Abstract
Plants are continuously exposed to a wide variety of perturbations including variation of temperature and/or light, mechanical forces, gravity, air and soil pollution, drought, deficiency or surplus of nutrients, attacks by insects and pathogens, etc., and hence, it is essential for all plants to have survival sensory mechanisms against such perturbations. Consequently, plants generate various types of intracellular and intercellular electrical signals mostly in the form of action and variation potentials in response to these environmental changes. However, over a long period, only certain plants with rapid and highly noticeable responses for environmental stresses have received much attention from plant scientists. Of particular interest to our recent studies on ultra fast action potential measurements in green plants, we discuss in this review the evidence supporting the foundation for utilizing green plants as fast biosensors for molecular recognition of the direction of light, monitoring the environment, and detecting the insect attacks as well as the effects of pesticides, defoliants, uncouplers, and heavy metal pollutants.
Key Words: plant signaling, plant electrophysiology, action potential, biosensor
Introduction
A biosensor is defined as a device that either detects, records and transmits information related to a physiological change/process in a biological system, or uses biological materials to monitor the presence of various chemicals in a substance. In most successful biosensors, the principle behind the determination of a chemical or biological molecule is the specific interaction of such an analyte molecule with the biological material present in the biosensor probe device. Even though a variety of biological materials and transduction methods has been investigated in the development of novel biosensors, the most successful commercial systems include immobilized enzymes as the biological material and an electrochemical transducer.1,2 As an alternative to enzyme-based biosensors, a plant-tissue based biosensor was first developed in early eighties by immobilizing slices of yellow squash tissue as a CO2 gas sensor.3 Since then, a variety of plant and animal-tissues has been incorporated into various electrochemical transducers to detect and quantify a range of biologically important analytes including drugs, hormones, toxicants, neurotransmitters and amino acids.4–7 A detailed discussion on biosensors utilized in chemical or biological analysis is beyond the scope of this review. Based on our investigations on fast bioelectrochemical signaling events in green plants and similar examples reported in the literature by other plant scientists, here we discuss the evidence supporting the foundation for utilizing the entire green plant as a fast biosensor for monitoring the environmental perturbations in the close vicinity of a living plant.
Nerve cells in animals and phloem cells in plants share one fundamental property: they possess excitable membranes through which electrical excitations can propagate in the form of action potentials.8–12 Plants generate bioelectrochemical signals that resemble nerve impulses, and are present in plants at all evolutionary levels. Prior to the morphological differentiation of nervous tissues, the inducement of nonexcitability after excitation and the summation of subthreshold irritations were developed in the vegetative and animal kingdoms in protoplasmatic structures.13
The cells, tissues, and organs of plants transmit electrochemical impulses over short and long distances. It is conceivable that action potentials are the mediators for intercellular and intracellular communication in response to environmental irritants.14–21 Action potential is a momentary change in electrical potential on the surface of a cell that takes place when it is stimulated, especially by the transmission of an impulse.21 The variation potential (VP) also called slow hydraulic wave has been described as a gradual change in the electrical potential in plants in response to injurious stimulation such as wounding, crushing and a thermal shock.18 Little is known about the origin of the variation potential.
Initially, plants respond to irritants at the site of stimulation; however, excitation waves can be distributed across the membranes throughout the entire plant. Bioelectrical impulses travel from the root to the stem and vice versa. Chemical treatment, intensity of the irritation, mechanical wounding, previous excitations, temperature, and other irritants influence the speed of propagation.8,18,19
Conductive bundles of vegetative organisms sustain the flow of material and trigger the conduction of bioelectrical impulses. This feature supports the harmonization of biological processes involved in the fundamental activity of vegetative organisms.
The conduction of bioelectrochemical excitation is a rapid method of long distance signal transmission between plant tissues and organs. Plants quickly respond to changes in luminous intensity, osmotic pressure, temperature, cutting, mechanical stimulation, water availability, wounding, and chemical compounds such as herbicides, plant growth stimulants, salts, and water.8,18–21 Once initiated, electrical impulses can propagate to adjacent excitable cells. The change in transmembrane potential creates a wave of depolarization or action potential, which affects the adjoining resting membrane.21
The phloem is a sophisticated tissue in the vascular system of higher plants. Representing a continuum of plasma membranes, the phloem is a potential pathway for transmission of electrical signals. It consists of two types of conducting cells: the characteristic sieve-tube elements, and the companion cells. Sieve-tube elements are elongated cells that have end walls perforated by numerous minute pores through which dissolved materials can pass. Sieve-tube elements are connected in a vertical series known as sieve tubes. The companion cells have nuclei and they are adjacent to the sieve-tube elements. It is hypothesized that they control the process of conduction in the sieve tubes. Thus, when the phloem is stimulated at any point, the action potential is propagated over the entire cell membrane and along the phloem with a constant voltage.
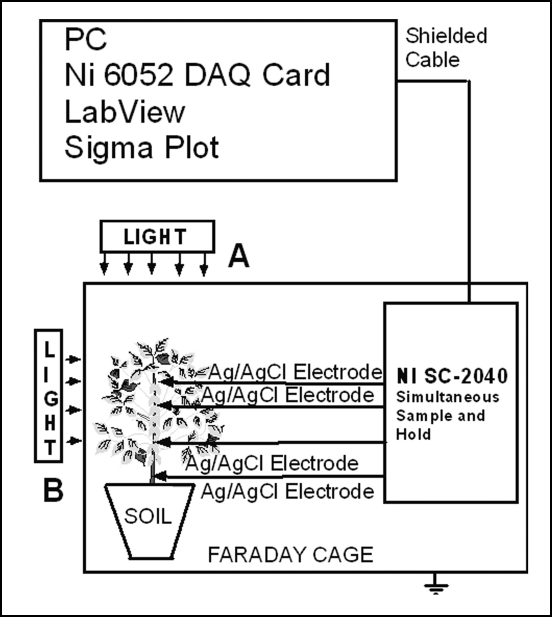
Electrical potentials have been measured at the tissue and whole plant level by using the experimental set-up described in Figure 1. Measurements were taken inside a Faraday cage mounted on a vibration-stabilized table. An IBM-compatible microcomputer with multi I/O plug-in data acquisition board NI 6052E DAQ (National Instruments) was interfaced through a NI SC-2040 Simultaneous Sample and Hold (National Instruments). The multifunction NI 6052E data acquisition board provides high resolution and a wide gain range and supports continuous, high-speed data acquisition. Single channels can be sampled at any gain up to 333 k samples/s. The digitized data includes negligible time skew (less than 50 ns) between channels. Measuring signals were recorded as ASCII files using LabView (National Instruments) software. Non-polarizable reversible Ag/AgCl electrodes were used to measure the electrical signals. The temperature was held constant since these electrodes are sensitive to the temperature. Ag/AgCl electrodes were prepared from Teflon coated silver wire (A-M Systems, Inc.). Plants were irradiated in directions A or B at different wavelengths using narrow band pass interference filters from GS Edmund Scientific (Barrington, NJ) with a central wavelength tolerance of ±1 nm.
Figure 1.
Experimental set-up for measuring electrical signals in green plants.
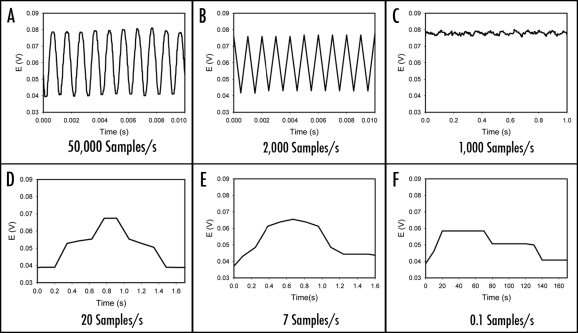
When electrochemical signals are measured, it is extremely important to take into consideration of the sampling rate that determines how often the measurement device samples an incoming analog signal. According to the sampling theorem, the original analog signal must be adequately sampled in order to be properly represented by the sampled signal. If the sampling rate is too slow, the rapid changes in the original signal in between any two consecutive samples cannot be accurately recorded. As a result, higher frequency components of the original signal will be misrepresented as lower frequencies. In signal processing, this problem is known as aliasing. According to the Nyquist Criterion, the sampling frequency must be at least twice the bandwidth of the signal to avoid aliasing. As illustrated in Figure 2A, a sinusoidal signal with 1 kHz frequency can be uniquely reconstructed from the digitized signal when sampled at a rate of 50,000 samples/s, a sampling rate well above the Nyquist frequency limit of 2,000 samples/s. However, when the sampling frequency is at the Nyquist frequency limit, the distortion begins as shown in Figure 2B. According to Figure 2C–2F, it is obviously impossible to reconstruct the original signal when it is under sampled at any frequency below the Nyquist Criterion limit, for example, at a sampling rate of 1000, 20, 7 or 0.1 samples/s, respectively. Undersampling may result in the mispresentation of the measured signal.
