Abstract
Melatonin (N-acetyl-5-methoxytryptamine), a well-known animal hormone, was discovered in plants in 1995 but very little research into it has been carried out since. It is present in different parts of all the plant species studied, including leaves, stems, roots, fruits and seeds. This brief review will attempt to provide an overview of melatonin (its discovery, presence and functions in different organisms, biosynthetic route, etc.) and to compile a practically complete bibliography on this compound in plants. The common biosynthetic pathways shared by the auxin, indole-3-acetic, and melatonin suggest a possible coordinated regulation in plants. More specifically, our knowledge to date of the role of melatonin in the vegetative and reproductive physiology of plants is presented in detail. The most interesting aspects for future physiological studies are presented.
Key Words: antioxidant, auxin, flowering, growth, IAA, melatonin, plant hormone, reproductive development, rooting, vegetative development
Melatonin (N-acetyl-5-methoxytryptamine), an “old friend” and well known as an animal hormone but “new” to plant biology is arousing great interest due to its broad distribution in the biological kingdom and the recent data on its possible physiological role in plants. Many studies on melatonin, as a phytochemical compound with potentially interesting health-related properties, have recently appeared, but no more than 15–20 papers with a plant physiological focus have been published since 1995. Besides mentioning the most interesting data on melatonin related with plants, this review will hopefully trigger more studies into this molecule to deepen our understanding of the different physiological roles that it might play in plants. We shall briefly look at the well-known function of melatonin in vertebrates, its discovery in plants and other organisms, and its presence in plants as a possible medicinal phytochemical. The joint biosynthetic pathways of melatonin and the auxin indole-3-acetic acid (IAA) will be described. Thus, we reveal the new and emerging field of melatonin studies in plants, the limited physiological data available and its possible role in plants.
Melatonin: Discovery and Functions
Melatonin is an indolic compound (biogenic indoleamine) related structurally with other important substances, such as tryptophan, serotonin, indole-3-acetic acid (IAA), etc. (Fig. 1). Melatonin was discovered in 1958 from the bovine pineal gland and identified as N-acetyl-5-methoxy-tryptamine by Lerner and coworkers.1 It was named melatonin because of its ability in certain fish, reptiles and amphibians to lighten skin.1,2 Today, melatonin is known as a biological modulator of mood, sleep, retina physiology, sexual behavior, seasonal reproductive physiology, circadian rhythms and immunological enhancement. In mammals, melatonin is secreted from the pineal gland at night, from where it diffuses into the cerebro-spinal fluid and the blood stream, although levels quickly drop during the day. Thus, a role in photoperiodic regulation has been demonstrated because of the duration and timing of the melatonin signal. Also, serum melatonin levels in humans vary markedly with age, showing consistent circadian pulses until the mid 20's, after which the pulses decline with age until the 60's.2–9
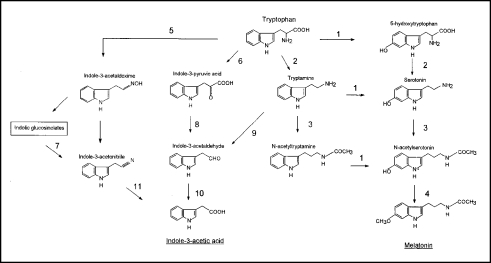
Figure 1.
Tryptophan-dependent biosynthetic pathway of IAA in plants and of melatonin in mammals. The enzymes for the respective steps are: 1, Tryptophan 5-hydroxylase (EC 1.14.16.4); 2, Tryptophan decarboxylase (EC 4.1.1.28); 3, Serotonin N-acetyltransferase (EC 2.3.1.87); 4, Hydroxyindole O-methyltransferase (EC 2.1.1.4); 5, Cytochrome P450 hydroxylase (CYP79 family); 6, Tryptophan aminotransferase (EC 2.6.1.27); 7, Myrosinase (EC 3.2.1.147); 8, Indolepyruvate decarboxylase (EC 4.1.1.74); 9, Monoamine oxidase (EC 1.4.3.4); 10, Indoleacetaldehyde oxidase (EC 1.2.3.7) and 11, Nitrilase (EC 3.5.5.1).
Another recent and important action attributed to melatonin is its scavenging capacity (antioxidant activity) against biological free radicals, such as reactive oxygen and nitrogen species, including the hydroxyl radical, singlet oxygen, peroxyl radical, hydrogen peroxide, peroxynitrite anion and nitric oxide. In this respect, it is interesting that melatonin shows certain peculiarities as an antioxidant, the melatonin molecule presenting no pro-oxidative effects, while melatonin-intermediate products show antioxidant properties and an important synergistic action with other antioxidants, such as ascorbic acid, glutathione, etc.10–21 The effect of melatonin on the photoperiodic (circadian) rhythms together with its antioxidant properties explain why it is sometimes taken as a food supplement (see below).
