Abstract
Recent studies suggest that plant roots can avoid competition with other roots of the same plant, but the mechanism behind this behavior is yet largely unclear and their effects on plant performance hardly studied. We grew combinations of two ramets of Trifolium repens in a single pot that were either intact, disconnected for a shorter or longer time, or that belonged to different genotypes. Interconnected ramets developed lower root length and mass than any other combination of ramets, supporting the notion that self/non-self discrimination in T. repens was based entirely on physiological coordination between different roots that develop on the same plant, rather than biochemical allorecognition. These responses were consistent among eight field-collected genotypes, suggesting that self/non-self discrimination is a common feature in wild populations of white clover. There were no significant treatment x genotype interactions suggesting that genetic variation for self/non-self discrimination may be limited. Self-interactions resulted in lower to similar shoot biomass and number of ramets, but higher flowering probabilities, compared to non-self interactions. Thus, our results demonstrated that the performance consequences of self/non-self discrimination may be more complicated than previously thought.
Key Words: biomass allocation, clonal plants, competition, flowering, phenotypic plasticity, physiological coordination, plant growth, Trifolium repens, self/non-self discrimination
Introduction
Competition is one of the most prominent determinants of plant performance.1–5 The most successful competitors are those individuals that capture the greatest proportion of shared resources.1,6–8 However, increased allocation to resource capturing organs may come at a cost of reduced reproduction and fitness.9–11 Accordingly, selection should favor mechanisms that minimize competition between parts of the same genetic individual or “self-competition” (e.g., refs. 12 and 13).
While the potential to minimize self-competition has been considered to be relatively limited in plants, recent evidence has shown that plant roots are able to differentially respond according to the identity of their neighbors. Mahall and Callaway14–16 found that roots of the desert shrub Ambrosia dumosa responded to contacts with roots of different Ambrosia plants by decreasing their rate of elongation, but showed no such response when contacting other roots of the same individual plant. By contrast, Falik et al17 have shown that roots of Pisum sativum decreased root growth in the presence and in the direction of their own roots compared to roots of other plants. These kinds of responses have now been demonstrated for a number of species, including Glycine max;11 Phaseolus varigaris;18 Fragaria chiloensis;19 Miscanthus sinensis20 and Buchloe dactyloides.21
The mechanism behind self/non-self (S/NS) root discrimination is still obscure. In Pisum sativum, Fragaria chiloensis and Buchloe dactyloides the mechanism is largely or wholly based on physiologically coordinated responses among different roots that develop on the same plant,17,19,21 but the responses of Miscanthus sinensis20 could be interpreted as being based on biochemical allorecognition, i.e., immunological-like recognition.22
The few studies that have looked at shoot biomass or fruit production as fitness measures emphasized reduced plant performance under non-self as compared to self interactions, which was interpreted as a cost of an enhanced root allocation under non-self interactions.11,18,19 These results suggest that reduced root growth under self interactions might save resources under competition with self, a reduction that is not evolutionary stable under competition with non-self neighbors.11,19,23–25 It is yet unknown how widespread the effects of S/NS root discrimination on plant performance are among plant species, and among different genotypes of the same species.
Here we present the results of experiments designed to study the nature and consequences of S/NS root discrimination in the widely-studied white clover (Trifolium repens L.). Using T. repens ramets of known genotypic identities collected from wild populations, we pursued the following three objectives. First, we investigated the ability of T. repens to discriminate between self and non-self roots. Using two different genotypes allowed us to assess whether root discrimination in this species is based on physiological coordination between different roots of the same plant or biochemical allorecognition. Second, we tested how common self/non-self discrimination was among a wider variety of field-collected T. repens genotypes. Finally, by measuring shoot biomass, number of ramets and number of flowers we tested the hypothesis that the cost of enhanced root proliferation under non-self interactions compromised plant performance, as compared to self interactions.
Materials and Methods
Plant material.