Figure 2.
Reconstructed 1 kHz sinusoidal signal from the digitized signal sampled at: (A) 50,000 samples/second, (B) the Nyquist rate of 2,000 samples/second; (C–F) Aliased 1 kHz sinusoidal signals due to undersampling at 2000, 1000, 20, 7 and 0.1 samples/second, respectively.
Ionic Stimulation
At the cellular level, electrical potentials exist across membranes, and thus between cellular and specific compartments. Figures 3 and 4 show solitary waves induced due to the deposition of a drop (100 µL) of KCl aqueous solution on the leave of Brassica juncea (Fig. 3) and tomato L. pennellii (Fig. 4). It is known, that 0.01–1 M KCl or 0.5–1 M NaCl solutions can induce action potentials in higher plants without generation of variation potentials.19,20,22–25 As reported in different publications, the speed of propgation of action potential induced by a drop of KCl solution varies from 0.25 cm/s to 15 m/s. The amplitude of the resting potential of plant cells also depends on KCl concentration.19,20,25 Electrolytic species such as K+, Ca2+, H+, Na+ and Cl− are actively involved in the establishment and modulation of electrical potentials. The highly selective ion channels serve as natural nanodevices. Voltage gated ion channels, as nanopotentiostats, regulate the flow of electrolytic species and determine the membrane potential.21,26
Figure 3.
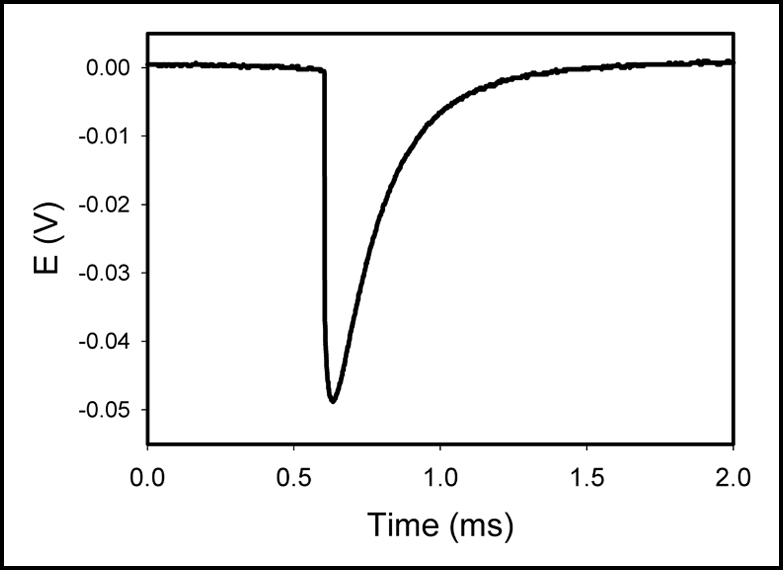
Electrical signals induced in Brassica juncea due to deposition of 100 µL of 1.0 M KCl solution on the leaf. The frequency of scanning (Data Acquisition Card NI-PCI-6115) was 1,000,000 samples per second. The volume of soil was 0.5 L. The soil around the plant was treated with water every day. Room temperature was 22°C. Humidity was 45–50%. The distance between Ag/AgCl electrodes was 2.5 cm.
Figure 4.
Electrical signals induced in tomato plant due to deposition of 100 µL of 1.0 M KCl solution on the leaf. The frequency of scanning (Data Acquisition Card NI-PCI-6115) was 400,000 samples per second. The volume of soil was 0.5 L. The soil around the plant was treated with water every day. Room temperature was 22°C. Humidity was 45–50%. The distance between two channels was 2.4 cm.
The cells of many biological organs generate an electric potential that may result in the flow of an electric current. Electrical impulses may arise spontaneously or they may result from stimulation. Once initiated, they can propagate to the adjacent excitable cells. The change in transmembrane potential creates a wave of depolarization, or action potential, that affects the adjoining membrane. As a result, when the phloem is stimulated at any point, the action potential is propagated over the entire cell membrane and along the phloem with a constant voltage.27
Plants are constantly responding with the external world in order to maintain homeostasis. Internal biological processes and their concomitant responses to the environment are closely associated with the phenomenon of excitability in plant cells. The extreme sensitivity of the protoplasm to chemical effects is the foundation for excitation. The excitable cells, tissues and organs alter their internal condition and external reactions under the influence of environmental factors, referred to as irritants; this excitability can be monitored. Plants generate different types of extracellular electrical events in connection to environmental stress.8,10,26–34 Recent findings have indicated that plants may use a common defense system to respond to various abiotic and biotic stresses, such as heat, cold, drought, flooding, osmotic shock, wounding, high light intensity, UV-radiation, ozone, and pathogens. Using cDNA microarrays, a large number of genes have been found to be coordinately regulated and overlap under different stresses.
Plants as Biosensors for Monitoring Atmospheric Electrochemistry: Effects of the Electrical Double Layer of the Earth and Acid Rain
The existence of ions in the atmosphere is the fundamental reason for atmospheric electricity. The voltage between the earth's surface and the ionosphere is approximately 40 kV, with an electrical current of approximately 2000 A, and a current density around 5 pA/m2. The earth is an electrode immersed in a weak gaseous electrolyte, the naturally ionized atmosphere. The earth's surface is negatively charged. The electrostatic field strength at the earth's surface is around 110–220 V/m, and depends on time of a day. It is approximately 110 V/m, however, at 7 pm GMT the electrostatic field strength is about 220–250 V/m. Oceans, lakes, and rivers cover a significant part of the earth, and their surface is also charged negatively against the atmosphere. Electrical polarity in soybean, potato, tomato and cacti coincides with the electrical field of the electric double layer of earth: negative in roots and positive at the top of the plants. Atmospheric change of the electrostatic field strength at 7 pm GMT does not induce action potentials or change in the variation potential of soybean or potato plants.
Acid rain is the most serious environmental problem and has impact on agriculture, forestry, and human health.35,36 Chemical reactions involving aerosol particles in the atmosphere are derived from the interaction of gaseous species with the liquid water. These reactions are associated with aerosol particles and dissolved electrolytes. For example, the generation of HONO from nitrogen oxides takes place at the air/water interface of seawater aerosols or in clouds. Clouds convert between 50% and 80% of SO2 to H2SO4. This process contributes to the formation of acid rain. Acid rain exerts a variety of influences on the environment by greatly increasing the solubility of different compounds, thus directly or indirectly affecting many forms of life.
Acid rain has a pH below 5.6. Sulfuric acid and nitric acid are the two predominant acids in acid rain. Approximately, 70% of the acid content in acid rain is sulfuric acid, with nitric acid contributing to the rest 30%. Spraying the soybean plant with an aqueous solution of H2SO4 in the pH region from 5.0 to 5.6 does not induce action potentials. However, action potentials were generated in soybean either by spraying the leaves of the plant (1 mL) or deposition of 10 µL drops of aqueous solution of H2SO4 or HNO3 in the pH region from 0 to 4.9 on leaves (Fig. 5). The duration of single action potentials after spraying the plant with HNO3 and H2SO4 was 0.2 s and 0.02 s, respectively.35,36
Figure 5.
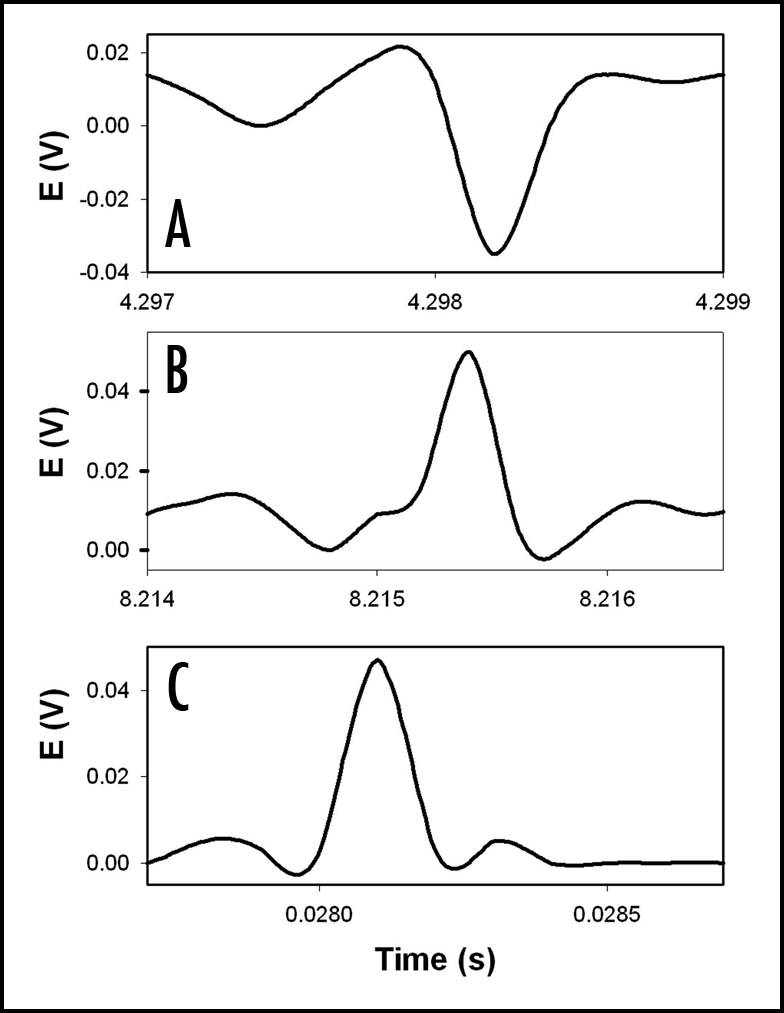
The potential difference between two silver/silver chloride electrodes in the stem of soybean (Glycine max (L) Merrill, cultivar Hutchenson) 1 (A), 15 (B), and 48 (C) hours after spraying 1 mL of 0.05 M sulfuric acid onto the plant. The distance between silver/silver chloride electrodes was 5 cm. The frequency of scanning was 10,000 samples per second. The volume of soil was 0.5 L. The soil around the plant was treated with water every day. Room temperature was 22°C. Humidity was 45–50%.
