Abstract
Formation of large perinuclear brefeldin A (BFA)-induced compartments is a characteristic feature of root apex cells, but it does not occur in shoot apex cells. BFA-induced compartments have been studied mostly using low resolution fluorescence microscopy techniques. Here, we have employed a high-resolution ultrastructural method based on ultra rapid freeze fixation of samples in order to study the formation of BFA-induced compartments in intact maize root epidermis cells in detail. This approach reveals five novel findings. Firstly, plant TGN/PGN elements are not tubular networks, as generally assumed, but rather vesicular compartments. Secondly, TGN/PGN vesicles interact with one another extensively via stalk-like connections and even fuse together via bridge-like structures. Thirdly, BFA-induced compartments are formed via extensive homotypic fusions of the TGN/PGN vesicles. Fourthly, multivesicular bodies (MVBs) are present within the BFA-induced compartments. Fifthly, mitochondria and small vacuoles accummulate abundantly around the large perinuclear BFA-induced compartments.
Key Words: brefeldin A, BFA-induced compartments, golgi, endosomes, root apex
Introduction
Formation of large perinuclear brefeldin A (BFA)-induced compartments is a characteristic feature of root apex cells, but it does not occur in shoot apex cells.1–10 In the root apex, BFA-induced compartments are characteristic for cells of the meristem and transition zone, but not for cells in the elongation region.1 BFA-induced compartments start to be formed within 15–20 minutes of exposure to BFA and reach full size at about 120 minutes of BFA treatment. At this time, they are visible in the light microscope as compact and roundish structures closely apposed to the nuclear surfaces,1 suggesting that there are strong attractive forces between the BFA-induced compartments and the nuclear surface. This causes their progressive fusion and, at the end, one or two large compartments are found closely apposed to the nuclear surface.1,3,8,11 Importantly, these large BFA-induced compartments can resolve back to the normal endomembrane configuration in recovering cells after BFA washout (reviewed in ref. 12).
Originally, BFA-induced compartments were proposed to represent aggregated vesicles and compartments derived from disintegration and vesiculation of Golgi stacks (reviewed in ref. 12). This early interpretation, based entirely on the observation that the disintegrating Golgi stacks localize closely to the assembling compartments, has been accepted for almost ten years.1,6,12 It could be abandoned only after the recent breakthrough studies on plant endocytosis and endosomes, revealing that endosomes and endosomal trans/ post-Golgi networks are the prevalent membrane source feeding into the BFA-induced compartments.5,6,13 On the other hand, in accordance with the situation in animal and yeast cells, cis cisternae of Golgi stacks of BFA-treated suspension plant cells merge with ER (reviewed in ref. 12). Recently, we have discovered that cell plates of cytokinetic cells are formed by progressive fusions of secretory endosomes2,10 and that the early cell plates show similarities to BFA-induced compartments.10 Moreover, cytokinetic plant cells develop BFA-induced compartments at their growing edges.2,10
So far, studies on BFA-induced compartments in intact roots have employed low resolution fluorescence microscopy techniques. Here, we have accomplished a high-resolution ultrastructural study based on ultra rapid freeze fixation of root epidermis samples. This approach reveals five major novel findings. Firstly, plant TGN/PGN elements are not tubular networks, as generally assumed, but rather vesicular compartments. Secondly, these TGN/PGN vesicles interact with one another extensively, via stalk-like connections, and even fuse together via bridge-like structures. Thirdly, BFA-induced compartments are formed via extensive homotypic fusions of the TGN/PGN vesicles. Fourthly, multivesicular bodies are present within the BFA-induced compartments. Fifthly, mitochondria and small vacuoles accummulate abundantly around these large BFA- induced compartments.