White clover, Trifolium repens L., is a short-lived perennial legume, native to Eurasia. It has been deliberately introduced worldwide as a forage crop.26,27 It is very common in pasture and forage mixes but also occurs as a significant weed of lawns, turfgrass, and orchards. T. repens can colonize bare soil patches and gain local dominance by clonal spread.28
Genotypes of T. repens were collected from natural populations in floodplains of the main tributary of the River Rhine, east and west of Nijmegen in the summer of 2001. Stock plants of each of the genotypes were grown under uniform conditions in a garden (in summer) or in an unheated greenhouse (in winter) at the Radboud University Nijmegen, The Netherlands. Plants were propagated in early 2002 to create a number of different mother plants for each of the genotypes. Experimental ramets were then selected from these mother plants based on size uniformity of their shoots and roots.
Experiment 1: Testing for self/non-self discrimination and its effects.
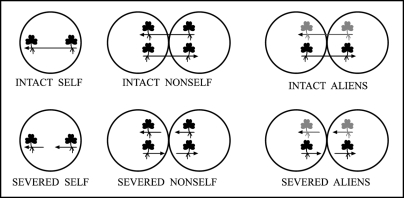
Connected ramet pairs of T. repens with shoots and roots of similar sizes were planted in pots so that each pot contained two ramets from either the same mother plant (SELF), or from two different mother plants belonging to the same (NON-SELF) or different (ALIEN) genotypes (Fig. 1). The stolons connecting the ramet pairs were either left intact (INTACT SELF, INTACT NON-SELF, INTACT ALIEN) or severed (SEVERED SELF, SEVERED NON-SELF, SEVERED ALIEN), producing two genetically identical but physiologically separate individuals. Throughout, experimental ramet severing was carried out immediately before the onset of the experiment.
Figure 1.
The experimental design. Trifolium repens ramet pairs with roots and shoots of similar size were grown so each of their roots was confronted with another root that belonged to either the same (SELF) or to another physiological individual of the same (NON-SELF) or of a different genotype (ALIEN). The ramet connections were either left INTACT or SEVERED shortly before the experiment. The arrow represents the growth direction of the stolon. By reversing the growth direction, a younger ramet always competes with an older ramet in a single pot. Different shades represent different genotypes.
Differences in root growth between INTACT SELF and INTACT NON-SELF indicate that the discrimination has a physiological basis. Differences between INTACT ALIEN and INTACT NON-SELF plants may suggest the involvement of biochemical allorecognition, i.e., S/NS discrimination that is based on the genetic identity of the competing plants. If all three treatments are different from each other both physiological and genetic mechanisms could be involved.
In Buchloe dactyloides, Gruntman and Novoplansky21 showed that cuttings originating from adjacent nodes become progressively alienated from each other and eventually relate to each other as genetically alien. By comparing INTACT SELF and SEVERED SELF we test whether disconnecting ramets results in S/NS discrimination, as compared to INTACT NON-SELF in which the two experimental plants originated from different but genetically identical mother plants, separated a few months before the experiment started. Furthermore, comparing INTACT NON-SELF and INTACT ALIEN to SEVERED NON-SELF and SEVERED ALIENS, respectively, allowed us to trace down and control for possible effects of plant severing (due to wounding) that are unrelated to root discrimination.
All these tests (all six treatments in Fig. 1) were carried out on one T. repens genotype (genotype A) using a second genotype (genotype B) as a competitor in the ALIEN treatments (Fig. 1). The SELF treatments were also conducted on genotype B. This design allowed us to (a) investigate whether genotype B also exhibited self/non-self discrimination, and (b) to control for potential inherent differences in ramet sizes and growth rates between the genotypes that could have been mistakenly interpreted as caused by the main treatments. All treatments were replicated 8–16 times. The experiment started on 13 September 2002 and lasted 91 days.
Aboveground plant biomass and number of ramets, fitness measures that are commonly used in clonal plants (e.g. refs. 29 and 30), were measured at harvest.