Electrical Signals Induced by Pesticides, Uncouplers, Protonophores and Heavy Metal Pollutants
In photosynthetic pathways, the radiation from the sun excites photosynthetic pigments. This excitement compels the pigments to donate electrons to the electron acceptors in the electron transport chain. Pheophytin is the first molecule to receive this energized electron. Proton gradients across the thylakoid membrane are established as a result of the charge transfer in the electron transport chain associated with sequential redox reaction. The energy produced from this gradient is used to drive ATP synthesis.37 It is at this stage where uncouplers have the ability to separate the flow of electrons in the electron transport chain and the H+ pump in ATP synthesis.8,37,38 Uncouplers are preventing the energy transfer from electron transport chain to ATPase. They are thought to uncouple oxidative phosphorylation.
Uncouplers are generally weak acids. These chemicals are often used to inhibit photosynthetic water oxidation due to their ability to become oxidized by the manganese cluster of the O2-evolving complex of photosystem II (PSII) and chloroplast.39 Most protonophoric uncouplers, widely used in photosynthesis research, are oxidized by the manganese cluster of the PSII O2-evolving complex in chloroplasts and inhibit photosynthetic water oxidation. Oxidized uncouplers can be reduced by the membrane pool of plastoquinone, leading to formation of an artificial cyclic electron transfer chain around PSII involving uncouplers as redox carriers. Protonophores such as carbonylcyanide m-chlorophenylhydrazone (CCCP), 2,3,4,5,6-pentachlorophenol (PCP), and 4,5,6,7-tetrachloro-2-trifluoromethylbenzimidazole (TTFB) inhibit the Hill reaction with K3Fe(CN)6 in chloroplast and cyanobacterial membranes. Inhibition of the Hill reaction by uncouplers reaches maximum when the pH corresponds to the pK values of these compounds.
Uncouplers promote autooxidation of the high-potential form of cytochrome b559 and partially convert it to lower potential forms. Protonophores uncouple electron transport, accelerate the deactivation of the S-2 and S-3 states on the donor side, and facilitate the oxidation of cytochrome b559 on the acceptor side of PSII.
Once oxidized, uncouplers can then be reduced by plastoquinone, thereby facilitating the formation of artificial cyclic electron transport chain around photosystem II involving uncouplers as redox carriers. Autooxidation of high potential cytochrome b559 isenhanced by the presence of uncouplers. Cytochrome b559 is also converted to low potential forms in the presence of chemical uncouplers.
Protonophores: (1) uncouple electron transport and H+ pump in ATP synthesis, (2) accelerate the deactivation of the S-2 and S-3 states on the donor side, and (3) facilitate oxidation of cytochrome b559 on the acceptor side of photosystem II. Although the interaction of proton-conducting ionophores with photosynthetic electron transport has been extensively studied during the past decade, the mode of action of protonophores remained uncertain. Electrochemical measurements in real time are required for a better understanding of the molecular mechanism of action of protonophores.
Pesticides PCP, 2,4-dinitrophenol (DNP), CCCP and carbonylcyanide-4-trifluoromethoxyphenylhydrazone (FCCP) act as insecticides and fungicides. PCP is the primary source of dioxins found in the environment. This pollutant is a defoliant and herbicide. PCP is utilized in termite control, wood preservation, seed treatmentand snail control. The pesticide DNP is used to manufacture dye and wood preservative. DNP is often found in pesticide runoff water. The electrochemical effects of CCCP, PCP, DNP and FCCP have been evaluated on soybean plants.14,15,40–44
CCCP decreased the variation potentials of soybean from 80–90 mV to 0 mV after 20 hours. CCCP induced fast action potentials in soybean with an amplitude of 60 mV.41 The maximum speed of propagation was 25 m/s. Exudation is a manifestation of the positive root pressure in the xylem. After treatment with CCCP, the exudation from cut stems of the soybean remains the same. Therefore, the addition of CCCP did not cause a change in the pressure, although it may influence the zeta potential due to depolarization.41
The addition of aqueous solution of PCP also causes the variation potential in soybeans to stabilize at 0 mV after 48 hours. Rapid action potentials are induced. These action potentials last for 2 ms, and have amplitudes of 60 mV. The speed of propagation is 12 ms−1; after 48 hours, the speed increased to 30 ms−1.
DNP induces fast action potentials and decreases the variation potential to zero in soybeans.14 The addition of aqueous DNP to the soil induces fast action potentials in soybeans. After treatment with an aqueous solution of DNP, the variation potential, measured between two Ag/AgCl electrodes in a stem of soybean, slowly decreases from 80–90 mV (negative in the root, positive on the top of the soybean) to 0 during a 48-hour time frame. The duration of single action potentials, 24 hours after treatment by DNP, varies from 3 s to 0.02 s. The amplitude of action potentials is about 60 mV. The maximum speed of action potential propagation is 1 m/s. After two days, the variation potential stabilized at 0. Fast action potentials were generated in soybean, with an amplitude of about 60 mV, 0.02 s duration time, and a speed of 2 m/s. Fromm and Spanswick studied the inhibiting effects of DNP on the excitability of willow by recording the resting potential in the phloem cells.45 In willow, 10−4 M DNP rapidly depolarized the membrane potential by about 50 mV.
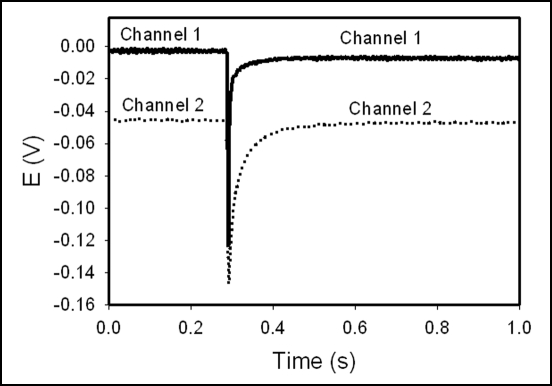
The FCCP also induced action potentials in soybean (Fig. 6). The maximum speed of these action potentials within 20 hours after the treatment was 10 ms−1. After 100 hours, the action potentials were still being produced. The amplitude of 60 mV remained constant. The duration was 0.3 ms, and the speed of propagation was 40 ms−1.15
Figure 6.
Potential difference between two Ag/AgCl electrodes in the stem of soybean (Glycine max (L) Merrill, cultivar Hutchenson) four (A) and 20 (B) hours after adding 30 mL of 10−6 M FCCP to soil. Distance between electrodes was 7 cm. The plants were given water every other day and kept at 24°C. Volume of soil was 0.5 L. Humidity was 45–50%.