MATERIAL AND METHODS
Roots of 5 days old seedlings of Zea mays were treated with 10−4M BFA (Sigma, Taufkirchen, Germany) for 10, 20, 30, 45, 60 and 90 minutes. Thereafter, a section of the central part of the root tip was transferred to a specimen holder filled with 20% bovine serum albumine (Sigma, Taufkirchen, Germany) and cryofixed with a high pressure freeze fixation apparatus (HPM 010, BAL-TEC, Liechtenstein, Germany). This extremely rapid fixation procedure allows excellent ultrastructural preservation of plant cells and their endomembrane systems. Subsequently, the specimens were cryosubstituted with 0.25% glutaraldehyde (SIGMA, Taufkirchen, Germany) and 0.1% uranyl acetate (Chemapol, Czech Republic) in acetone at −80°C for 4 days using special cryosubstitution equipment (FSU, BAL-TEC, Liechtenstein), and finally embedded in HM20 (Polysciences Europe, Eppelheim Germany) at −20°C. Ultrathin sections were poststained with uranyl acetate and lead citrate in an EM-Stain apparatus (Leica, Bensheim, Germany) and subsequently observed with an EM 900 transmission electron microscope (Carl Zeiss SMT, Oberkochen, Germany). Micrographs were taken with a Variospeed SSCCD SM-1k-120 camera (TRS, Dünzelbach, Germany). Three different roots for each treatment were analyzed and there were no qualitative differences scored between the individual roots and cells.
Results
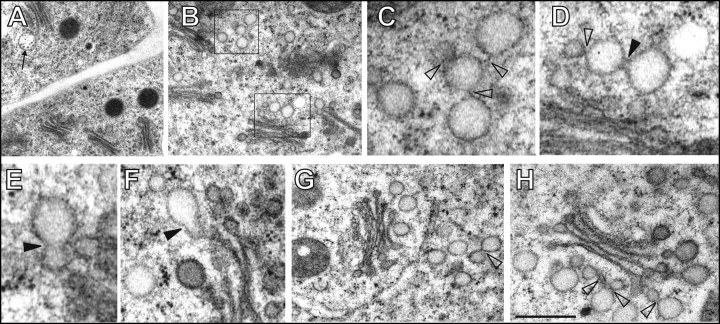
Root apex epidermis cells are highly cytoplasmic with only few vacuoles and abundant mitochondria and Golgi stacks (Fig. 1A,B). Golgi stacks have some 3–5 cisternae, coated vesicles closely associated with cis- and median cisternae, and prominent electron-transparent round and pear-shaped vesicular structures are loosely associated with their trans-sides (Fig. 1B–H). These trans/post-Golgi network (TGN/PGN) vesicles show coating at their surfaces and have mostly lighter contents (Fig. 1D–H). However, in several cases, we also observed groups of TGN/PGN compartments located independently from Golgi stacks (Fig. 1B,C,G). Characteristically, they communicate with each other via distinct stalk-like connections and partial bridge-like fusions (Fig. 1C–H). Interestingly, limited fusion among these TGN/PGN vesicles occurs in control cells. Both, pear-shaped vesicles and partially fused roundish vesicles are present, reaching sizes about 120–150 nm. Ocassionally, MVBs were visible near the TGN/PGN vesicles suggesting close communication between these two organelles (Fig. 1A).
Figure 1.
Control cells of maize root epidermis. (A,B) Numerous Golgi stacks with loosely associated vesicles of trans/post-Golgi network (TGN/PGN) (boxed areas in B) and multivesicular bodies (MVBs, indicated by arrow in A). (C,D) Higher magnification views of TGN/PGN vesicles from boxed areas in part B reveal stalk-like connections (empty arrowheads) and bridge-like partial fusions (filled arrowheads). (E,F) Pear-shaped vesicles resulting from the advanced fusion between TGN/PGN vesicles (filled arrowheads). (G,H) Abundant TGN/PGN compartments near or at some distance from Golgi stacks (see also B and C). Arrowheads indicate bridge-like connections. Bar = 1.2 µm for A; 1 µm for B; 0.25 µm for C and F; 0.3 µm for D; 0.2 µm for E; 0.5 µm for G and 0.35 µm for H.
