Germany). Aliquots from each sample were diluted 1:10 with PBS for protein estimation using the BCA method. Samples were diluted with H2O to reach equal amounts of protein in each sample and boiled at 95°C for 10 min in Laemmli buffer. Equal amounts of protein (15 µg) of each sample were loaded and electrophoretically separated on 8% Tris/HCl polyacrylamide gels and transferred to nitrocellulose membranes (Protran, Schleicher & Schuell, Dassel, Germany). All samples from the stressed and control mice were run together on one gel. Membranes were blocked in 4% milk powder (Frema Reform, DE-VAU-GE, Lüneburg, Germany) in PBS/0.05% Tween 20 for 2 h at room temperature (20–25°C) and incubated with primary antibodies in blocking buffer for 2 h at room temperature (20–25°C). The following primary antibodies were used in this study: polyclonal rabbit anti glucocorticoid receptor (M-20; 1:2000; Santa Cruz Biotechnology, Heidelberg, Germany), polyclonal rabbit anti-NCAM (1:2000),25 polyclonal rabbit anti-L1 (1:2000),26 polyclonal rabbit anti-CHL1 (1:1000; R & D Systems, Minneapolis, MN) and polyclonal rabbit anti-βIII tubulin (1:5000; Covance, Munich, Germany). Membranes were washed four times with PBS/0.05% Tween 20 and incubated with anti-rabbit HRP conjugated secondary antibody (1:20000; Dianova, Hamburg, Germany) for 1.5 h at room temperature (20–25°C). Membranes were washed four times with PBS/0.05% Tween 20 and blots were developed using chemiluminescence detection reagents (GE Healthcare, Munich, Germany). All membranes were stripped and re-probed with anti-βIII tubulin antibodies to ensure that all wells were equally loaded. Western blots were scanned and densitometric analysis was performed using TINA Image software (Version 2.0).
Abbreviations
- NCAM
neural cell adhesion molecule
- CHL1
close homolog of L1
- PSA
polysialic acid
- HRP
horse radish peroxidase
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6013
References
- 1.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 2.Heffelfinger AK, Newcomer JW. Glucocorticoid effects on memory function over the human life span. Dev Psychopathol. 2001;13:491–513. doi: 10.1017/s0954579401003054. [DOI] [PubMed] [Google Scholar]
- 3.Brunson KL, Chen Y, Avishai Eliner S, Baram TZ. Stress and the developing hippocampus: a double-edged sword? Mol Neurobiol. 2003;27:121–136. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 5.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 6.Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci USA. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donohue HS, Gabbott PL, Davies HA, Rodriguez JJ, Cordero MI, Sandi C, Medvedev NI, Popov VI, Colyer FM, Peddie CJ, Stewart MG. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 8.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 9.Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E. Chronic stress decreases the number of parvalbuminimmunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology. 2005;30:67–79. doi: 10.1038/sj.npp.1300581. [DOI] [PubMed] [Google Scholar]
- 10.Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- 11.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport. 2006;17:593–597. doi: 10.1097/00001756-200604240-00008. [DOI] [PubMed] [Google Scholar]
- 13.Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- 14.Touyarot K, Sandi C. Chronic restraint stress induces an isoform-specific regulation on the neural cell adhesion molecule in the hippocampus. Neural Plast. 2002;9:147–159. doi: 10.1155/NP.2002.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venero C, Tilling T, Hermans Borgmeyer I, Schmidt R, Schachner M, Sandi C. Chronic stress induces opposite changes in the mRNA expression of the cell adhesion molecules NCAM and L1. Neuroscience. 2002;115:1211–1219. doi: 10.1016/s0306-4522(02)00543-2. [DOI] [PubMed] [Google Scholar]
- 16.Laifenfeld D, Karry R, Grauer E, Klein E, Ben Shachar D. Antidepressants and prolonged stress in rats modulate CAM-L1, laminin, and pCREB, implicated in neuronal plasticity. Neurobiol Dis. 2005;20:432–441. doi: 10.1016/j.nbd.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Sandi C, Loscertales M. Opposite effects on NCAM expression in the rat frontal cortex induced by acute vs. chronic corticosterone treatments. Brain Res. 1999;828:127–134. doi: 10.1016/s0006-8993(99)01346-3. [DOI] [PubMed] [Google Scholar]
- 18.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai K, Migita O, Toru M, Arinami T. An association between a missense polymorphism in the close homologue of L1 (CHL1, CALL) gene and schizophrenia. Mol Psychiatry. 2002;7:412–415. doi: 10.1038/sj.mp.4000973. [DOI] [PubMed] [Google Scholar]
- 20.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 21.Tamashiro KL, Nguyen MM, Sakai RR. Social stress: from rodents to primates. Front Neuroendocrinol. 2005;26:27–34. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Jakovcevski M, Schachner M, Morellini F. Individual variability in the stress response of C57BL/6J male mice correlates with trait anxiety. Genes Brain Behav. 2007;7:235–243. doi: 10.1111/j.1601-183X.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 23.Bartolomucci A, Palanza P, Parmigiani S, Pederzani T, Merlot E, Neveu PJ, Dantzer R. Chronic psychosocial stress downregulates central cytokines mRNA. Brain Res Bull. 2003;62:173–178. doi: 10.1016/j.brainresbull.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Bartolomucci A, Palanza P, Sacerdote P, Panerai AE, Sgoifo A, Dantzer R, Parmigiani S. Social factors and individual vulnerability to chronic stress exposure. Neurosci Biobehav Rev. 2005;29:67–81. doi: 10.1016/j.neubiorev.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J Cell Biol. 1986;103:2439–2448. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathjen FG, Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984;3:1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raone A, Cassanelli A, Scheggi S, Rauggi R, Danielli B, De Montis MG. Hypothalamus-pituitary-adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience. 2007;146:1734–1742. doi: 10.1016/j.neuroscience.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;1:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 29.Welzl H, Stork O. Cell adhesion molecules: key players in memory consolidation? News Physiol Sci. 2003;18:147–150. doi: 10.1152/nips.01422.2002. [DOI] [PubMed] [Google Scholar]
- 30.Garner B, Wood SJ, Pantelis C, van den Buuse M. Early maternal deprivation reduces prepulse inhibition and impairs spatial learning ability in adulthood: no further effect of post-pubertal chronic corticosterone treatment. Behav Brain Res. 2007;17:323–332. doi: 10.1016/j.bbr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Oitzl MS, Workel JO, Fluttert M, Frösch F, De Kloet ER. Maternal deprivation affects behaviour from youth to senescence: amplification of individual differences in spatial learning and memory in senescent Brown Norway rats. Eur J Neurosci. 2000;12:3771–3780. doi: 10.1046/j.1460-9568.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- 32.Luine V, Martinez C, Villegas M, Magariños AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- 33.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 34.Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- 35.Kosten TA, Galloway MP, Dunam RS, Russell DS, D'Sa C. Repeated unpredictable stress and antidepressants differentially regulate expression of the Bcl-2 family of antiapotopic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301527. in press. [DOI] [PubMed] [Google Scholar]
- 36.Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of lateonset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 41.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 42.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]