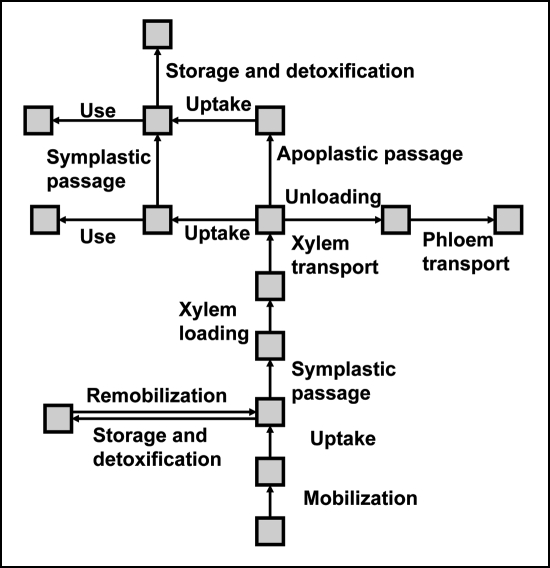
Constant release of hazardous metal pollutants into the environment has become a global problem. Contamination of soil, ground and surface waters with such pollutants can negatively affect all levels of an ecosystem, and thus, the clean up of contaminated soils and waters is one of the most important challenges the environmental scientists face today. By its very nature, the traditional clean-up methods are very expensive and time consuming. For example, the general approaches of clean-up of contaminated soil include: (i) treatment of soil itself in the ground; (ii) excavation of soil followed by either the treatment or relocation to a hazardous waste landfill; or (iii) containment of soil in place in order to prevent further leaching of contaminant into groundwater by rainfall, and/or direct contact with any living thing including plants. Considering the long list of polluted sites that have already been identified and the additional sites being added to the list as a result of continuous evaluation process, the estimated cost for environmental restoration will be well over several billion dollars. These issues have created the urgent need for development of effective, low-cost and sustainable clean-up technologies for environmental restoration. One such growing concept is the development of plant-based clean-up technologies for removal or detoxification of contaminants from soils and surface waters known as phytoremediation.46–50 To date, a diverse group of plants has been identified as capable of accumulating exceptionally high concentrations of metallic elements in their aboveground tissues. Most research on these so-called metal-hyperaccumulating plants has centered on thorough understanding of mechanisms of metal uptake, chelation, translocation, and sequestration. Such recent progress has led to valuable insights into the molecular understanding of metal accumulation and tolerance in hyperaccumulating plants. However, the knowledge on the genetic basis of such processes still remains limited.51 As illustrated in Figure 7, metal accumulation takes place in several steps: mobilization, uptake and sequestration, xylem transport, unloading, trafficking and storage.49 It is understood that every step has tremendous impact on the rate of metal accumulation process. In addition, there are several studies reported in the literature on the mechanisms of chemical defense against insect herbivores and pathogens.52–57
Figure 7.
The pathway of heavy metal accumulation in hyperaccumulating plants. Modified from reference 49.
These interesting observations together with our ongoing investigations on the role of ion channels in intercellular and intracellular bioelectrochemical signaling in green plants17,18,21 have inspired our attention on the use of hyperaccumulating plants as models for studying the impact of transition metals as ion channel blockers in green plants. Several transition metal ions are known to be the ion channel blockers for voltage-gated calcium ion channels at low concentrations.58,59 Presently, several experiments are underway to determine the impact of ion channel blockers on signal transduction process in green plants. For example, as shown in Figure 3, electrical signals are induced in Brassica juncea due to deposition of 100 µL of 1.0 M KCl solution on the leaf. The plant treated with potassium ion channel blocker, tetraethylammonium chloride (TEACl), failed to propagate such electrical activity after deposition of 100 µL of 1.0 M KCl solution on the leaf (Ranatunga DRA, Volkova-Gugeshashvili MI, and Volkov AG; unpublished results).
Insect-Induced Electrochemical Signals in Potato Plants
Volkov and Haack were the first to afford a unique opportunity to investigate the role of electrical signals induced by insects in long- distance communication in plants.32,60
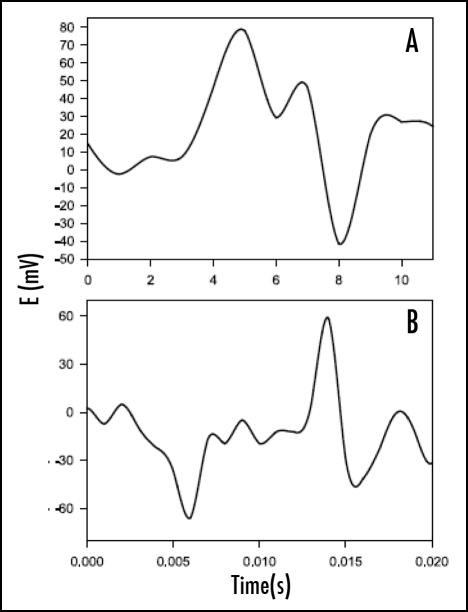
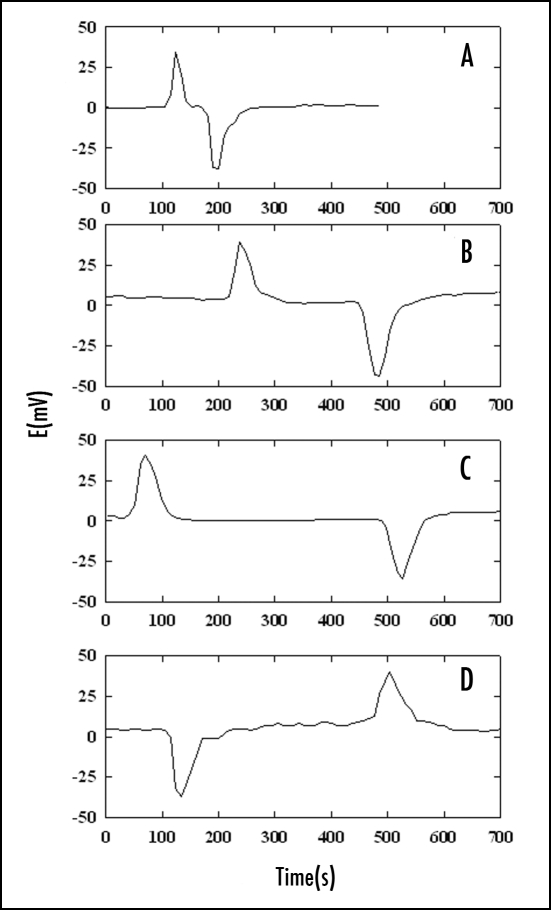
Action and resting potentials were measured in potato plants (Solanum tuberosum L.) in the presence of leaf-feeding larvae of the Colorado potato beetle (Leptinotarsa decemlineata (Say); Coleoptera: Chrysomelidae). When the larvae were allowed to consume upper leaves of the potato plants, after 6–10 hours, action potentials with amplitudes of 40 ± 10 mV were recorded every 2 ± 0.5 hours during a two-day test period. The resting potential decreased from 30 mV to a steady state level of 0 ± 5 mV. Figure 8 shows that positive spikes and negative humps appeared during measurement of electrical potential difference between two reversible silver chloride electrodes. The action potential induced by the Colorado potato beetle in potato plants propagates slowly and hence, the speed of propagation can be measured with two Ag/AgCl electrodes. The action potential propagates from plant leaves with Colorado potato beetles down the stem, and to the potato tuber.28 The speed of propagation of the action potential does not depend on the location of a working electrode in the stem of the plant or tuber, or the distance between the working and reference electrodes.60
Figure 8.
The action potentials in the stem of a potato plant (Solanum tuberosom L.) with Colorado beetles on the young terminal leaves. Distance between electrodes: (A) 3.5 cm; (B) 12 cm; (C) 22 cm; (D) 20 cm. The reference silver/silver chloride electrode was inserted in the stem between the cotyledons. The working silver/silver chloride electrode was inserted into the stem (A–C) or the tuber (D). The frequency of scanning was 10,000 samples per second. The volume of soil was 0.5 L. The soil around the plant was treated with water every day. Room temperature was 22°C. Humidity was 45–50%.
Molecular Recognition of the Direction of Light by Green Plants
Light is important for plant development by influencing nearly all aspects of the life cycle from germination to flowering. Plants perceive light ranging from ultraviolet to far-red light by specific photoreceptors. Natural radiation simultaneously activates more than one photoreceptor in higher plants. These receptors initiate distinct signaling pathways leading to wavelength-specific light responses. The three classes of plant photoreceptors that have been identified at the molecular level are phototropins, cryptochromes, and phytochromes.
Phototropin is a blue light (360–500 nm) flavoprotein photoreceptor responsible for phototropism and chloroplast orientation. The phototropins, such as phot1 and phot2, are a family of flavoproteins that function as the primary photoreceptors in plant phototropism and in intracellular chloroplast movements. Phot1 contains two 12 kD flavin mononucleotide binding domains. LOV1 (light, oxygen, and voltage) and LOV2 are found within its N-terminal region and a C-terminal serine/threonine protein kinase domain. The protein conformation changes in light-activated phototropin. Phot1 and phot2 bind FMN and undergo light- dependent autophosphorylation. Phot2 is localized in the plasma membrane.61,62
Phytochrome (phy) is a protein photoreceptor that regulates many aspects of plant development. Plant phytochromes are also light-modulated protein kinases that process dual ATP-dependent autophosphorylation and protein phosphotransferase activities.63
Cryptochromes (cry1 and cry2) are flavoproteins in the family of photoreceptors responsible for photomorphogenesis.64,65 They perceive (UV-A) light as well as blue light (360–500 nm). Although cryptochromes and phototropin share many similarities, they have different transduction pathways. Cry1 plays a significant role in the synthesis of anthocyanin and in the entrainment of circadian rhythms. Cry2 plays a part in the photoperiodic flowering and cotyledon expansion. Cryptochromes were predominantly found in the nucleus. Stomatal opening is also stimulated by blue light and UV irradiation. Zeaxanthin has been proposed to be the blue/green light photoreceptor.66
Phototropism is one of the best-known plant tropic responses. A positive phototropic response is characterized by a bending or turning toward the source of light. When plants bend or turn away from the source of light, the phototropic response is considered negative. A phototropic response is a sequence of the four following processes: reception of the directional light signal, signal transduction, transformation of the signal to a physiological response, and the production of directional growth response.