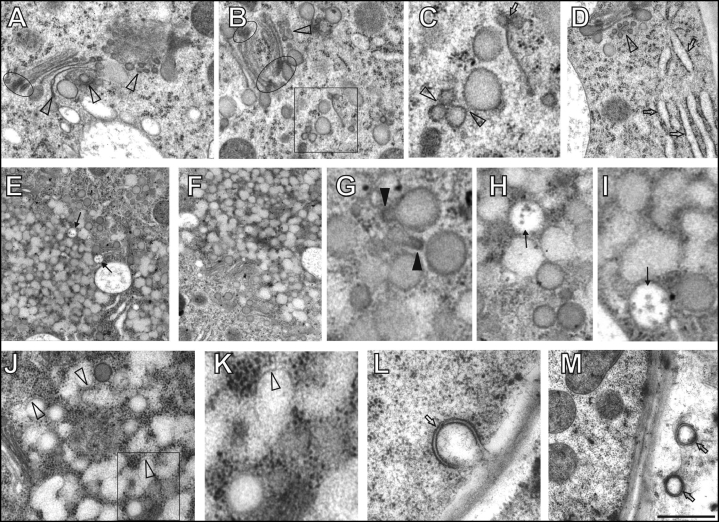
Brefeldin A (BFA) is a well characterized drug which inhibits ADP ribosylation factor-guanine-nucleotide exchange factor (ARF-GEF) resulting in a rapid block of secretory vesicle trafficking in both plants and animals (for plant cells see ref. 12). Already after 10 minutes of BFA exposure, some trans-Golgi cisternae become bent, shed off from Golgi stacks, and are progressively transformed into small vesicles (Fig. 2A–D). Moreover, TGN/PGN compartments, which are loosely associated with the trans Golgi face in control cells, leave this location and start to accumulate in distinct aggregates (Fig. 2D). They also increase their interaction and/or fusion activities (Fig. 2C). Conspicuous is the inflation of ER elements (Fig. 2D). After 20 minutes of treatment, prominent BFA-induced compartments are already scored within root epidermis cells (Figs. 2E,F). These are presumably formed from the TGN/PGN compartments which fuse together (Fig. 2G–I), often via tubular protrusions covered with prominent coating (Fig. 2G). After 30 minutes, massive homotypic fusions among TGN/PGN vesicles, which progressively loose their coat, are obvious in the centre as well as periphery of BFA-induced compartments (Fig. 2J–K). These findings indicate that BFA-induced uncoating of TGN/PGN vesicles is associated with massive homotypic vesicle fusions within maturing BFA-induced compartments. Within these compartments, intact non-fused multivesicular bodies (MVBs) are entrapped (Fig. 2E, H–I). At peripheries of the BFA-induced compartments, vesicles arrive and continue to fuse (Fig. 2H), driving the progressive enlargement of these compartments. During continued merging of newly arrived TGN/PGN vesicles into the BFA-induced compartments, they lose their coating (Fig. 2J,K). Inflated cis-cisternae of disintegrating Golgi stacks are located at the peripheries of BFA-induced compartments (Fig. 2E,F). Autophagosome-like compartments enclosed with double-membranes (Fig. 2L,M) are also sometimes found in the BFA-treated cells.
Figure 2.
Maize root epidermis cells treated with brefeldin A (BFA) for 10–30 minutes. (A–D) After 10 minutes of BFA treatment, progressive degradation of trans Golgi cisternae via vesiculation (arrowheads in A, B and D) and bending was obvious (A). Boxed area in B is presented as C. Note that occasionally trans Golgi cisternae can be lost (C, arrow) and inflated ER elements appear early after BFA treatment (D, arrows). Elipses in A and B highlight prominent dense coating of edges of cis and median Golgi cisternae. (C) Coated vesicles enhanced their contacts and fusion events (arrowheads). (E–I) 20 minutes of BFA treatment. (E–F) Note progressive fusions of TGN/PGN vesicles in the centre and periphery of BFA compartments while MVBs retain their structural identity (arrows in E). Note accumulation of Golgi stacks surrounding BFA-induced compartments in F. (G) Fusing TGN/PGN vesicles typically show pear-shaped forms with coated protrusions (filled arrowheads). (H,I): Detail views of fused TGN/PGN vesicles and unfused MVBs (arrows). (J–M) 30 minutes of BFA treatment. Boxed area in J is presented as K. (J,K) Most of the TGN/PGN vesicles fuse together extensively and almost lose their identity as well as coatings (empty arrowheads). (L,M) Autophagosome-like compartments enclosed with double-membranes (arrows). Bar = 0.73 µm for A and M; 0.65 µm for B; 0.26 µm for C and I; 0.80 µm for D; 1.35 µm for E; 1.25 µm for F; 015 µm for G; 0,40 µm for H; 0.50 µm for J; 0.20 µm for K and 0.27 µm for L.