From 1980 onwards, the detection of melatonin in non-vertebrates (insects, crustaceans, planarians, etc.) began to be reported. In insects particularly, circadian rhythms with nocturnal maxima were described. Nevertheless, in many species arrhythmicity or diurnal peaks of melatonin have been reported. In 1991, melatonin was detected in the unicellular algae Gonyaulax polyedra and, later, in other dinoflagellates and green algae.22–24 Melatonin was also detected in bacteria and fungi, but has been investigated in very few species. An excellent review of melatonin in non-vertebrates with a broad reference list can be consulted in Hardeland and Poeggeler.25
In vascular plants, melatonin was first detected in 1995, when several groups found it mainly in mono- and dicotyledon edible plant families.26–29 Since then, melatonin has been detected and quantified in roots, shoots, leaves, fruits and seeds of a considerable variety of plant species. The most common techniques used to measure melatonin are the radioimmunoassay and high performance liquid chromatography with electrochemical or fluorescence detection. Lastly, high performance liquid chromatography coupled to mass- spectrometry identification is a powerful and essential tool for the precise determination of melatonin in plant samples.26–31 The levels of melatonin in plant organs vary considerably, from picograms to micrograms per gram of plant material. Generally, seeds and leaves present the highest level of melatonin and fruits the lowest.26,28,32–35 Nevertheless, more specific analyses of melatonin are needed, taking into account variables such as the variety, agronomic cultivation conditions, extraction protocols and the technique used for measuring purposes. Until now, studies of melatonin in plants have concentrated on measuring its levels and its possible implications in human food, since melatonin from plant foods is absorbed from the gastrointestinal tract and incorporated in the blood stream; it also crosses the blood-brain barrier and the placenta, while at subcellular level, it is mainly incorporated in the nucleus and mitochondria.36–38 The possibility of modulating blood melatonin levels in mammals and birds through the ingestion of plant-derived foods has led to numerous studies where melatonin is considered as a health-related phytochemical. Table 1 presents some of the most characteristic studies on melatonin, and shows the plant species analyzed, the technique used and the principal aim of the studies. Of particular interest is its presence in medicinal herbs, where it is traditionally used as a sleep modulator in human sleeping disorders, as an anti-depressant or to combat jet-lag. Some herbs (medicinal or not) present high levels of melatonin, in the order of µg/g dry weight, as is the case of Hypericum perforatum (St. John's wort), Tanacetum parthenium (feverfew) and some Chinese medicinal herbs.39–42
Table 1.
Characteristic studies on melatonin in plants, as a healthcare phytochemical and as a molecule of interest for plant physiology
| Plant Species | Analytical Method | Objective | Reference (Year) |
| Phytochemical studies | |||
| 24 edible species | Radioimmunoassay and HPLC-FLU | Level of melatonin in foodstuffs | 28 (1995) |
| 5 edible plant species | Radioimmunoassay and GC-MS | ” | 26 (1995) |
| Tanacetum parthenium Hypericum perforatum Scutellaria baicalensis Scutellaria lateriflora | Radioimmunoassay and HPLC-EC | Level of melatonin in medicinal herbs | 39 (1997) |
| 15 seeds of edible plants | Radioimmunoassay and HPLC-EC | Level of melatonin and seed protection | 33 (2000) |
| Prunus cerasus, fruit | HPLC-EC | Level of melatonin | 32 (2001) |
| 64 Chinese medicinal herbs | HPLC-FLU and HPLC-MS | ” | 40 (2003) |
| Portulaca oleracea | HPLC-EC and GC-MS | ” | 42 (2005) |
| Plant physiology studies | |||
| Chenopodium rubrum, shoots | Radioimmunoassay and HPLC-MS | Rhythms in melatonin levels | 63 (1997) |
| ” | HPLC-MS | Light/dark photoperiod in melatonin levels | 64 (2001) |
| ” | Radiolabel melatonin-Liquid scintillation | Effect of exogenous applied melatonin in flowering | 65 (2003) |
| Pharbitis nil, seedlings | Radioimmunoassay and GC-MS | Melatonin in different photoperiods | 35 (2001) |
| Lycopersicon esculentum, fruit | Melatonin at different ripening stage | ||
| Hypericum perforatum, cultured cells | HPLC-EC and HPLC-MS | Metabolism of melatonin | 54 (2000) |
| Hypericum perforatum, cultured cells | HPLC-EC and HPLC-MS | Melatonin in organogenesis | 79 (2001) |
| Hypericum perforatum, flower | HPLC-EC and HPLC-MS | Melatonin in flower development | 70 (2002) |
| Daucus carota, cultured cells | - | Effect of exogenous melatonin as protector | 71 (2004) |
| Lupinus albus, hypocotyl | HPLC-EC and HPLC-MS | Melatonin as growth promoter | 75 (2004) |
| Triticum aestivum, coleptile, root | HPLC-EC and HPLC-MS | Melatonin as growth promoter in coleoptiles | 76 (2005) |
| Avena sativa, coleoptile, root | Melatonin as growth inhibitor in roots | ||
| Hordeum vulgare, coleoptile, root | |||
| Phalaris canariensis, coleoptile, root |
HPLC, high performance liquid chromatography; GC, gas chromatography; FLU, fluorescence detection; EC, electrochemical detection; MS, mass spectrometry.
Melatonin and IAA: Joint Biosynthetic Pathways
The de novo biosynthesis of IAA in plants has been extensively studied and two main pathways have been established, one tryptophan-dependent and the other tryptophan-independent.43–46 Figure 1 shows a schematic representation of the biosynthesis of IAA, in which the tryptophan-dependent pathway is depicted. In the other pathway, present in bacteria, fungi and plants, IAA is synthetized from chorismate, generating, indole-3-glycerol-phosphate, which is converted into tryptophan and IAA.
The tryptophan-dependent biosynthetic pathway of IAA is induced by many factors, including light, temperature, wounding and pathogen infection. In plants, tryptophan is converted to IAA through three possible routes: (a) the indole-3-acetaldoxime, (b) the indole-3-pyruvic acid and (c) the tryptamine pathways. This last appears as a loop, taking in indole-3-acetaldehyde as a common metabolite with the indole-3-pyruvic acid route. In the indole-3-acetaldoxime pathway, the intermediate indole-3-acetonitrile can be converted to IAA through the action of the enzyme nitrilase. This pathway (characteristic of Brassicaceae) is also fed from the indolic glucosinolate pool through the action of myrosinases. The indole-3-acetamide pathway (no picture in Fig. 1) has mainly been described in bacteria.