Inside the Faraday cage the soybean plant was irradiated in the direction A (Fig. 1) with white light for two days with a 12:12 hr light:dark photoperiod prior to the conduction of experiments. Action potentials are not generated when the lights are turned off and on. Changing the direction of irradiation from direction A to direction B generates action potentials in soybean approximately after 1–2 min (Fig. 9). These action potentials depend on the wavelength of irradiation light. Irradiation at wavelengths 400–500 nm induces fast action potentials in soybean with duration time of about 0.3 ms; conversely, the irradiation of soybean in the direction B at wavelengths between 500 and 630 nm fails to generate action potentials. Irradiation at wavelengths between 500 nm and 700 nm does not induce phototropism. Irradiation of soybean by blue light induces positive phototropism.67
Figure 9.
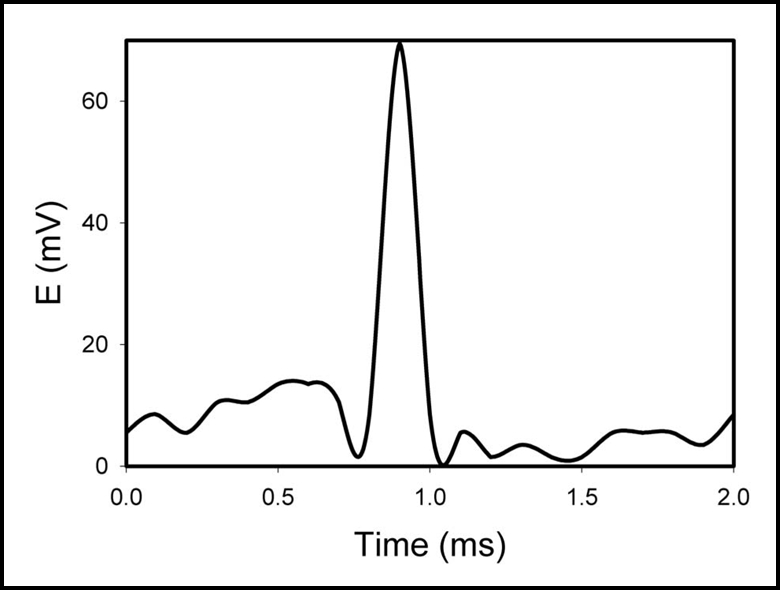
Action potentials in soybean (Glycine max (L) Merrill) induced by changing the direction of white light irradiation from the direction A to B as shown on Figure 1. Irradiance was 10 mE/m2s. Distance between electrodes was 5 cm. The soil was preliminary treated by water every day. Volume of soil was 0.5 L. Frequency of scanning was 50,000 samples/s.
The sensitive membranes in phloem cells facilitate the passage of electrical excitations in the form of action potentials. The action potential has a stereotyped form and an essentially fixed amplitude—an “all or none” response to a stimulus. Each impulse is followed by the absolute refractory period.27 The fiber cannot transmit a second impulse during the refractory period. The integral organism of a plant can be maintained and developed in a continuously varying environment only if all cells, tissues, and organs function in concordance.
These propagating excitations are modeled theoretically as traveling wave solutions of certain parameter-dependent non-linear reaction-diffusion equations coupled with some non-linear ordinary differential equations. These traveling wave solutions can be classified as single and multiple loop pulses, fronts and backs of periodic waves of different wave speed. This classification is matched by the classification of the electrochemical responses observed in plants. The experimental observations also show that under the influence of various pathogens, the shapes and speeds of the electrochemical responses undergo changes. From the theoretical perspective, the changes in the shapes and wave speeds of the traveling waves can be accounted by appropriate changes in parameters in the corresponding non-linear differential equations.
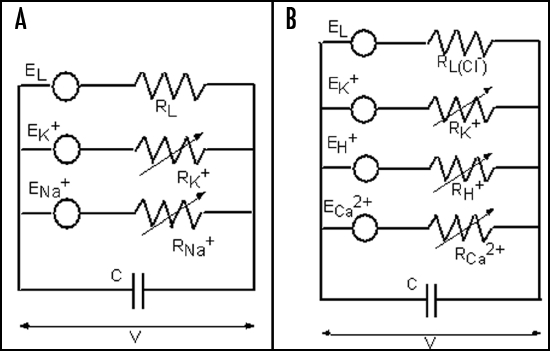
Hodgkin and Huxley's membrane model accounts for K+, Na+ and ion leakage channels in squid giant axons (Fig. 10A). The membrane resting potential for each ion species is treated like a battery and a variable resistor models the degree to which the channel is open.
Figure 10.
The Hodgkin-Huxley (HH) equivalent circuit for an axon (A) and the modified HH circuit for sieve tubes in phloem (B).
Fromm and Spanswick discovered that the electric stimulation of the plant is followed by ion shifts, which is most striking in the phloem cells.45 The amount of cytoplasmic calcium increased slightly while the content of K+ and Cl− was diminished after stimulation. Such evidence leads to the conclusion that Ca2+ influx as well as K+ and Cl− efflux is involved in the propagation of action potentials. In an axon there is the K+ and Na+ transmembrane transport; conversely, in phloem cells the K+, Ca2+ and more than likely H+ channels are involved in this process (Fig. 10B).
Babourina et al. have found that blue light induces significant changes in activity of H+ and Ca2+ transporters within the first 10 minutes of exposure to blue light onset, peaking between 3 and 5 minutes.68 Blue light induced the opening of potassium and anion channels in plants and plant cells.
Some voltage-gated ion channels work as plasma membrane nanopotentiostats. A blocker of K+ ion channels such as tetraethylammonium chloride (TEACl) stops the propagation of action potentials in soybean induced by blue light and inhibits phototropism in soybean plants. Voltage-gated ionic channels control the plasma membrane potential and the movement of ions across membranes; thereby, regulating various biological functions. These biological nanodevices play vital roles in signal transduction in higher plants.
TEACl blocks voltage-gated potassium channels.26 The propagation of action potentials with a constant amplitude and speed depends on the work of ion channels. TEACl inhibits the propagation of action potentials induced by blue light as well as phototropism in soybean plants.
The duration of the action potential is not influenced by the location of the working electrode in the stem or leaves of the plant, or on the distance between the working and reference electrodes. Action potentials take an active part in the expedient character of response reactions of plants as a reply to external changes. These impulses transfer a signal about the changes of conditions in a conducting bundle of a plant from the root system to the point of growth and from the point of growth to the root system. Solitary waves due to impulses generated by changes in environmental conditions function as carriers of information in soybean.
Green plants sense many parameters in order to adapt to the environment. Figure 11 illustrates the mechanism of biosignaling in green plants.
Figure 11.
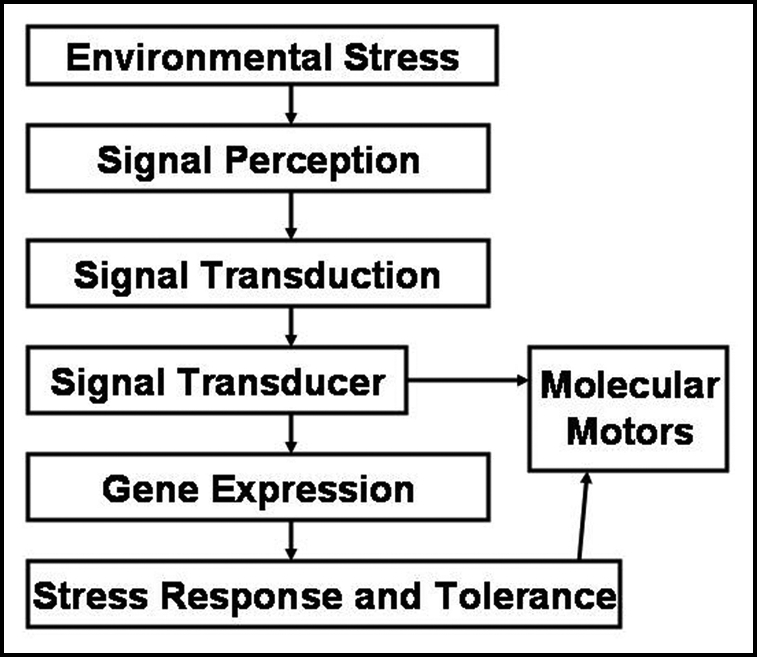
Mechanism of biosignaling in green plants.