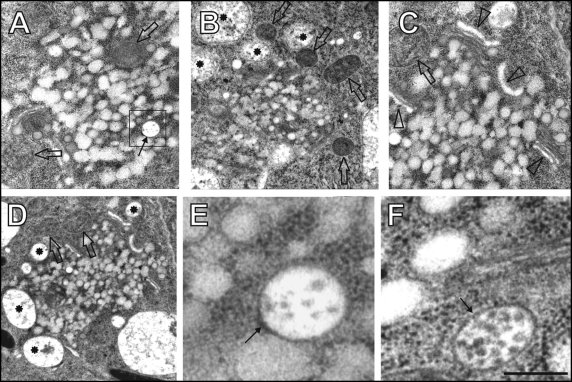
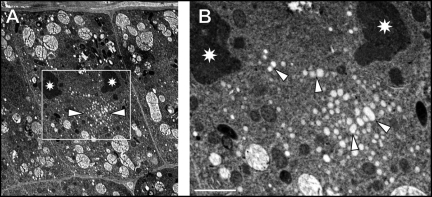
After 60 minutes of BFA treatment, fused TGN/PGN vesicles aggregate within the core of the BFA-induced compartments and lose their structural identity (Fig. 3A–C). At this time point, BFA-induced compartments are surrounded by curled Golgi stacks with dilated cis-cisternae (Fig 3C), small vacuoles containing vesicular inclusions, and with mitochondria (Fig. 3A–D). In contrast to the extensively fused TGN/PGN vesicles, MVBs are located both within the core or at peripheries of BFA compartments, keep their integrities and do not undergo heterotypic fusions with fused TGN/PGN compartments (Fig. 3A,E,F). Few ribosomes and some cytoplasmic material is visible in cytoplasmic space between the fusing vesicles forming local electron-dense areas (Fig. 3E,F). In cells undergoing cytokinesis, homotypically fused large TGN/PGN vesicles are organized in form of the BFA-induced compartments (Fig. 4A,B; arrowheads) occur between the postmitotic telophasic nuclei (Fig. 4A,B).
Figure 3.
60 minutes of brefeldin A (BFA) treatment. (A–D) Large BFA-induced compartments containing clusters of fused TGN/PGN vesicles and MVBs (arrow in A) as well as peripherally associated mitochondria (empty arrows), small vacuoles (stars), and Golgi stacks having inflated cis-cisternae (arrowheads),. Boxed area in A is presented as E. (E,F) Detail views on MVBs (arrows). Bar = 0.83 µm for A and C; 1.35 µm for B; 1.48 µm for D; 0.17 µm for E; 0.2 µm for F.
Figure 4.
Sixty minutes of brefeldin A (BFA) treatment. (A) Overview of cytokinetic root epidermal cell showing BFA-induced compartment (indicated by arrowheads) in between telophasic nuclei (stars). The boxed area in A is presented as (B). Note several fusions between TGN/PGN vesicles indicated by arrowheads in B. Bar = 4.58 µm for A; 1.52 µm for B.
Discussion
In our study, we have revealed that the TGN/PGN elements are represented by vesicles, rather than by tubular networks. These TGN/PGN vesicles are interconnected by stalk-like structures and bridge-like partial fusions. Recently, a similar appearance of the TGN/PGN elements was reported for Arabidopsis cells.14,15 Relevant in this respect is that living animal cells overexpressing Rab5 GTPase form enlarged endosomes via homotypic fusion events mediated by bridge-like fusion intermediates.16 Similar bridge-like fusion events occur also between phagosomes and late endosomes.17
TGN/PGN vesicles, which partially fuse together already in control cells, initiate the formation of BFA-induced compartments by their excessive fusions forming an amorphous mass of interconnected vesicles (e.g., Fig. 2E,F and Fig. 3A). As another original finding of this study, we show that the BFA-induced compartments contain also MVBs. Intact MVBs in BFA-treated plant cells were reported already earlier18 but their localization within and around BFA-induced compartments is a novel finding. In this respect, it is important to keep in mind that TGN/PGN vesicles, as well as MVBs, emerge as integral parts of the endocytic and secretory networks in plant cells.6,19 Recently, this new endocytic concept of the TGN6,19 was confirmed for the TGN/PGN compartments by Dettmer et al. (2006)13 who suggested that TGN/PGN act as early endosomes in plants. This is further supported by recent results suggesting that late endosomal MVBs function as secretory organelles in both animal20 as well as plant21 cells. Moreover, even early endosomes seem to act as secretory organelles as far as cell plate formation is concerned.2,10,13 There is growing evidence for a tight interaction between endocytic and exocytic pathways in plant cells including tip-growing cells like pollen tubes and root hairs.22–25 This is also seen on the molecular level, where Rab11 is important for pollen tube tip-growth22 as well as for homotypic fusion of endosomes.20