Experiment 2: Testing for self/non-self discrimination in a larger selection of genotypes.
In order to test the prevalence of self/non-self discrimination in Trifolium repens, we repeated part of the treatments of experiment 1 using eight different field-collected genotypes. The above mentioned INTACT SELF, INTACT NON-SELF and INTACT ALIEN treatments were used to test whether root discrimination was based on physiological coordination or biochemical allorecognition. Because the first experiment showed some severing effects, we also included the SEVERED SELF and SEVERED ALIEN treatments. In the ALIEN treatments four unique genotype pairs were tested: A–B, C–D, E–F and G–I, with genotypes A and B as a repetition of the first experiment. Each treatment was replicated 3–10 times. The second experiment, carried out under identical conditions as the first experiment, started on 6 January 2003 and lasted 88 days.
Growth conditions, plant processing and statistical analyses.
The experiments were carried out in a controlled growth chamber (Geerlings Koeltechniek, Rijswijk, The Netherlands). Supplemental lighting with fluorescent tubes and additional high pressure sodium lamps yielded 250 µmol m−2 s−1 for 16 h per day and a constant temperature of 20°C. Ramet pairs with 25 mm long shoots (petioles) and 5 mm long roots were cut from the mother plants. They were rooted in pots by pinning them down to the soil medium using plastic-coated metal wires. We used square 10 × 10 × 10 cm plastic pots filled with a 1:1 mixture of fine vermiculite and sieved potting soil. When plants were grown in pot pairs (Fig. 1), the relative positions of the pots were fixed by gluing them to each other. Special care was given to ensure that the position of the two ramets in relation to the pot's rim and their neighbors was similar in all treatments, irrespective of whether the ramets were connected or not. A 1 cm thick layer of black polyethylene beads covered the pot's surface in order to avoid the rooting of daughter ramets. T. repens stolons grow out laterally and were arranged in such a way that leaf overtopping and hence above-ground competition was avoided. The pots containing the ramet pairs were randomly arranged on benches in the growth chamber. The plants were irrigated by tap water every 3–4 days. To increase the probability of effective root competition no additional nutrients were supplemented.
The plants were harvested following initial leaf senescence. Since no meaningful shading developed among neighboring plants during the experiments it is assumed that leaf senescence developed mainly due to nutrient limitation. The substrate was carefully washed and the roots of the two ramets were separated. Total root length was estimated from scans of fresh roots using DT-scan software (Delta-T, Cambridge, UK). Shoot and root biomasses were determined after drying the plants at 70°C for at least three days. Statistical analyses were conducted using SYSTAT 10.0. All dependent variables were tested for normality and log-transformed when they did not meet the assumptions of parametric statistics. We used one-way ANOVA's to test for treatment effects in Experiment 1, and two-way ANOVA's (treatment × genotype) in experiment 2. Differences between individual treatment averages were further assessed by Tukey HSD comparisons.
Results
Self/non-self discrimination.
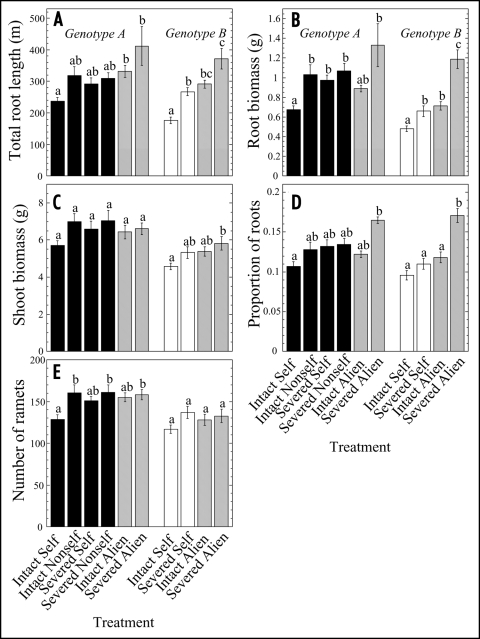
By the end of experiment 1, INTACT NON-SELF plants had on average 30% greater root length and 53% greater root biomass than INTACT SELF plants (Fig. 2). Physiological integration among interconnected ramets thus had a significant effect on root responses. Severing the connection produced interactions similar to NON-SELF interactions: SEVERED SELF and SEVERED NON-SELF plants developed similar root length and biomass as INTACT NON-SELF. In addition, root responses in INTACT ALIEN were similar to INTACT NON-SELF, suggesting that biochemical allorecognition had no significant role in the observed discrimination. However, there was an interaction between alien and severing effects in genotype B (Fig. 2): in SEVERED ALIEN, plants developed greater root length and biomass than in INTACT ALIEN. A similar trend in Genotype A was not significant.