Gravitropism in Plants
Gravitropism is the ability of plant organs for directional growth as a result of the gravitational force of the earth. Naturally, the powerful gravitational force dictates most of other environmental stimuli and influences the growth and development of a plant. Primary roots grow downward reaching for water and mineral ions and shoots grow upward facilitating the efficient photosynthesis. These two differential growth patterns, toward and away from the earth's center of gravity, are known as positive and negative gravitropism, respectively.
Since the early postulation on the importance of the root tip, the cap, as the essential element for graviperception to generate the physiological signal,69 developments have been made in the understanding of physiological and molecular processes fundamental to the root gravitropism.70–75. Understanding the details of gravitropism at the molecular level is essential since the perceived gravitropic stimulus triggers several important physiological processes including signal formation, intracellular and intercellular signal transduction, followed by growth control leading to bending and reorientation of the responding organ.
Recently, the more intelligent behavior of plant root apices as command centers has been critically discussed and reviewed based on the new information obtained from electrophysiology and cell/molecular biology of higher plants.76,77 As the authors emphasized, this would definitely lead to a breakthrough in ‘nervous plant biology’ as well as new discoveries closing the gap between the intelligent behavior of plants and animals.
In literature, the mechanism of gravitropism is explained by two long-surviving hypotheses: the Cholodney-Went hypothesis and the starch-statolith hypothesis. The Cholodny-Went hypothesis suggests that the gravitropic curvature is due to lateral transport of auxin across the gravistimulated plant organs resulting asymmetric growth. According to the starch-statolith hypothesis, the gravity perception in roots occurs in the root cap due to sedimentation of starch-filled amyloplasts within the cells in the columella, the central region of the root cap.78 Laser ablation experiments to remove the innermost columella cells with the highest amyloplast-sedimentation velocities in the root cap of Arabidopsis primary cells have resulted the strongest inhibitory effect in response to gravity.79
It is suggested that the sedimenting amyloplasts can disrupt the local actin filaments in the plant cytoskeleton resulting the activation of mechanosensitive ion channels in the plasma and/or intracellular membrane,80 followed by rapid increases in cytoplasmic ion levels.81 Despite the ongoing studies, fully understanding of the mechanism by which the physiological or biochemical signal is generated from the physical signal due to amyloplast sedimentation still remains unanswered.
Even though the measurement of electrical potentials induced due to gravitropism in higher plants has long been known,82 the concept has been rarely studied until recently. For example, fast extracellular gravielectric potentials with maximum amplitude of 17 mV have been observed in soybean hypocotyls due to directional change in the gravity vector.83 A transient of rapid surface potential with about 10 mV has resulted in gravistimulated bean epicotyls approximately after 30–120 s following gravistimulation.84 Recently, the current understanding of the electrophysiology of plant gravitropism is reviewed by Stankovic.75 A detailed understanding of plant's response to gravity should provide valuable insights into future research programs in space plant biology and horticulture.
Mechanosensation in Plants
Plant response to mechanical stimulation has long been known.69 Perhaps all plants can react in response to the mechanical stimuli, only certain plants with rapid and highly noticeable touch-stimulus response have received much attention; for example, the trap closure of Venus' flytrap Dionaea muscipula.85–87 In contrast, such actions in their counterparts with only very slow response to the perturbation may not be immediately recognized.
Mechanosensation is a physiological process by which a distortion of the cell membrane is converted into an electrical and/or biochemical signal. Since mechanical forces are all over the place, it would have been essential for all living cells including the earliest microorganisms on earth to have a survival mechanism against these forces. For this reason, mechanosensation is considered to have evolved as one of the oldest sensory mechanisms in living organisms.
The concept of mechanosensitive ion channels was primarily developed based on studies of specialized mechanosensory neurons.88–90 Due to the applied mechanical force on the cell membrane, these mechanically-gated channels capable of converting mechanical stress into electrical or biochemical signals are activated. As a result, they act as molecular transducers and play a vital role in regulating various physiological processes responsible for growth and development in all forms of life, as well as monitoring the surrounding environmental challenges for survival; for example, turgor control in plants, and touch and hearing in animals.91 Although the current understanding of the structure and function of mechanosensitive ion channels found in living organisms is limited, significant progress has recently been made in the area of evolutionary origins of mechanosensitive ion channels.92–95
The patch-clamp technique96 has provided the tool for identification of two basic types of mechanosensitive ion channels found in living cells: stretch-activated and stretch-inactivated ion channels.97 Cosgrove and Hedrich98 studied the mechanosensory channels in the plasma membrane of guard cells of Vicia faba L. using the patch-clamp technique, and identified three coexisting stretched-activated calcium, potassium and chloride ion channels. It has been found that such mechanosensitive ion channels play a vital role in the physiological function of the plant by controlling the ion transport across the plasma membrane, and hence influencing volume and turgor regulation of guard cells.
Action potentials induced by mechanical wounding have been measured by Volkov and Haack32,60 in the stem tissue of potato plants (Solanum tuberosum L.) using a few Ag/AgCl electrodes and an amplifier-recording system. Mechanical wounding due to a pinch along the midrib in the center of the young terminal leaflet by forceps induced the propagation of an action potential along the stem, probably via the phloem.
It is considered that the reactive oxygen intermediates, such as superoxides, hydrogen peroxide and hydroxyl radicals, play a vital role in the stress-resistance network.99 For instance, when a pathogen attack occurs at the plant, an instantaneous local production of these intermediates is induced at the site of attack in order to initiate the local defense mechanism. The electrical signals originated at the site of attack will be used to communicate with other parts of the plant. Further attacks are prevented due to systemic rapid release of reactive oxygen intermediates by distal cells.
Future Perspective
Green plants interfaced with a computer through data acquisition systems can be used as fast biosensors for monitoring the environment; detecting the effects of pollutants, pesticides and defoliants; predicting and monitoring climate changes; and in agriculture, directing and fast controlling of conditions influencing the harvest. The use of new computerized methods provides opportunities for detection of fast action potentials in green plants in real time.