The biosynthetic pathway of melatonin has been clearly determined in mammals, including humans, and other groups (amphibian, reptilian, avian) (Fig. 1).4,6,47–53 Melatonin is synthetized from serotonin (5-hydroxy tryptamine) via an acetylation reaction catalyzed by arylalkylamine N-acetyltransferase, also called serotonin N-acetyltransferase (NAT) (EC 2.3.1.87), a regulated enzyme that controls melatonin biosynthesis. The last step is catalyzed by hydroxyindole O-methyltransferase (HIOMT) (EC 2.1.1.4). Serotonin is generated from 5-hydroxytryptophan in a reaction catalyzed by the aminoacid decarboxylase (EC 4.1.1.28). This enzyme also acts on tryptophan, giving rise to tryptamine. Tryptamine can be transformed into serotonin by the action of tryptophan 5-hydroxylase (EC 1.14.16.4), which can also act on tryptophan, forming 5-hydroxytryptophan. Tryptamine is also a substrate of NAT, forming N-acetyltryptamine, which can be hydroxylated to form N-acetylserotonin. All the enzymes shown have been isolated and characterized in animal tissues, but not in plant material. One exception is the enzyme tryptophan decarboxylase (Fig. 1), which has been studied in many plant species, according to InterPro, UniProt, IntEnz and Brenda databases. This pyridoxal-dependent decarboxylase is present in all the kingdoms, from bacteria to human, and has been related, in plants, with tryptamine formation (IAA biosynthesis) but not with the formation of serotonin. This enzyme has been characterized in Arabidopsis thaliana, Oryza sativa, Catharanthus roseus, Papaver somniferum and others. The other melatonin biosynthesis enzymes, tryptophan 5-hydroxylase, serotonin N-acetyltransferase and hydroxyindole O-methyltransferase (Fig. 1) do not appear to have been studied in plants according to the above mentioned databases. Obviously, the generic activities (hydroxylases, acetyltransferases, methyltransferases) have been extensively described in plants in diverse metabolic pathways, but never in relation with melatonin metabolism. The biosynthesis of melatonin and IAA presents some common precursors, such as tryptophan and tryptamine (see Fig. 1). As regards the routes, while the catalyzed steps to form IAA in plants have largely been elucidated by radioactive and biochemical assays, our knowledge on melatonin biosynthesis in plants is practically nil. In this respect, the work of Murch's group on melatonin biosynthesis in plants is unique.54,55 In this study, the radioactivity from 14C-tryptophan was recovered as 14C-indole-3-acetic acid, 14C-tryptamine, 14C- 5-hydroxytryptophan, 14C-serotonin and 14C-melatonin in 1 hour-treated in vitro Hypericum perforatum L. plants, showing the interrelation between melatonin and IAA metabolism. Also, some enzymological data on serotonin biosynthesis in walnut seeds have been published.56 Luckily, the investigations into melatonin in mammals and other groups are well advanced and much of the information can be applied to melatonin metabolism in plants.
An interesting aspect is the relation between structure and activity. Auxinic compounds such as IAA and phenylacetic acid, or other synthetic auxinic-plant growth regulators such as α-naphthalene acetic acid and 2,4-dichlorophenoxy acetic acid, present a strong negative charge on the carboxyl group of the side chain that is separated from a weaker positive charge on the ring structure by a distance of about 0.5 nm. This charge separation seems to be an essential structural requirement for auxin activity.57–61 In the case of melatonin, crystallographic data point to a distance between the indolic ring and the carbonyl group of ∼0.52 nm (Arnao MB, unpublished data). In 1994, Edgerton and colleagues62 proposed a set of molecular requirements for auxin activity based on studies of the capacity of distinct analogs of IAA to bind to auxin-binding protein-1 (ABP-1). Basically, there are three requirements: the existence of a planar aromatic ring, a carboxylic acid-binding site (electronegative charge region) and a hydrophobic transition region that separates the two binding sites. Melatonin seemingly fulfils these conditions (the carbonyl group may simulates the electronegative charge region), although specific experiments still need to be carried out to confirm this.
Possible Physiological Functions of Melatonin in Plants
As mentioned above, the relation between melatonin and vascular plants has been studied almost exclusively from a phytochemical viewpoint. However, the limited number of papers published cover the approaches followed to discover a possible role for melatonin in plants. These approaches look at its role: (1) in reproductive development, including circadian rhythms; (2) in cell protection, and (3) in vegetative development. In this section we present the most relevant data for each. Table 1 shows some of the most relevant studies on melatonin in physiological processes, including the plant species, the technique used and the main objectives of the studies.
Role of melatonin in reproductive development and circadian rhythms.