Figure 2.
Effects of self/non-self discrimination in Trifolium repens in Experiment 1 as expressed by total root length (A), root biomass (B), shoot biomass (C), proportion of total plant biomass allocated to roots (D) and number of ramets (E) of two different genotypes (A and B). Treatments are explained in Figure 1. Values given are means (± S.E.) per plant, the two originally connected ramets together (n = 12–16).
Shoot growth.
While only genotype B produced significantly greater shoot biomass in SEVERED ALIEN than in INTACT SELF, shoot growth increased rather than decreased under all nonself and alien interactions in Experiment 1 (Fig. 2C). The number of ramets showed similar trends as shoot biomass, and the NONSELF and the SEVERED ALIEN treatments resulted in significantly larger genotype A plants than the INTACT SELF treatment. The markedly enhanced root growth under non-self and alien interactions resulted only in a marginally higher allocation of biomass to roots (Fig. 2D). This allocation response was not significant with the exception of the SEVERED ALIEN treatment.
Prevalence of self/non-self discrimination among T. Repens genotypes.
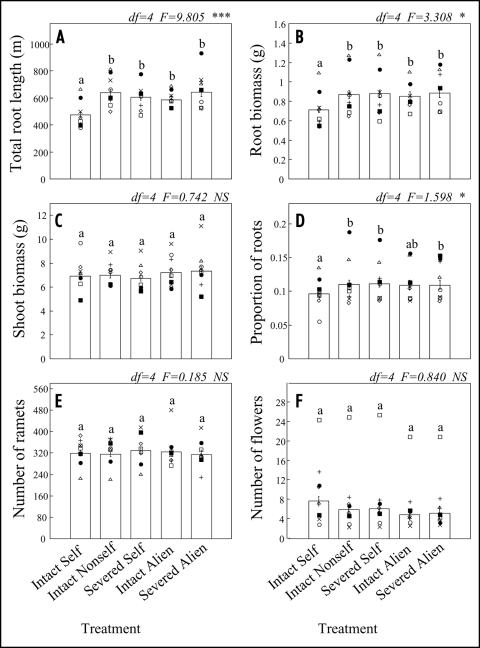
The responses of the eight genotypes to the treatments were largely consistent with the results of the first experiment (Fig. 3). Across all genotypes, NON-SELF, SEVERED and ALIEN interactions resulted in significantly greater root length and biomass compared to INTACT SELF. Although genotypes differed in root lengths and root biomass, there were no significant treatment × genotype interactions for these variables (Table 1), indicating that genotypes could not be segregated according to their self-non-self discrimination. In contrast to the results of the first experiment, no additional root biomass was produced under SEVERED ALIEN over all genotypes, even though genotype B demonstrated the same trend as in experiment 1 (Fig. 3B).
Figure 3.
Effects of self/non-self discrimination in Trifolium repens in Experiment 2 as expressed by total root length (A), root biomass (B), shoot biomass (C), proportion of total plant biomass allocated to roots (D), number of ramets (E) and number of flowers (F). The treatments are explained in Figure 1. Each symbol represents the mean value of a single genotype (n = 3–10). The genotypes also used in the Experiment 1 are indicated by the symbols solid circle (genotype A) and solid square (genotype B). The bars represent the mean (± S.E.) per plant (i.e., the two originally connected ramets together) averaged over all eight genotypes. The letters denote Tukey HSD grouping, based on a model in which genotypic differences are ignored. Table 1 gives the results of a 2-way ANOVA with treatment and genotypes as independent factors.