Acknowledgements
This work has been supported by the National Science Foundation grant DMR-0521611, National Institutes of Health grant G11-HD37200-06 and NASA grant NAG8-1888.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3000
References
- 1.Volkov AG, Deamer DW, Tanelian DI, Markin VS. Liquid Interfaces in Chemistry and Biology. New York: J Wiley; 1998. [Google Scholar]
- 2.Volkov AG, editor. Liquid Interfaces in Chemical, Biological, and Pharmaceutical Applications. New York: M Dekker; 2001. [Google Scholar]
- 3.Kuriyama S, Rechnitz GA. Plant tissue-based bioselective membrane electrode for glutamate. Anal Chim Acta. 1981;131:91–96. [Google Scholar]
- 4.Sidwell JS, Rechnitz GA. Progress and challenges for biosensors using plant tissue materials. Biosensors. 1986;2:221–233. [Google Scholar]
- 5.Wijesuriya DC, Rechnitz GA. Biosensors based on plant and animal tissues. Biosensors & Bioelectronics. 1993;8:155–160. doi: 10.1016/0956-5663(93)85027-l. [DOI] [PubMed] [Google Scholar]
- 6.He X, Rechnitz GA. Plant tissue-based fiber-optic pyruvate sensor. Anal Chim Acta. 1995;316:57–63. [Google Scholar]
- 7.Liawruangrath S, Oungpipat W, Watanesk S, Liawruangrath B, Dongduen C, Purachat P. Asparagus-based amperometric sensor for fluoride determination. Anal Chim Acta. 2001;448:37–46. [Google Scholar]
- 8.Ksenzhek OS, Volkov AG. Plant Energetics. San Diego: Academic Press; 1998. [Google Scholar]
- 9.Bertholon M. De l'electricite des vegetaux : ouvrage dans lequel on traite de l'electricite de l'atmosphere sur les plantes, de ses effets sur l'economie des vegetaux, de leurs vertus medicaux. Paris: PF Didotjeune; 1783. (Fre). [Google Scholar]
- 10.Bose JC. Transmission of stimuli in plants. Nature. 1925;115:457. [Google Scholar]
- 11.Volkov AG. Green plants: Electrochemical interfaces. J Electroanal Chem. 2000;483:150–156. [Google Scholar]
- 12.Volkov AG, Mwesigwa J. Interfacial electrical phenomena in green plants: action potentials. In: Volkov AG, editor. Liquid Interfaces in Chemical, Biological, and Pharmaceutical Applications. New York: M Dekker; 2001. pp. 649–681. [Google Scholar]
- 13.Goldsworthy A. The evolution of plant action potentials. J Theor Biol. 1983;103:645–648. [Google Scholar]
- 14.Mwesigwa J, Collins DJ, Volkov AG. Electrochemical signaling in green plants: effects of 2,4-dinitrophenol on resting and action potentials in soybean. Bioelectrochem. 2000;51:201–205. doi: 10.1016/s0302-4598(00)00075-1. [DOI] [PubMed] [Google Scholar]
- 15.Shvetsova T, Mwesigwa J, Volkov AG. Plant electrophysiology: FCCP induces fast electrical signaling in soybean. Plant Science. 2001;161:901–909. [Google Scholar]
- 16.Sinukhin AM, Britikov EA. Action potentials in the reproductive system of plant. Nature. 1967;215:1278–1280. [Google Scholar]
- 17.Volkov AG. Electrophysiology and Phototropism. In: Balushka F, Mancuso S, Volkman D, editors. Communication in Plants. Neuronal Aspects of Plant Life. Berlin, New York: Springer-Verlag; 2006. pp. 351–367. [Google Scholar]
- 18.Volkov AG, editor. Plant Electrophysiology. New York, Berlin: Springer; 2006. [Google Scholar]
- 19.Opritov VA, Pyatygin SS, Retivin VG. Bioelectrogenesis in Higher Plants. Moscow: Nauka; 1991. [Google Scholar]
- 20.Sinyukhin AM, Gorchakov VV. Action potentials of higher plants not possessing motor activity. Russian J Plant Physiol. 1966;11:840–846. [PubMed] [Google Scholar]
- 21.Volkov AG, Brown CL. Electrochemistry of Plant Life. In: Volkov AG, editor. Plant Electrophysiology. Berlin, New York: Springer; 2006. [Google Scholar]
- 22.Mamulashvili GG, Krasavina MS, Lyalin OO. Comparative study of electrical activity of the root and stem of plants. Russ J Plant Physiol. 1972;19: 51–57. [Google Scholar]
- 23.Mamulashvili GG, Krasavina MS, Lyalin OO. Role of different stem tissues in transmission of excitation. Russ J Plant Physiol. 1973;20:442–450. [Google Scholar]
- 24.Vyskrebentseva EI, Sinyukhin AM, Krasavina MS. Effect of adenine on conductivity of action potentials arising in pumkin stems under potassium starvation. Sov. Plant Physiol. 1970;17:845–850. [Google Scholar]
- 25.Jacobson SL. Effect of ionic environment on the response of the sensory hair of Venus's-flytrap. Can J Bot. 1974;52:1293–1302. [Google Scholar]
- 26.Volkov AG, Dunkley T, Labady A, Brown C. Phototropism and electrified interfaces in green plants. Electrochim Acta. 2005;50:4241–4247. [Google Scholar]
- 27.Volkov AG, Shvetsova T, Markin VS. Waves of excitation and action potentials in green plants. Biophys J. 2002;82:218a. [Google Scholar]
- 28.Volkov AG, Jovanov E. Electrical signaling in green plants: action potentials. In: Jan J, Kozumplik J, Provaznik J, editors. Analysis of Biomedical Signals and Images. Brno: Vutum Press; 2002. pp. 36–38. [Google Scholar]
- 29.Fromm J, Bauer T. Action potentials in maize sieve tubes change phloem translocation. J Experimental Botany. 1994;45:463–469. [Google Scholar]
- 30.Davies E. New functions for electrical signals in plants. New Phytol. 2004;161:607–610. doi: 10.1111/j.1469-8137.2003.01018.x. [DOI] [PubMed] [Google Scholar]
- 31.Volkov AG, Labady A, Thomas D'J, Shvetsova T. Green plants as environmental biosensors: electrochemical effects of carbonyl cyanide 3-chlorophenylhydrazone on soybean. Analytical Sci. 2001;17:i359–i362. [Google Scholar]
- 32.Volkov AG, Haack RA. Bioelectrochemical signals in potato plants. Russ J Plant Physiol. 1995;42:17–23. [Google Scholar]
- 33.Lautner S, Grams TEE, Matyssek R, Fromm J. Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol. 2005;138:2200–2209. doi: 10.1104/pp.105.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stankovic B, Davies E. Wounding evokes rapid changes in tissue deformation, electrical potential, transcription, and translation in tomato. Plant & Cell Physiol. 1997;39:268–274. [Google Scholar]
- 35.Shvetsova T, Mwesigwa J, Labady A, Kelly S, Thomas D'J, Lewis K, Volkov AG. Soybean electrophysiology: Effects of acid rain. Plant Science. 2002;162:723–731. [Google Scholar]
- 36.Volkov AG, Mwesigwa J, Jovanov E, Labady A, Thomas D'J, Lewis K, Shvetsova T. In: Cerutti S, Akay M, Mainardi LT, Sato S, Zywietz C, editors. Acid Rain Induces Action Potentials in Green Plants; Proceedings IV International Workshop on Biosignal Interpretation BSI2002; 2002. pp. 13–17. [Google Scholar]
- 37.Yaguzhinsky LS, Boguslavsky LI, Volkov AG, Rakhmaninova AB. Synthesis of ATP coupled with action of membrane protonic pumps at the octane-water interface. Nature. 1975;259:494–496. doi: 10.1038/259494a0. [DOI] [PubMed] [Google Scholar]
- 38.Volkov AG, editor. Interfacial Catalysis. New York: M Dekker; 2003. [Google Scholar]
- 39.Volkov AG. Oxygen evolution in the course of photosynthesis. Bioelectrochem Bioenerg. 1989;21:3–24. [Google Scholar]
- 40.Volkov AG, Collins DJ, Mwesigwa J. Plant electrophysiology: pentachlorophenol induces fast action potentials in soybean. Plant Science. 2000;153:185–190. doi: 10.1016/s0168-9452(99)00271-x. [DOI] [PubMed] [Google Scholar]
- 41.Labady A, Thomas D'J, Shvetsova T, Volkov AG. Plant electrophysiology: excitation waves and effects of CCCP on electrical signaling in soybean. Bioelectrochem. 2002;57:47–53. doi: 10.1016/s1567-5394(01)00175-x. [DOI] [PubMed] [Google Scholar]
- 42.Volkov AG, Mwesigwa J. Electrochemistry of soybean: effects of uncouplers, pollutants, and pesticides. J Electroanal Chem. 2001;496:153–157. [Google Scholar]
- 43.Volkov AG, Mwesigwa J, Shvetsova T. Soybean as an environmental biosensor: Action potentials and excitation waves. In: Butler M, Vanysek P, Yamazoe N, editors. Chemical and Biological Sensors and Analytical Methods II. Pennington: The Electrochemical Society; 2001. pp. 229–238. [Google Scholar]
- 44.Volkov AG, Dunkley TC, Labady AJ, Ruff D, Morgan SA. Electrochemical signaling in green plants induced by photosensory systems: Molecular recognition of the direction of light. In: Bruckner-Lea C, Hunter G, Miura K, Vanysek P, Egashira M, Mizutani F, editors. Chemical Sensors VI: Chemical and Biological Sensors and Analytical Methods. Pennington: The Electrochemical Society; 2004. pp. 344–353. [Google Scholar]
- 45.Fromm J, Spanswick R. Characteristics of action potentials in willow (Salix viminalis L.) J Exp Bot. 1993;44:1119–1125. [Google Scholar]
- 46.Chaney RL. Plant uptake of organic waste. In: Parr JE, Marsh PB, Kla JM, editors. Land treatment of hazardous wastes. Park Ridge, IL: Noyes Data; 1983. pp. 50–76. [Google Scholar]
- 47.Salt DE, Smith RD, Raskin I. Phytoremediation. Ann Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- 48.Kramer U, Chardonnens AN. The use of transgenic plants in the bioremediation of soils contaminated with trace elements. Appl Microbiol Biotechnol. 2001;55:661–672. doi: 10.1007/s002530100631. [DOI] [PubMed] [Google Scholar]
- 49.Clemens S, Plamgren MG, Kramer U. A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci. 2002;7:309–315. doi: 10.1016/s1360-1385(02)02295-1. [DOI] [PubMed] [Google Scholar]