Importantly, the partial fusions of TGN/PGN elements in both controls and BFA-treated cells, resemble the membraneous structures involved in the formation of the early cell plate during plant cytokinesis.10,14,26–29 Similar to the early cell plates, BFA-induced compartments represent pectin and xyloglucan-enriched cell wall ‘islands’ within the cytoplasm.2 The most important difference is that the phragmoplast array of microtubules forces the membrane cisterna of the cell plate into a thin and flat structure. This ultimately spans the whole diameter of dividing cell; whereas the BFA-induced compartments are of roundish shapes, closely apposed to the nuclear surface (for maize root cells see refs. 1 and 2). All these features are reminiscent of so-called ‘compound exocytosis’ which was recently proposed as a new type of regulated secretion in animal cells based on secretory endosomes.30–31 The fusigenic TGN/PGN compartments in plant cells, initiating and driving the formation of BFA-induced compartments, are of endosomal nature and are obviously accomplishing ‘compound exocytosis’ in plant cells.10,13
Why do the BFA-induced compartments fuse progressively together and associate closely with the nuclear surface? And why do they form only in specific types of root cells? These urgent questions need to be addressed next in the coming years. There are several emerging clues pointing into directions towards which future studies should move. For instance, endosomes in animal cells are well-known to act as motile signalling platforms for communication between the cell periphery and the nucleus.32–35 Moreover, several endocytic proteins enter the nucleus and regulate both the chromatin structure and gene expression.34 This has also been shown in plants, where the classical trithorax-like nuclear protein ATX1 has been localized also to the plasma membrane, especially at the cell-cell contact domains and bulges/tips of root hairs, and endosome-like PI5P enriched vesicles.36 In addition, perinuclear BFA-induced compartments resemble clusters of starvation-induced acidic vesicles, known as autolysosomes in plant cells, as well as aggresomes of animal cells; all of which accumulate at perinuclear locations.37–40 Plant endosomes are participating in autolysosome formation.39–40 Besides their perinuclear locations, there are also several interesting ultrastructural similarities between acidic vesicles of starvation-induced autolysosomes and BFA-induced compartments (this study; see also ref. 38).
It is intriguing that only root cells, but not shoot cells,4,7 form large perinuclear BFA-induced compartments. Cell-cell communication via endosome-based vesicle recycling across the cell wall at end-poles in root tissue has features resembling those of neuronal synapses in animals.41,42 Indeed, recent studies confirmed that components of the putative auxin transporter machinery PIN1 accumulate at plant synapses41,42 in dependency on cell-cell contacts,9 as it is the case in neuronal and immunological synapses.43 Interestingly in this respect, polar auxin transport is inhibited within a few minutes of exposure to BFA in both suspension cells44 and intact root apices.45 Besides auxin transporting plant synapses in root tissue,46 which determine and depend on cell polarity,9,46 another type of cell-cell contact is formed between a penetrating fungal pathogen and the plant host resembling the immunological synapse of animal cells.42 To prevent the access of these pathogens into plant cells, the latter assemble papilla21,47 via the fusion of multivesicular bodies21 and presumably also other endosomes filled with cell wall material and reactive oxygen species.6,21 Interestingly, a SNARE-protein termed PEN1, which drives the papilla formation47 is an endosomal SNARE that localizes not only to endosomes, but also to endosomal cell plates, and BFA-induced compartments in dividing root cells (Boris Voigt, Hans Thordal-Christensen, Diedrik Menzel, Frantisek Baluska, manuscript in preparation). So obviously, compound exocytosis in plants is operating in those situations when rapid cell wall assembly is essential. It seems to be achieved via secretory endosomes enriched with cell wall pectins and xyloglucans.1,2,10 In cytokinetic plant cells, compound exocytosis-like drives cell plate formation; while in plant cells under pathogen attack, compound exocytosis drives papilla formation. In addition, BFA induces intracellular compound exocytosis resulting in the formation of BFA-induced compartments in dividing root cells.