It is entirely comprehensible that, as a first hypothesis, melatonin in plants was thought to have a similar function to that observed in mammals. Thus, the first experimental study of the physiological role of melatonin in plants was to test its possible involvement as a regulatory molecule in circadian rhythms and in aspects connected with photoperiodicity, such as flowering. In this physiological process, photoreceptors, vernalization, hormones and circadian rhythms are integrated in a regulated-complex network. In 1997, Kolar and colleagues63 presented a study in which the presence of melatonin was confirmed in cultivated 15 day-old plants of Chenopodium rubrum L. using liquid chromatography/tandem mass spectrometry and radioimmunoassays. The authors studied the possible changes in the melatonin levels in light/dark cycles of 12 hours. An oscillating behaviour was observed, showing low or undetectable melatonin levels during the light period and a considerable increase in darkness, when a maximum concentration of 240–550 pg/g fresh weight was observed. This increase during darkness and the range of melatonin concentrations, similar to those observed in animals, opened up interesting expectations. Nevertheless, in a subsequent work, in which plants were exposed to different photoperiodic profiles (6, 12 and 18 h of darkness), no changes in the duration of the melatonin increase with the photoperiod applied was observed, although the maximum of melatonin always occurred after lights off, it being concluded that melatonin synthesis is not directly light-regulated but shows a circadian rhythm, as in animals.64 In a recent paper, this same group, studied the effect of the exogenous application of melatonin on the flowering of the short-day plant Chenopodium rubrum.65 Melatonin treatment provoked neither toxic effects nor changes in the shape, color or number of leaves compared with the control plants. Only exogenous melatonin at 100 and 500 µM (much above physiological levels) had a weak inhibitory effect on flowering, but not on the timing and rhythm (phase, period) of the process. Futhermore, 3H-melatonin applied to the apex and cotyledons of young plants remained practically unmetabolized and was only minimally transported to other regions after 37 h. Thus, whether or not melatonin affects flowering remains unclear.65,66
Another group, that of van Tassel and colleagues, investigated the possible variations in melatonin concentrations in two physiological models, Pharbitis nil Choisy seedlings under light/dark photoperiod and tomatoes (Lycopersicum esculentum Mill.) in different ripening stages. In the first, no differences in the melatonin content appeared with respect to the light/dark cycle, the concentration remaining constant throughout. In the case of tomatoes, mature red fruits presented a slightly higher level of melatonin, although not to a statistically significant extent. Thus, the authors concluded, there was no conclusive evidence relating melatonin levels and photoperiod or ripening.35
Role of melatonin in cell protection.
In part as a consequence of the numerous works on the protective role played by melatonin in animal materials, a similar experimental approach has been applied to plant material. Protection against free radical damage is an important aspect of this indoleamine.10–21 Some interesting studies with non-vertebrates have been made.25 In plants, no clear evidence supporting the protective function of melatonin has, to date, been found. Several hypotheses, however, exist, but only based on melatonin levels in plant organs. Thus, Hardeland34,67 hypothezed on the possible role of melatonin as a dormancy maintenance agent in seeds due to the high concentration observed. Also, some relation between the different melatonin content of two cultivars of Nicotiana tabacum and its susceptibility to ozone has been suggested.26 Manchester et al.33 analyzing 15 seeds of edible plants, proposed a germ tissue protection role for melatonin. A role as antioxidant against the oxidation of lipid vesicles of Gonyaulax polyedra cysts during photoperiodically-induced encystment has also been proposed.68,69
An interesting experimental approach was that of Murch and Saxena,70 who studied the possible protective role of melatonin during flower development of Hypericum perforatum L. A profile of indole levels (IAA, serotonin and melatonin) throughout flower development indicated that serotonin and melatonin present higher concentrations at given stages, and that the higher melatonin level coincided with the maximum regeneration potential of isolated anthers. The authors suggested that melatonin could act as a stress-protecting agent, providing an adaptative mechanism to ensure reproduction.
An interesting effect of melatonin as a protector of cold-induced apoptosis in carrot suspension cells has recently been suggested.71 In this work, (the nearest to demonstrating a protective role in plants), the anti-apoptotic effect of melatonin could not be experimentally related with reactive oxygen species generation. Nevertheless, an important effect of melatonin as membrane integrity factor (in nuclear and plasmatic membranes) in these cultured cells was demonstrated. This effect of melatonin was explained in animal cells as reducing lipid peroxidation and supporting the optimal fluidity of membranes.72 More relevant was the finding that the pretreatment of carrot suspension cells by melatonin significantly increased the level of the polyamines, putrescine and spermidine. Although the authors suggested that the effect of melatonin on polyamine levels may be related with some photoperiodic events, a possible interrelation between auxin, melatonin and polyamines is more probable. In general, the application of hormones that stimulate plant development (auxins, cytokinins and gibberellins) increases the content of polyamines in plant tissues, while inhibitory hormones (abscisic acid and ethylene) decrease their content, which suggests a possible physiological activity for melatonin.
Role of melatonin in vegetative development.
Its chemical structure (indole derivative) and biosynthetic pathway (from tryptophan) suggest that melatonin might be related with IAA, as some authors have previously suggested.55,65,73 IAA is an auxin involved in many physiological actions, but one of the most typical is its role as growth promotor.74
As regards the numerous hypotheses concerning melatonin's possible role as a hormonal agent, we recently demonstrated that it does in fact act as a growth promoter, which is the first physiological role attributed to it.75 In this study, we used three typical longitudinal-growth bioassays applied to young etiolated Lupinus albus L. hypocotyls: section growth, de-rooted hypocotyl growth and apical decapitated- hypocotyl growth with hormone restoration in agar blocks. All bioassay data pointed to the stimulatory effect of melatonin on the growth potential with respect to control bioassays in the concentration range applied (10 nM to 0.1 mM). The optimum growth-promoting effect was determined at 10 µM melatonin. The same paper compared the effect of melatonin with IAA in order to quantify its physiological action. Thus, taking the optimum degree of growth promotion obtained with IAA as 100, the optimum effect of melatonin varied from 22 to 63%, according to the bioassay used. The presence and identification of endogenous melatonin and IAA in the hypocotyls were confirmed by liquid chromatography with tandem mass spectrometry. Also, we studied the distribution of melatonin and IAA in different zones of the hypocotyls (apical, central and basal), demonstrating that melatonin and IAA coexist in the tissues, and that a similar concentration gradient existed for both indoles.