- 50.Kramer U. Phytoremediation: novel approaches to cleaning up polluted soils. Curr Opin Biotechnol. 2005;16:133–141. doi: 10.1016/j.copbio.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Pollard AJ, Powell KD, Harper FA, Smith JAC. The genetic basis of metal hyperaccumulation in plants. Crit Rev Plant Sci. 2002;21:539–566. [Google Scholar]
- 52.Pollard AJ, Baker AJM. Deterrence of herbivory by zinc hyperaccumulation in Thlaspi caerulescens (Brassicaceae) New Phytol. 1997;135:655–658. [Google Scholar]
- 53.Martens SN, Boyd RS. The defensive role of Ni hyperaccumulation by plants: a field experiment. Amer J Botany. 2002;89:998–1003. doi: 10.3732/ajb.89.6.998. [DOI] [PubMed] [Google Scholar]
- 54.Behmer ST, Lloyd CM, Raubenheimer D, Stewart-Clark J, Knight J, Leighton RS, Harper HA, Smith JAC. Metal hyperaccumulation in plants: mechanisms of defence against insect herbivores. Functional Ecology. 2005;19:55–66. [Google Scholar]
- 55.Boyd RS. Hyperaccumulation as a plant defensive strategy. In: Brooks RR, editor. Plants that hyperaccumulate heavy metals. Wallingford, UK: CAB International; 1998. pp. 181–201. [Google Scholar]
- 56.Boyd RS, Shaw JJ, Martens SN. Nickel hyperaccumulation defends Streptanthus polygaloides (Brassicaceae) against pathogens. Amer J Botany. 1994;81:294–300. [Google Scholar]
- 57.Ghaderian SM, Lyon AJE, Baker AJM. Seedling mortality of metal hyperaccumulator plants resulting from damping-off by Pythium spp. New Phytol. 2000;146:219–224. doi: 10.1046/j.1469-8137.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 58.Hille B. Ion Channels of Excitable Membranes. 3rd Ed. Sunderland, MA: Sinaur; 2001. [Google Scholar]
- 59.Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 60.Volkov AG, Haack RA. Insect induced bioelectrochemical signals in potato plants. Bioelectrochem Bioenerg. 1995;35:55–60. [Google Scholar]
- 61.Casal JJ. Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol. 2000;71:1–11. doi: 10.1562/0031-8655(2000)071<0001:pcppii>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 62.Short TW, Briggs WR. The transduction of blue light signals in higher plants. Ann Rev Plant Physiol Plant Mol Biol. 1994;45:143–171. [Google Scholar]
- 63.Quail PH. An emerging molecular map of the phytochromes. Plant Cell Environ. 1997;20:657–665. [Google Scholar]
- 64.Ahmad M, Jarillo JA, Smirnova O, Cashmore AR. Cryptochrome blue-light photoreceptors implicated in phototropism. Nature. 1998;392:720–723. doi: 10.1038/33701. [DOI] [PubMed] [Google Scholar]
- 65.Cashmore AR, Jarillo JA, Wu YJ, Liu D. Cryptochromes: blue light receptors for plants and animals. Science. 1999;284:760–765. doi: 10.1126/science.284.5415.760. [DOI] [PubMed] [Google Scholar]
- 66.Frechilla S, Talbott LD, Bogomolni RA, Zeiger E. Reversal of blue light-stimulated stomatal opening by green light. Plant Physiol. 2000;122:99–106. doi: 10.1093/pcp/41.2.171. [DOI] [PubMed] [Google Scholar]
- 67.Volkov AG, Dunkley TC, Morgan SA, Ruff D, II, Boyce Y, Labady AJ. Bioelectrochemical Signaling in Green Plants Induced by Photosensory Systems. Bioelectrochem. 2004;63:914. doi: 10.1016/j.bioelechem.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 68.Babourina O, Newman I, Shabala S. Blue light-induced kinetics of H+ and Ca2+ fluxes in etiolated wild-type and phototropin-mutant Arabidopsis seedlings. Proc Natl Acad Sci USA. 2002;99:2433–2438. doi: 10.1073/pnas.042294599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darwin C. The Power of Movement in Plants. London: John Murray; 1880. [Google Scholar]
- 70.Chen AM, Rosen ES, Masson PH. Update: gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tasaka M, Kato T, Fukaki H. The endodermis and shoot gravitropism. Trends Plant Sci. 1999;4:103–107. doi: 10.1016/s1360-1385(99)01376-x. [DOI] [PubMed] [Google Scholar]
- 72.Blancaflor EB, Masson PH. Plant Gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol. 2003;133:1677–1690. doi: 10.1104/pp.103.032169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morita MT, Tasaka M. Gravity sensing and signaling. Curr Opin Plant Biol. 2004;7:712–718. doi: 10.1016/j.pbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Davies E, Stankovic B. Electrical signals, the cytoskeleton, and gene expression: a hypothesis on the coherence of the cellular responses to environmental insult. In: Balushka F, Mancuso S, Volkman D, editors. Communication in Plants. Neuronal Aspects of Plant Life. Berlin, New York: Springer-Verlag; 2006. pp. 309–320. [Google Scholar]
- 75.Stankovic B. Electrophysiology of plant gravitropism. In: Volkov AG, editor. Plant Electrophysiology. Berlin, New York: Springer-Verlag; 2006. [Google Scholar]
- 76.Baluska F, Mancuso S, Volkmann D, Barlow P. Root apices as plant command centers: the unique ‘brain-like” status of the root apex transition zone. Biologia (Bratisl) 2004;13(Suppl):1–13. [Google Scholar]
- 77.Baluska F, Volkmann D, Menzel D. Plant synapses: actin-based domains for cell-to-cell communication. Trends Plant Sci. 2005;10:106–111. doi: 10.1016/j.tplants.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Sack FD. Plant gravity sensing. Intl Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- 79.Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoder TL, Zeng HQ, Todd P, Staehelin LA. Amyloplast sedimentation dynamics in maize columella cells support a new model for gravity-sensing apparatus of roots. Plant Physiol. 2001;125:1045–1060. doi: 10.1104/pp.125.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boonsirichai K, Guan C, Chae R, Masson PH. Root gravitropism: an experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Ann Rev Plant Biol. 2002;53:421–447. doi: 10.1146/annurev.arplant.53.100301.135158. [DOI] [PubMed] [Google Scholar]
- 82.Bose JC. Comparative electro-physiology, a physico-physiological study. London, New York: Longmans, Green & Co.; 1907. [Google Scholar]
- 83.Tanada T, Vinten-Johansen C. Gravity induces fast electrical field change in soybean hypocotyls. Plant Cell Environ. 1980;3:127–130. [Google Scholar]
- 84.Shigematsu H, Toko K, Matsuno T, Yamafuji K. Early gravi-electrical responses in bean epicotyls. Plant Physiol. 1994;105:875–880. doi: 10.1104/pp.105.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fagerberg WR, Allain D. A quantitative study of tissue dynamics during closure in the traps of venus's flytrap Dianaea muscipula ellis. Amer J Botany. 1991;78:647–657. [Google Scholar]
- 86.Braam J. In touch: plant responses to mechanical stimuli. New Phytol. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 87.Forterre Y, Skotheim JM, Dumais J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433:421–425. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- 88.Katz B. Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol (Lond) 1950;111:261–282. doi: 10.1113/jphysiol.1950.sp004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loewenstein WR. The generation of electric activity in a nerve ending. Ann NY Acad Sci. 1959;81:367–387. doi: 10.1111/j.1749-6632.1959.tb49320.x. [DOI] [PubMed] [Google Scholar]
- 90.Detweiler PB. Sensory transduction. In: Patton HD, Fuchs AF, Hille B, Sher AM, Streiner R, editors. Textbook of Physiology, Excitable Cells and Neurophysiology. Philadelphia: Saunders Publishing; 1989. pp. 98–129. [Google Scholar]
- 91.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 92.Kloda A, Martinac B. Common evolutionary origins of mechanosensitive ion channels in Archaea, bacteria and cell-walled Eukarya. Archaea. 2002;1:35–44. doi: 10.1155/2002/419261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kloda A, Martinac B. Mechanosensitive channels of bacteria and archaea share a common ancestral origin. Eur Biophys J. 2002;31:14–25. doi: 10.1007/s002490100160. [DOI] [PubMed] [Google Scholar]
- 94.Martinac B, Kloda A. Evolutionary origins of mechanosensitive ion channels. Progress Biophys Mol Biol. 2003;82:11–24. doi: 10.1016/s0079-6107(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 95.Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117:2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 96.Hamill OP, Marty A, Neher E, Sackmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch Eur J Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 97.Sachc F, Morris CE. Mechanosensitive ion channels in nonspecialized cells. Rev Physiol Biochem Pharmacol. 1998;132:1–77. doi: 10.1007/BFb0004985. [DOI] [PubMed] [Google Scholar]
- 98.Cosgrove DJ, Hedrich R. Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta. 1991;186:143–153. doi: 10.1007/BF00201510. [DOI] [PubMed] [Google Scholar]
- 99.Pastori GM, Foyer CH. Common components, networks, and pathways of cross-tolerance to stress, the central role of “redox” and abscisic acid-mediated controls. Plant Physiol. 2002;129:460–468. doi: 10.1104/pp.011021. [DOI] [PMC free article] [PubMed] [Google Scholar]