An intriguing possibility would be that the active plant synapses recycle their components and transported cargo (e.g., auxin and pectin) via recycling pathways which get transformed into perinuclear BFA-induced compartments. This scenario is strongly supported by the entrapping of recycling cell wall pectins, xyloglucans, putative auxin transporters, and of auxin itself, within the perinuclear BFA-induced compartments.1,2,5,8,48 A similar scenario is valid also for neuronal synapses which are build from prefabricated TGN-derived vesicular packets equipped with many components of the presynaptic active zone.49,50 In polarized neurons, TGN elements are localized independently, at the synapse,51 whereas the Golgi apparatus is associated with nucleus. Similarly in polarly growing plant cells, like root hairs, endosomes and TGN elements accumulate at the very tips of growing cells, while Golgi stacks are localized more basally.52 As root synapses are generated during cytokinesis from endosomal/TGN compartments,2,10 resembling the BFA-induced compartments described here, we can suggest that neuronal and plant synapses are generated via similar processes. Finally, our study provides the first ultrastructural evidence strongly supporting the view that the BFA-induced compartment is indeed an endocytic hybrid organelle composed of fused TGN/PGN vesicles, as well as structurally independent MVBs, all representing secretory endosomal organelles in plants. With this ultrastructural study, we are complementing recent avalanche of data on the BFA-induced compartments obtained in planta at the level of light microscopy.
Acknowledgements
This work was supported by grants from Deutsches Zentrum für Luft- und Raumfahrt (DLR, Bonn, Germany). F.B. and J.S. were also supported by Grant Agencies Vega (Project Nr.2/5085/25) and APVT, Bratislava(APVT-51-002302) and G.H. was supported by grant SFB 648/Z1 from the Deutsche Forschungsgemeinschaft.
Abbreviations
- BFA
brefeldin A
- MVBs
multivesicular bodies
- TGN
trans-Golgi network
- PGN
post-Golgi network
- ER
endoplasmic reticulum;
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2996
References
- 1.Baluska F, Hlavacka A, Samaj J, Palme K, Robinson DG, Matoh T, McCurdy DW, Menzel D, Volkmann D. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells: insights from brefeldin A-induced compartments. Plant Physiol. 2002;130:422–431. doi: 10.1104/pp.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baluska F, Liners F, Hlavacka A, Schlicht M, Van Cutsem P, McCurdy D, Menzel D. Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma. 2005;225:141–155. doi: 10.1007/s00709-005-0095-5. [DOI] [PubMed] [Google Scholar]
- 3.Grebe M, Xu J, Mobius W, Ueda T, Nakano A, Geuze HJ, Rook MB, Scheres B. Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6 positive early endosomes. Curr Biol. 2003;13:1378–1387. doi: 10.1016/s0960-9822(03)00538-4. [DOI] [PubMed] [Google Scholar]
- 4.Russinova E, Borst J-W, Kwaaitaal M, Caño-Delgado A, Yin Y, Chory J, de Vries SC. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1) Plant Cell. 2004;16:3216–3229. doi: 10.1105/tpc.104.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samaj J, Baluska F, Voigt B, Schlicht M, Volkmann D, Menzel D. Endocytosis, actin cytoskeleton and signalling. Plant Physiol. 2004;135:1150–1161. doi: 10.1104/pp.104.040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samaj J, Read ND, Volkmann D, Menzel D, Baluska F. The endocytic network in plants. Trends Cell Biol. 2005;15:425–433. doi: 10.1016/j.tcb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Bloch D, Lavy M, Efrat Y, Efroni I, Bracha-Drori K, Abu-Abied M, Sadot E, Yalovsky S. Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol Biol Cell. 2005;16:1913–1927. doi: 10.1091/mbc.E04-07-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paciorek T, Zazímalová E, Ruthhardt N, Petrásek J, Stierhof Y-D, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, Friml J. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 9.Boutté Y, Crosnier MT, Carraro N, Traas J, Satiat-Jeunemaitre B. The plasma membrane recycling pathway and cell polarity in plants: studies on PIN proteins. J Cell Sci. 2006;119:1255–1265. doi: 10.1242/jcs.02847. [DOI] [PubMed] [Google Scholar]