Later, we investigated the possible growth-promoting activity of melatonin in some monocots. The data obtained in Triticum aestivum L., Avena sativa L., Hordeum vulgare L. and Phalaris canariensis L. confirmed the growth-promoting action of melatonin in coleoptile longitudinal-growth assays.76 In these cases, melatonin presented a growth-promoting activity in coleoptiles of around 10, 20, 31 and 55% with respect to IAA for oat, wheat, canarygrass and barley, respectively. We demonstrated that, in the same concentration range as IAA, melatonin had a strong inhibitory effect on root growth. Thus, the effect of IAA as growth promotor in aerial organs (coleoptile, hypocotyl, etc.) and growth inhibitor in root, was matched by melatonin.77 In the monocots assayed, similar levels of melatonin and IAA were recorded, although, in barley and oat, the melatonin concentration in etiolated coleoptiles was higher than that of IAA.
The action mechanism of IAA could be mediated by cell wall proton extrusion through the activation and/or new synthesis of plasma membrane H+-ATPases. We demonstrated that a slight acidification of the media during coleoptile growth-promotion was produced by melatonin, as happens with IAA,76 which pointing to possible coparticipation with IAA.78
However, the possible involvement of some melatonin catabolites in these auxin-like responses should be considered. For example, 5-methoxyindole-3-acetic acid has been described as an inactive catabolite of melatonin in the retina of Xenopus laevis,4,11 although it seems unlikely that a minimal amount of the in situ generated catabolite can resemble the auxin activity of a similar concentration of IAA. Also, melatonin can be very stable when infiltrated in plant tissues (more than 24 h without being metabolized).65
At present, investigations are under way into other possible physiological actions of melatonin related with auxins in vegetative development. Treatment with melatonin at physiological concentrations induced the formation of root primordia in adventitious and lateral roots, affecting the length and number of roots and the rate of growth, in a similar way to IAA. Also, melatonin seems to have a similar effect on cellular expansion (Arnao MB, unpublished data). These data show that melatonin could play some role in in vivo organogenesis, in the same way as IAA. This last aspect was also investigated using in vitro cultured explants of Hypericum perforatum, where an increase in the melatonin concentration of the culture medium was correlated with a high de novo root formation in explants. A possible role for serotonin and melatonin as regulators of plant growth and morphogenesis was postulated.79
Future Perspectives
Although interesting results have been obtained in the past ten years,80 the data on melatonin in plants are clearly incomplete and more extensive studies are needed to elucidate the role of this compound in plants.
We list below some possible ways forward for the study of physiological aspects of melatonin in plants:
All considerations with respect to the metabolism of melatonin in plants are still open. Investigations to transfer our knowledge of animal melatonin metabolism to plants are a priority. Such investigations should be centered on: isolating and characterizing the enzymes of biosynthesis and their modulators; the biosynthetic origin at tissular and cellular level; the mode and pathway of melatonin transport; its possible conjugated compounds (some have been described in animal cells) and it catabolic pathway(s). Finally, its interaction with IAA metabolism should also be investigated.
The existing data on the possible role of melatonin in circadian rhythms and photoperiodic aspects are very interesting but these investigations should be extended to other plant species with the aim of confirming this physiological action. Perhaps, the relation between melatonin and phytochromes/cryptochromes (with related members in many phylogenetic organisms) will open up new perspectives in the possible role of melatonin in flowering, tuberization, vernalization, dormancy and some stress-situations.
As regards vegetative development, more studies on melatonin as growth modulator are necessary, investigating possible ATPase activation, its cellular localization and cell transporter mechanisms. The involvement of melatonin in organogenesis, both in vivo and in vitro, seems probable. The possible implication of melatonin catabolites in these plant responses should be clearly elucidated.
Exploratory data on apical dominance, melatonin's role in tropisms (photo-, geo- and others) and its relation with other hormones, especially ethylene, will possibly throw light on the role of melatonin in plants.
The possible presence of specific melatonin receptors in plant cells cannot be discarded. In animals, various subtypes of receptors (ML1 and ML2) have been characterized, and their genes have been sequenced.81–83 Another perspective is the possible interaction of melatonin with the postulated auxin receptor ABP-1 or with the recently identified intracellular auxin receptor TIR-1.62,84
The consideration of melatonin as an universal antioxidant due to its broad phylogenetic distribution is an interesting aspect that should be investigated specifically in plants. More physiological studies on the role of melatonin as protective agent against reactive oxygen/nitrogen species and other environmental contaminants is an important field to study.
To conclude, further studies will probably identity additional physiological processes in which melatonin is involved, pointing to a specific role in conjunction with other classical hormones such as auxin.