Table 1.
Tests of effects of self/non-self discrimination in Trifolium repens as expressed by root and shoot variables in Experiment 2
| Root length | Root biomass | Shoot biomass | Proportion of roots | Number of ramets | Number of flowers | |||||||||||||
| df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | |
| Treatment | 3 | 11.15 | <0.001 | 3 | 5.138 | 0.002 | 3 | 0.82 | 0.439 | 3 | 5.825 | 0.001 | 3 | 1.23 | .299 | 3 | 6.113 | <0.001 |
| Genotype | 7 | 8.25 | <0.001 | 7 | 13.08 | <0.001 | 7 | 3.44 | 0.002 | 7 | 9.268 | <0.001 | 7 | 3.94 | <0.001 | 7 | 25.167 | <0.001 |
| T * G | 21 | 1.27 | 0.208 | 21 | 1.26 | 0.210 | 21 | 0.92 | 0.383 | 21 | 1.57 | 0.063 | 21 | 1.17 | 0.285 | 21 | 1.32 | 0.173 |
| Error | 217 | 217 | 217 | 217 | 217 | 146 | ||||||||||||
Overall, there were no treatment effects on plant performance in the second experiment (Figs. 3C and E). Trends in experiment 1 of enhanced shoot biomass and number of ramets under NON-SELF, SEVERED or ALIEN interactions were not discernable, although there were some differences in response among individual genotypes (marginally significant treatment x genotype interaction for number of ramets; Table 1). The number of flowers per plant varied an order of magnitude among genotypes (Fig. 3F) and was significantly different among treatments in a two-way ANOVA (Table 1). Although the Tukey grouping in Figure 3F (in which these genotypic differences were not incorporated) did not reveal differences between individual treatments, NON-SELF, SEVERED and ALIEN interactions all resulted in 30% fewer flowers compared to INTACT SELF. These shifts in flowering were not due to (small) changes in number of ramets among treatments. An analysis of number of flowers with number of ramets as a covariate still revealed a significant treatment effect (p < 0.01), with INTACT ALIEN significantly lower than INTACT SELF (results not shown).
Discussion
Self/non-self root discrimination in Trifolium Repens.
Our results show that roots of Trifolium repens are able to discriminate between self and non-self neighbors and develop smaller root systems in the presence of other roots of the same physiological individual. Roots of ramets of the same genotype severed from each other immediately before the experiment or separated months in advance, as well as roots of different genotypes elicited similarly higher root growth compared to roots that encountered roots of connected ramets (Fig. 2A and B). Our results thus support the notion that coordination among roots of the same physiological individual plant rather than biochemical allorecognition, was responsible for S/NS root discrimination in T. repens. We are the first to demonstrate the effects of self/non-self root discrimination in this species, despite the enormous attention it enjoyed in ecology and agronomy, and the first to show that the responses were consistent among eight different field-collected genotypes. Our results thus suggest that self/non-self discrimination is a widespread phenomenon in wild populations of T. repens. Non-self interactions may lead to considerably higher rooting densities (and less flowering—see below) in white clover populations, especially where they consist of intermingled genotypes and fragmented clones.