- 10.Dhonukshe P, Baluska F, Hlavacka A, Schlicht M, Samaj J, Friml J, Gadella TWJ., Jr Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell. 2006;10:137–150. doi: 10.1016/j.devcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 12.Nebenführ A, Ritzenthaler C, Robinson DG. Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002;130:1102–1108. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seguí-Simarro JM, Staehelin LA. Cell cycle-dependent changes in Golgi stacks, vacuoles, clathrin-coated vesicles and multivesicular bodies in meristematic cells of Arabidopsis thaliana: a quantitative and spatial analysis. Planta. 2006;223:223–236. doi: 10.1007/s00425-005-0082-2. [DOI] [PubMed] [Google Scholar]
- 15.Preuss ML, Schmitz AJ, Thole JM, Bonner HKS, Otegui MS, Nielsen E. A role for the RabA4b effector protein PI-4Kβ1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol. 172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts RL, Barbieri MA, Pryse KM, Chua M, Morisaki JH, Stahl PD. Endosome fusion in living cells overexpressing GFP-Rab5. J Cell Sci. 1999;112:3667–3675. doi: 10.1242/jcs.112.21.3667. [DOI] [PubMed] [Google Scholar]
- 17.Stockinger W, Zhang SC, Trivedi V, Jarzylo LA, Shieh EC, Lane WS, Castoreno AB, Nohturfft A. Differential requirements for actin polymerization, calmodulin and Ca2+ define distinct stages of lysosome/phagosome targeting. Mol Biol Cell. 2006;17:1697–1710. doi: 10.1091/mbc.E05-12-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004;16:672–693. doi: 10.1105/tpc.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uemura T, Ueda T, Ohniwa RL, Nakano A, Takeyasu K, Sato MH. Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct Funct. 2004;29:49–65. doi: 10.1247/csf.29.49. [DOI] [PubMed] [Google Scholar]
- 20.Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 21.An Q, Hückelhoven R, Kogel K-H, van Bel AJE. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 2006;8:1009–1019. doi: 10.1111/j.1462-5822.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- 22.de Graaf BH, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu HM. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell. 2005;17:2564–2579. doi: 10.1105/tpc.105.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voigt B, Timmers A, Samaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H, Nakano A, Baluska F, Menzel D. Actin-based motility of endosomes is linked to polar tip-growth of root hairs. Eur J Cell Biol. 2005;84:609–621. doi: 10.1016/j.ejcb.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Kong L, Li Y, Samaj J, Baluska F, Lin J. Effects of brefeldin A on pollen germination and tube growth: antagonistic effects on endocytosis and secretion. Plant Physiol. 2005;139:1692–1703. doi: 10.1104/pp.105.069765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ovecka M, Lang I, Baluska F, Ismail A, Illes P, Lichtscheidl IK. Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma. 2005;226:39–54. doi: 10.1007/s00709-005-0103-9. [DOI] [PubMed] [Google Scholar]
- 26.Samuels AL, Giddings TH, Jr, Staehelin LA. Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol. 1995;130:1345–1357. doi: 10.1083/jcb.130.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seguí-Simarro JM, Austin JR, II, White EA, Staehelin LA. Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell. 2004;16:836–856. doi: 10.1105/tpc.017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otegui MS, Staehelin LA. Electron tomographic analysis of post-meiotic cytokinesis during pollen development in Arabidopsis thaliana. Planta. 2004;218:501–515. doi: 10.1007/s00425-003-1125-1. [DOI] [PubMed] [Google Scholar]
- 29.Otegui MS, Mastronarde DN, Kang B-H, Bednarek SY, Staehelin LA. Three-dimensional analysis of syncytial-type cell plates during endosperm cellularisation visualized by high resolution electron tomography. Plant Cell. 2001;13:2033–2051. doi: 10.1105/TPC.010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickett JA, Edwardson M. Compound exocytosis. Mechanisms and functional significance. Traffic. 2006;7:1–8. doi: 10.1111/j.1600-0854.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 31.Sokac AM, Bement WM. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Mol Biol Cell. 2006;17:1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benmerah A. Endocytosis: signaling from endocytic membranes to the nucleus. Curr Biol. 2004;14:R314–R316. doi: 10.1016/j.cub.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 33.Giri DK, Ali-Seyed M, Li L-Y, Lee D-F, Ling P, Bartholomeusz G, Wang S-C, Hung MC. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25:11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, Yang R, Fan F, Chen X, Pei G, Ma L. A nuclear function of β-arrestin 1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Chang JS, Henry K, Geli MI, Lemmon SK. Cortical recruitment and nuclear-cytoplasmic shuttling of Scd5p, a protein phosphatase-1-targeting protein involved in actin organization and endocytosis. Mol Biol Cell. 2006;17:251–262. doi: 10.1091/mbc.E05-10-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Venegas R, Sadder M, Hlavacka A, Baluska F, Xia Y, Lu G, Firsov A, Sarath G, Moriyama H, Dubrovsky JG, Avramova Z. The Arabidopsis homolog of trithorax, ATX1, binds phosphatidylinositol 5-phosphate, and the two regulate a common set of target genes. Proc Natl Acad Sci USA. 2006;103:6049–6054. doi: 10.1073/pnas.0600944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Mata R, Gao Y-S, Sztul E. Hassles with taking out the garbage: aggravating aggresomes. Traffic. 2002;3:388–396. doi: 10.1034/j.1600-0854.2002.30602.x. [DOI] [PubMed] [Google Scholar]
- 38.Rose TL, Bonneau L, Der C, Marty-Mazars D, Marty F. Starvation-induced expression of autophagy-related genes in Arabidopsis. Biol Cell. 2006;98:53–67. doi: 10.1042/BC20040516. [DOI] [PubMed] [Google Scholar]
- 39.Yamada K, Fuji K, Shimada T, Nishimura M, Hara-Nishimura I. Endosomal proteases facilitate the fusion of endosomes with vacuoles at the final step of the endocytotic pathway. Plant J. 2005;41:888–898. doi: 10.1111/j.1365-313X.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- 40.Yano K, Matsui S, Tsuchiya T, Maeshima M, Kutsuna N, Hasezawa S, Moriyasu Y. Contribution of the plasma membrane and central vacuole in the formation of autolysosomes in cultured tobacco cells. Plant Cell Physiol. 2004;45:951–957. doi: 10.1093/pcp/pch105. [DOI] [PubMed] [Google Scholar]
- 41.Baluska F, Samaj J, Menzel D. Polar transport of auxin: carrier-mediated flux across the plasma membrane or neurotransmitter-like secretion? Trends Cell Biol. 2003;13:282–285. doi: 10.1016/s0962-8924(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 42.Baluska F, Volkmann D, Menzel D. Plant synapses: actin-based adhesion domains for cell-to-cell communication. Trends Plant Sci. 2005;10:106–111. doi: 10.1016/j.tplants.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Dustin ML, Colman DR. Neural and immunological synaptic relations. Science. 2002;298:785–789. doi: 10.1126/science.1076386. [DOI] [PubMed] [Google Scholar]
- 44.Delbarre A, Muller P, Guern J. Short-lived and phosphorylated proteins contribute to carrier-mediated efflux, but not to influx, of auxin in suspension-cultured tobacco cells. Plant Physiol. 1998;116:833–844. doi: 10.1104/pp.116.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mancuso S, Marras AM, Volker M, Baluska F. Non-invasive and continuous recordings of auxin fluxes in intact root apex with a carbon-nanotube-modified and self-referencing microelectrode. Anal Biochem. 2005;341:344–351. doi: 10.1016/j.ab.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 46.Barlow PW, Volkmann D, Baluska F. Polarity in roots. In: Lindsey K, editor. Polarity in Plants. Blackwell Publishing; 2004. pp. 192–241. [Google Scholar]
- 47.Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, Wanner G, Peck SC, Rdwards H, Ramonell K, Somerville CR, Thordal-Christensen H. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell. 2004;15:5118–5120. doi: 10.1091/mbc.E04-02-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlicht M, Strnad M, Scanlon MJ, Mancuso S, Hochholdinger F, Palme K, Volkmann D, Menzel D, Baluska F. Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signal Behav. 2006;1 doi: 10.4161/psb.1.3.2759. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- 50.Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]
- 51.Sytnyk V, Leshchyns'ka I, Delling M, Dityateva G, Dityatev A, Schachner M. Neural cell adhesion molecules promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts. J Cell Biol. 2002;159:649–661. doi: 10.1083/jcb.200205098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preuss ML, Schmitz AJ, Thole JM, Bonner HK, Otegui MS, Nielsen E. A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol. 2006;172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]