Acknowledgements
This work was supported by project MCyT-AGL2003-00638 (co-financed by FEDER) and by project Fundación SENECA 502/PI/04. J.H.R. has a contract with the University of Murcia.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2640
References
- 1.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, a pineal factor that lightens melanocytes. J Am Soc. 1958;80:2587. [Google Scholar]
- 2.Carlson N. Physiology of Behavior. 5th ed. Needham Heights, MA: Paramount Publishing; 1994. [Google Scholar]
- 3.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 4.Yu HS, Reiter RJ. Melatonin: Biosynthesis, physiological effects, and clinical applications. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- 5.Foulkes NS, Borjigin J, Snyder SH, Sassone-Corsi P. Rhythmic transcription: The molecular basis of circadian melatonin synthesis. Trends Neurosci. 1997;20:487–492. doi: 10.1016/s0166-2236(97)01109-0. [DOI] [PubMed] [Google Scholar]
- 6.Olcese J. Melatonin after four decades: An assessment of its potential. New York: Kluwer Academic; 2000. [Google Scholar]
- 7.Bartness TJ, Goldman BD. Mammalian pineal melatonin: A clock for all seasons. Experientia. 1989;45:939–945. doi: 10.1007/BF01953051. [DOI] [PubMed] [Google Scholar]
- 8.Reiter RJ. The melatonin rhythm: Both a clock and a calendar. Experientia. 1993;49:654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- 9.Humbert W, Pevet P. The decrease of pineal melatonin production with age. Ann NY Acad Sci. 1994;719:43–61. doi: 10.1111/j.1749-6632.1994.tb56819.x. [DOI] [PubMed] [Google Scholar]
- 10.Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocrine J. 1993;1:57–60. [Google Scholar]
- 11.Beyer CE, Steketee JD, Saphier D. Antioxidant properties of melatonin: An emerging mystery. Biochem Pharmacol. 1998;56:1265–1272. doi: 10.1016/s0006-2952(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ, Tan DX, Acuña-Castroviejo D, Burkhardt S, Karbownik M. Melatonin: Mechanisms and actions as an antioxidant. Curr Top Biophys. 2000;24:171–183. [Google Scholar]
- 13.Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species. Cell Biochem Biophys. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 14.Tan DX, Manchester LC, Reiter RJ, Plummer BF, Limson J, Weintraub ST, Qi W. Melatonin directly scavenges hydrogen peroxide: A potentially new metabolic pathway of melatonin biotransformation. Free Rad Biol Med. 2000;29:1177–1185. doi: 10.1016/s0891-5849(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 15.Reiter RJ, Burkhardt S, Cabrera J, Garcia JJ. Beneficial neurobiological effects of melatonin under conditions of increased oxidative stress. Curr Med Chem. 2002;2:45–58. [Google Scholar]
- 16.Reiter RJ, Oh CS, Fujimori O. Melatonin: Its intracellular and genomic actions. Trends Endocrinol Metabol. 1996;7:22–26. doi: 10.1016/1043-2760(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: A significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 18.Poeggeler B, Reiter RJ, Hardeland R, Tan DX, Barlow-Walden LR. Melatonin and structurally-related, endogenous indoles act as potent electron donors and radical scavengers in vitro. Redox Rep. 1996;2:179–184. doi: 10.1080/13510002.1996.11747046. [DOI] [PubMed] [Google Scholar]
- 19.Poeggeler B, Reiter RJ, Hardeland R, Sewerynek E, Melchiorri D, Barlow-Walden LR. Melatonin, a mediator of electron transfer and repair reactions, acts synergistically with the chain-breaking antioxidants ascorbate, trolox and glutathione. Neuroendocrinol Lett. 1995;2:87–92. [Google Scholar]
- 20.Cano A, Alcaraz O, Arnao MB. Free radical-scavenging activity of indolic compounds in aqueous and ethanolic media. Anal Bioanal Chem. 2003;376:33–37. doi: 10.1007/s00216-003-1848-7. [DOI] [PubMed] [Google Scholar]
- 21.Tan DX, Hardeland R, Manchester LC, Poeggeler B, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J Pineal Res. 2003;34:249–259. doi: 10.1034/j.1600-079x.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 22.Hardeland R, Fuhrberg B. Ubiquitous melatonin: Presence and effects in unicells, plants and animals. Trends Comp Biochem Physiol. 1996;2:25–45. [Google Scholar]
- 23.Fuhrberg B, Balzer I, Hardeland R, Werner A, Luning K. The vertebrate pineal hormone melatonin is produced by the brown alga Pterygophora californica and mimics dark effects on growth rate in the light. Planta. 1996;200:125–131. [Google Scholar]
- 24.Balzer B, Hardeland R. Photoperiodism and effects of indoleamines in a unicellular alga, Gonyaulax polyedra. Science. 1991;253:795–797. doi: 10.1126/science.1876838. [DOI] [PubMed] [Google Scholar]
- 25.Hardeland R, Poeggeler B. Nonvertebrate melatonin. J Pineal Res. 2003;34:233–241. doi: 10.1034/j.1600-079x.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 26.Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W. Melatonin in edible plants identified by radioimmunoassay and high performance liquid chromatography-mass spectrometry. J Pineal Res. 1995;18:28–31. doi: 10.1111/j.1600-079x.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 27.Kolar J, Machackova I, Illnerova H, Prinsen E, van Dongen W, van Onckelen HA. Melatonin in higher plant determined by radioimmunoassay and high performance liquid chromatography-mass spectrometry-mass spectrometry. Biol Rhythm Res. 1995;26:406–409. [Google Scholar]
- 28.Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int. 1995;35:627–634. [PubMed] [Google Scholar]