Our results are consistent with recent work with Pisum sativum and Buchloe dactyloides showing that inhibitory effects among roots of the same plant are rapidly lost when the connection between the roots is terminated by severing,17,21 though more rapidly than in Buchloa. Modular integration, also regarding root responses, has been observed before,31 but the surprising effect of s/ns discrimination as reported here is that physiological integration is a prerequisite for the discriminatory root responses to occur. This communication may be mediated by hormones such as auxin and cytokinins, with the shoot as an intermediary.32 A resource-based signal seems unlikely as discriminatory effects are demonstrated well before resources are limiting,17,18 and because the restricted root growth has led to reduced rather than increased shoot growth in our experiments (see below). Falik et al.17 and Gruntman and Novoplansky21 speculated that oscillations of hormones and/or electricity underlie the physiological identity of individual plants and their discriminatory responses toward self/non-self neighbors but the nature of these signals is still obscure and in an urgent need of further investigation.
Only few studies allowed a systematic comparison between effects of physiological coordination and biochemical allorecognition, and such a comparison suggests a near consensus that self/non-self discrimination is based on coordination among roots that develop on the same physiological individual.17,19,21 Only in the rhizomatous grass Miscanthus sinensis, self/non-self discrimination couldn't safely be assigned to either mechanistic category. Consistent among four different genotypes, root growth was more strongly inhibited upon encounters with roots of a genetically identical but physiologically separate individual than with roots of a different genotype.20 Either the s/ns mechanism in Miscanthus is physiological and the time of separation (that was done just before the experiment started; Nishiwaki A, personal communication) was not sufficient to alienate the individuals from each other (sensu21), or the mechanism was based on biochemical allorecognition. A similar apparent discrepancy was demonstrated in our first experiment, where severing connections enhanced root growth more strongly upon encounters with roots of a different than of the same genotype (Fig. 2A and B). Nevertheless, here too, variability in the alienation time of recently-severed ramets could account for this discrepancy. Further work is necessary in order to totally rule out the possible role of biochemical allorecognition in S/NS root discrimination.
Whatever the precise mechanism, it is remarkable that none of the measured plant characteristics showed a difference in response among the eight T. repens genotypes in Experiment 2 (Table 1), despite large differences in genotypic means, as significant Genotype × Environment effects are ubiquitous for ecologically relevant plastic traits. If more generally true, this result will have profound consequences for the evolution and ecological significance of this type of interaction. It would suggest that additive genetic variation for S/NS discrimination is lacking and that benefits and costs are equally shared among competing individuals, effectively prohibiting the evolution of these traits. However, whether this lack of genotypic differentiation in self/non-self discrimination has wider validity (see ref. 19) is in urgent need of further investigation.
Implications of self/non-self discrimination for growth and reproductive allocation.
We expected that enhanced root proliferation under non-self encounters would compromise shoot growth and reproduction, but this expectation was not borne out by the results. In both experiments, enhanced root proliferation under non-self interactions resulted in similar or higher shoot mass and number of ramets. This lack of cost of root growth occurred despite of the apparent intense belowground competition demonstrated by the leaf senescence that suggested strong nutrient limitation towards the end of the experiments. Yield density curves revealed that both T. repens genotypes interacted strongly when grown with a single neighbor under the conditions of our experiments (results not shown). The total shoot mass of two neighboring ramets was 70–94% that of four neighboring ramets growing under the same conditions in the same pots.
Our results seemingly challenge the evolutionary arguments rationalizing self/non-self discrimination and are in a potential conflict with the conclusions of a number of other experimental studies.11,18,19 However, a closer inspection of the results sheds light on the discrepancy with these results. Similar to our experiments, shoot mass at harvest was equal for plants experiencing self and non-self competition in both Glycine max and Phaseolus vulgaris,11,18 in spite of the smaller root systems produced under self interactions. Maina et al18 harvested plants at regular time intervals and showed that up to day 40, shoot mass was greater in plants interacting with roots of a different individual compared to plants interacting with their own roots. It was not until the last 20 d of the experiment that the shoot biomasses became equal. These results suggest that in the initial process of competition, when self/non-self discrimination has already begun, the larger roots take up more nutrients and enhance plant growth. Over longer periods, as competition for soil resources intensifies, costs of the larger root systems may become apparent and effects on plant performance may turn negative, as shown by Holzapfel and Alpert19 in Fragaria chiloensis. Nevertheless, further investigation will have to concentrate on studying the cumulative fitness effects of S/NS discrimination over realistic competitive regimes in the field and over much longer periods.