- 29.Van Tassel DL, Roberts NJ, O'Neill SD. Melatonin from higher plants: Isolation and identification of N-acetyl-5-methoxytryptamine. Plant Physiol. 1995;108S:101. [Google Scholar]
- 30.Harumi T, Matsushima S. Separation and assay methods for melatonin and its precursors. J Chromatogr B. 2000;747:95–110. doi: 10.1016/s0378-4347(00)00064-5. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, Zheng X, Xu Y, Zhou X. Rapid determination of serum melatonin by ESI-MS-MS with direct sample injection. J Pharm Biomed Anal. 2002;30:781–790. doi: 10.1016/s0731-7085(02)00387-4. [DOI] [PubMed] [Google Scholar]
- 32.Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ. Detection and quantification of the antioxidant melatonin in montmorency and balaton tart cherries. J Agric Food Chem. 2001;49:4898–4902. doi: 10.1021/jf010321+. [DOI] [PubMed] [Google Scholar]
- 33.Manchester LC, Tan DX, Reiter RJ, Park W, Monis K, Qi W. High levels of melatonin in the seeds of edible plants. Possible function in germ tissue protection. Life Sci. 2000;67:3023–3029. doi: 10.1016/s0024-3205(00)00896-1. [DOI] [PubMed] [Google Scholar]
- 34.Balzer I, Hardeland R. Melatonin in algae and higher plants. Possible new roles as a phytohormone and antioxidant. Bot Acta. 1996;109:180–183. [Google Scholar]
- 35.Van Tassel DL, Roberts NJ, Lewy A, O'Neill SD. Melatonin in plant organs. J Pineal Res. 2001;31:8–15. doi: 10.1034/j.1600-079x.2001.310102.x. [DOI] [PubMed] [Google Scholar]
- 36.Reiter RJ, Tan DX, Burkhardt S, Manchester LC. Melatonin in plants. Nutr Rev. 2001;59:286–290. doi: 10.1111/j.1753-4887.2001.tb07018.x. [DOI] [PubMed] [Google Scholar]
- 37.Benot S, Goberna R, Reiter RJ, Garcia-Mauriño S, Osuna C, Guerrero JM. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J Pineal Res. 1999;27:59–64. doi: 10.1111/j.1600-079x.1999.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 38.Reiter RJ, Manchester LC, Tan DX. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition. 2005;21:920–924. doi: 10.1016/j.nut.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Murch SJ, Simmons CB, Saxena PK. Melatonin in feverfew and other medicinal plants. Lancet. 1997;350:1598–1599. doi: 10.1016/S0140-6736(05)64014-7. [DOI] [PubMed] [Google Scholar]
- 40.Chen G, Huo Y, Tan DX, Liang Z, Zhang W, Zhang Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003;73:19–26. doi: 10.1016/s0024-3205(03)00252-2. [DOI] [PubMed] [Google Scholar]
- 41.Murch SJ, Vasantha-Rupasinghe HP, Goodenowe D, Saxena PK. A metabolomic analysis of medicinal diversity in Huang-qin (Scutellaria baicalensis Georgi) genotypes: Discovery of novel compounds. Plant Cell Rep. 2004;23:419–425. doi: 10.1007/s00299-004-0862-3. [DOI] [PubMed] [Google Scholar]
- 42.Simopoulos AP, Tan DX, Manchester LC, Reiter RJ. Purslane: A plant source of omega-3 fatty acids and melatonin. J Pineal Res. 2005;39:331–332. doi: 10.1111/j.1600-079X.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 43.Bandurski RS, Cohen JD, Slovin J, Reinecke DM. Auxin biosynthesis and metabolism. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. 2nd ed. Dordrecht: Kluwer Academic Publishers; 1995. pp. 39–65. [Google Scholar]
- 44.Normanly J, Slovin J, Cohen JD. Rethinking auxin biosynthesis and metabolism. Plant Physiol. 1995;107:323–329. doi: 10.1104/pp.107.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol. 2002;50:309–332. doi: 10.1023/a:1016024017872. [DOI] [PubMed] [Google Scholar]
- 46.Normanly J, Slovin J, Cohen JD. Auxin biosynthesis and metabolism. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. 3rd ed. Dordrecht: Kluwer Academic Publishers; 2004. pp. 36–62. [Google Scholar]
- 47.Reiter RJ. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 48.Huether G, Kochen W, Simat TJ, Steinhart H. Tryptophan, seronin and melatonin: Basic aspects and applications. Vol. 467. Dordrecht: Kluwer Academic Publishers; 1999. (Adv Exp Med Biol Series). [Google Scholar]
- 49.Abe M, Itoh M, Miyata M, Ishikawa S, Sumi Y. Detection of melatonin, its precursor and related enzyme activities in rabbit lens. Exp Eye Res. 1999;68:255–262. doi: 10.1006/exer.1998.0601. [DOI] [PubMed] [Google Scholar]
- 50.Tan DX, Manchester LC, Reiter RJ, Qi WB, Zhang M, Weintraub ST, Cabrera J, Sainz RM, Mayo JC. Identification of highly elevated levels of melatonin in bone marrow: Its origin and significance. Biochim Biophys Acta. 1999;1472:206–214. doi: 10.1016/s0304-4165(99)00125-7. [DOI] [PubMed] [Google Scholar]
- 51.Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, Maestroni GJM. Evidence for melatonin synthesis in mouse and human bone marrow cells. J Pineal Res. 2000;28:193–202. doi: 10.1034/j.1600-079x.2000.280401.x. [DOI] [PubMed] [Google Scholar]
- 52.Hardeland R, Reiter RJ, Poeggeler B, Tan DX. The significance of the metabolism of the neurohormone melatonin: Antioxidative protection and formation of bioactive substances. Neurosci Biobehav Rev. 1993;17:347–357. doi: 10.1016/s0149-7634(05)80016-8. [DOI] [PubMed] [Google Scholar]
- 53.Sugden D. Melatonin biosynthesis in the mammalian pineal gland. Experientia. 1989;45:922–932. doi: 10.1007/BF01953049. [DOI] [PubMed] [Google Scholar]
- 54.Murch S, KrishnaRaj S, Saxena PK. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John's wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000;19:698–704. doi: 10.1007/s002990000206. [DOI] [PubMed] [Google Scholar]
- 55.Murch S, Saxena PK. Melatonin: A potential regulator of plant growth and development? In vitro Cell Dev Biol Plant. 2002;38:531–536. [Google Scholar]
- 56.Schroder R, Abele C, Gohr P, Stuhlfauth-Roisch U, Grosse W. Latest on enzymology of serotonin biosynthesis in walnut seeds. In: Huether G, Kochen W, Simat TJ, Steinhart H, editors. Tryptophan, Seronin and Melatonin: Basic Aspects and Applications. Vol. 467. Dordrecht: Kluwer Academic Publishers; 1999. pp. 637–644. (Adv Exp Med Biol Series). [DOI] [PubMed] [Google Scholar]
- 57.Porter WL, Thimann KV. Molecular requirements for auxin action. Phytochemistry. 1965;4:229–243. [Google Scholar]
- 58.Elliot MC, Farrimond JA, Clack DW. Structural requirements for auxin activity. Plant Physiol. 1976;57:18. [Google Scholar]
- 59.Thimann KV. Auxin activity of some indole derivatives. Plant Physiol. 1958;33:311–321. doi: 10.1104/pp.33.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Findlay SP, Dougherty G. The activity of certain substituted indoleacetic acids as plant hormones in the pea test. J Biol Chem. 1950;183:361–364. [Google Scholar]
- 61.Katekar GF. Auxins: On the nature of the receptor site and molecular requirements for auxin activity. Phytochemistry. 1979;18:223–233. [Google Scholar]
- 62.Edgerton MD, Tropsha A, Jones AM. Modelling the auxin-binding site of auxin-binding protein-1 of maize. Phytochemistry. 1994;35:1111–1123. [Google Scholar]
- 63.Kolar J, Machackova I, Eder J, Prinsen E, van Dongen W, van Onckelen H, Illnerova H. Melatonin: Occurrence and daily rhythm in Chenopodium rubrum. Phytochemistry. 1997;44:1407–1413. [Google Scholar]
- 64.Wolf K, Kolar J, Witters, van Dongen W, van Onckelen H, Machackova I. Daily profile of melatonin levels in Chenopodium rubrum depends on photoperiod. J Plant Physiol. 2001;158:1491–1493. [Google Scholar]
- 65.Kolar J, Johnson CH, Machackova I. Exogenously applied melatonin affects flowering of the short-day plant Chenopodium rubrum. Physiol Plant. 2003;118:605–612. [Google Scholar]
- 66.Machackova I, Krekule J. Sixty-five years of searching for the signals that trigger flowering. Russ J Plant Physiol. 2002;49:451–459. [Google Scholar]
- 67.Hardeland R, Rodriguez C. Versatile melatonin: A pervasive molecule serves various functions in signalling and protection. Chronobiol Int. 1995;12:157–165. [Google Scholar]
- 68.Fuhrberg B, Hardeland R, Poeggeler B, Behrmann G. Melatonin rises dramatically in Gonyaulax polyedra exposed to decreased temperature: Implications for photoperiodic cyst induction. Biol Rhythm Res. 1995;26:391–396. [Google Scholar]
- 69.Fuhrberg B, Hardeland R, Poeggeler B, Behrmann G. Dramatic rises of melatonin and 5-methoxytryptamine in Gonyaulax exposed to decreased temperatures. Biol Rhythm Res. 1997;28:144–150. [Google Scholar]
- 70.Murch SJ, Saxena PK. Mammalian neurohormones: Potential significance in reproductive physiology of St. John's wort (Hypericum perforatum L.) Naturwissenschaften. 2002;89:555–560. doi: 10.1007/s00114-002-0376-1. [DOI] [PubMed] [Google Scholar]
- 71.Lei XY, Zhu RY, Zhang GY, Dai YR. Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: The possible involvement of polyamines. J Pineal Res. 2004;36:126–131. doi: 10.1046/j.1600-079x.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 72.García JJ, Reiter RJ, Guerrero JM, Escames G, Yu BP, Oh CS, Muñoz-Hoyos A. Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. J Pineal Res. 1997;408:297–300. doi: 10.1016/s0014-5793(97)00447-x. [DOI] [PubMed] [Google Scholar]
- 73.van Tassel DL, O'Neill SD. Putative regulatory molecules in plants: Evaluating melatonin. J Pineal Res. 2001;31:1–7. doi: 10.1034/j.1600-079x.2001.310101.x. [DOI] [PubMed] [Google Scholar]
- 74.Cleland RE. Auxin and cell elongation. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. 3rd ed. Dordrecht: Kluwer Academic Publishers; 2004. pp. 204–220. [Google Scholar]
- 75.Hernandez-Ruiz J, Cano A, Arnao MB. Melatonin: A growth-stimulating compound present in lupin tissues. Planta. 2004;220:140–144. doi: 10.1007/s00425-004-1317-3. [DOI] [PubMed] [Google Scholar]
- 76.Hernandez-Ruiz J, Cano A, Arnao MB. Melatonin acts as a growth-stimulating compound in some monocot species. J Pineal Res. 2005;39:137–142. doi: 10.1111/j.1600-079X.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- 77.Tanimoto E. Regulation of root growth by plant hormones. Roles for auxin and gibberellin. Crit Rev Plant Sci. 2005;24:249–265. [Google Scholar]
- 78.Hager A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J Plant Res. 2003;116:483–505. doi: 10.1007/s10265-003-0110-x. [DOI] [PubMed] [Google Scholar]
- 79.Murch SJ, Campbell SSB, Saxena PK. The role of serotonin and melatonin in plant morphogenesis: Regulation of auxin-induced root organogenesis in in vitro cultured plants of St. John's wort. In vitro Cell Dev Biol Plant. 2001;37:786–793. [Google Scholar]
- 80.Kolar J, Machackova I. Melatonin in higher plants: Occurrence and possible functions. J Pineal Res. 2005;39:333–341. doi: 10.1111/j.1600-079X.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 81.Reppert SM. Melatonin receptors: Molecular biology of a new family of G protein-coupled receptors. J Biol Rhytm. 1997;12:528–531. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- 82.Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: Cloning and classification of subtypes. Trends Pharm Sci. 1996;17:100–102. doi: 10.1016/0165-6147(96)10005-5. [DOI] [PubMed] [Google Scholar]
- 83.Von Gall Ch, Stehle JH, Weaver DR. Mammalian melatonin receptors: Molecular biology and signal transduction. Cell Tiss Res. 2002;309:151–162. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- 84.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]