In support of the hypothesis that self/non-self discrimination yields ecological benefits and in contrast to the results on shoot biomass and number of ramets, the number of flowers per plant was higher under intact self interactions than in all other treatments in our second experiment (Fig. 3F). This result was consistent for most genotypes, despite a ten-fold difference in flowering percentage. Gersani et al.11 and Maina et al.18 also found a higher number of fruits and greater pod mass under self- rather than non-self competition, as in our experiment largely due to a higher propensity of meristems to flower. However, it remains to be demonstrated that meristematic response is entirely or even partly due to resource shortage. In Phaseolus vulgaris a higher nutrient supply hardly increased the number of pods per unit of plant size, while the number of pods was 53% higher in similar-sized plants under self- than under non-self-competition,18 suggesting that nutrient availability and self/non-self discrimination affect flowering differently. There are no indications in this study, nor in ours, that resource shortage due restricted root production under self interaction was less severe than under non-self, severed, and alien interactions, explaining the flowering responses. Accordingly, a search into the physiological mechanism of self/non-self root discrimination should include reproductive responses as they may be directly influenced by the specific signaling involved.
Acknowledgements
We are grateful to Harry van de Steeg and Hannie de Caluwe for collecting the clover genotypes in the field and technical assistance, and to Josef Stuefer, Heidi Huber, Susan Kalisz, Maxine Watson and other members of the Experimental Plant Ecology group at the Radboud University Nijmegen for discussion. Liesje Mommer provided insightful comments on an earlier draft of the manuscript. O.F. was supported by a Huygens Fellowship from NUFFIC. The work of A.N. was partially supported by the Israel Science Foundation. This is publication no. 526 of the Mitrani Department of Desert Ecology.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2639
References
- 1.Tilman D. Resource Competition and Community Structure. Princeton: Princeton University Press; 1982. [PubMed] [Google Scholar]
- 2.Grace JB, Tilman D. In: Perspectives on plant competition. Grace JB, Tilman D, editors. San Diego: Academic Press; 1990. [DOI] [PubMed] [Google Scholar]
- 3.Casper BB, Jackson RB. Plant competition underground. Annu Rev Ecol Syst. 1997;28:545–570. [Google Scholar]
- 4.Grime JP. Plant strategies, vegetation processes, and ecosystem properties. 2nd ed. Chichester: John Wiley; 2001. [Google Scholar]
- 5.Keddy PA. Competition. 2nd ed. Dordrecht: Kluwer; 2001. [Google Scholar]
- 6.Mooney HA. Some contributions of physiological ecology to plant population biology. Syst Bot. 1976;1:269–283. [Google Scholar]
- 7.Robinson D, Hodge A, Griffiths BS. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proceedings of the Royal Society of London. 1999;266:431–435. [Google Scholar]
- 8.Moore PD. Ecology - Roots of diversity. Nature. 2003;424:26–27. doi: 10.1038/424026a. [DOI] [PubMed] [Google Scholar]
- 9.Novoplansky A, Sachs T, Cohen D, Bar R, Budenheimer J, Reisfeld R. Increasing plant productivity by changing the solar spectrum. Sol Energ Mater. 1990;21:17–23. [Google Scholar]
- 10.Schmitt J, McCormac AC, Smith H. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbors. Am Nat. 1995;146:937–953. [Google Scholar]
- 11.Gersani M, Brown JS, O'Brien EE, Maina GM, Abramsky Z. Tragedy of the commons as a result of root competition. J Ecol. 2001;89:660–669. [Google Scholar]
- 12.Grosberg RK, Hart MW. Mate selection and the evolution of highly polymorphic self/non-self recognition genes. Science. 2000;289:2111–2114. doi: 10.1126/science.289.5487.2111. [DOI] [PubMed] [Google Scholar]
- 13.Kimura M, Simbolon H. Allometry and life history of a forest under story palm Pinanga coronata (Arecaceae) on Mount Halimun, West Java. Ecol Res. 2002;17:323–338. [Google Scholar]
- 14.Mahall BE, Callaway RM. Root communication among desert shrubs. PNAS. 1991;88:874–876. doi: 10.1073/pnas.88.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahall BE, Callaway RM. Root communication mechanisms and intracommunity distributions of two Mojave Desert shrubs. Ecology. 1992;73:2145–2151. [Google Scholar]
- 16.Mahall BE, Callaway RM. Effects of regional origin and genotype on intraspecific root communication in the desert shrub Ambrosia dumosa (Asteraceae) Am J Bot. 1996;83:93–98. [Google Scholar]
- 17.Falik O, Raides P, Gersani M, Novoplansky A. Self/nonself discrimination in roots. J Ecol. 2003;91:525–531. [Google Scholar]
- 18.Maina GG, Brown JS, Gersani M. Intra-plant versus inter-plant root competition in beans: Avoidance, resource matching or tragedy of the commons. Plant Ecol. 2002;160:235–247. [Google Scholar]
- 19.Holzapfel C, Alpert P. Root cooperation between plants of the same clone. Oecologia. 2003;134:72–77. doi: 10.1007/s00442-002-1062-x. [DOI] [PubMed] [Google Scholar]
- 20.de Kroon H, Mommer L, Nishiwaki A. Root competition: Towards a mechanistic understanding. In: de Kroon H, Visser EJW, editors. Root Ecology. Berlin: Springer; 2003. pp. 215–234. [Google Scholar]
- 21.Gruntman M, Novoplansky A. Physiologically-mediated self/nonself discrimination in roots. PNAS. 2004;101:3863–3867. doi: 10.1073/pnas.0306604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixit R, Nasrallah JB. Recognizing self in the self-incompatibility response. Plant Physiol. 2001;125:105–108. doi: 10.1104/pp.125.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirmirani M, Oster G. Competition, kin selection, and evolutionary stable strategies. Theor Popul Biol. 1978;13:304–339. doi: 10.1016/0040-5809(78)90049-7. [DOI] [PubMed] [Google Scholar]
- 24.Iwasa Y, Cohen D, Leon JA. Tree height and crown shape, as results of competitive games. J Theor Biol. 1984;112:279–297. [Google Scholar]
- 25.Zhang DY, Sun GJ, Jiang XH. Donald's ideotype and growth redundancy: A game theoretical analysis. Field Crop Res. 1999;61:179–187. [Google Scholar]
- 26.Daday H. Gene frequencies in wild populations of Trifolium repens L. III. World distribution. Heredity. 1958;12:169–184. [Google Scholar]
- 27.Hayden KJ, Parker IM. Plasticity in cyanogenesis of Trifolium repens L.: inducibility, fitness costs and variable expression. Evol Ecol Res. 2002;4:155–168. [Google Scholar]
- 28.Fothergill M, Morgan CT, Jones S. Using leaf-mark material to monitor the morphology of white clover (Trifolium repens L.) at the clone and ramet level in grazed swards. Ann Botany. 2001;88:797–802. [Google Scholar]
- 29.Wikberg S. Fitness in clonal plants. Oikos. 1995;72:293–297. [Google Scholar]
- 30.Lenssen JPM, van Kleunen M, Fischer M, de Kroon H. Local adaptation of the clonal plant Ranunculus reptans to flooding along a small-scale gradient. J Ecol. 2004;92:696–706. [Google Scholar]
- 31.de Kroon H, Huber H, Stuefer JF, van Groenendael JM. A modular concept of phenotypic plasticity in plants. New Phytol. 2005;166:73–82. doi: 10.1111/j.1469-8137.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- 32.Sachs T. Pattern formation in plant tissues. Cambridge: Cambridge University Press; 1991. [Google Scholar]