Abstract
The maintenance of a cytosolic free calcium gradient (Ca2+]c) and vesicle secretion in the apex of pollen tubes is essential for growth. It has been postulated that high [Ca2+]c levels promote and confine vesicle fusion with the apical plasma membrane and in this study we performed a correlative analysis of both events using specific fluorescent dyes and confocal scanning microscopy. [Ca2+]c was imaged with Calcium Green-1 10 kDa dextran (CG-1) while secretory events were followed with FM1–43 or FM4–64 in pollen tubes undergoing normal growth and reorientation events.
During straight growth (no modification in direction), we found that changes in apical [Ca2+]c accompany changes in apical FM fluorescence indicating a tight coupling between [Ca2+]c and apical secretion. This coupling seems however to be perturbed during periods of reorientation of the pollen tube growth axis. Analysis of apical and sub-apical fluorescent signals during the reorientation events and subsequent re-entry in straight growth indicate that the increase in secretory events (higher fusion rate) precede the increases in [Ca2+]c that should be required for the transduction of the signal.
Based on these findings, we discuss a model for membrane secretion and recycling which considers the apical and sub-apical region as a functional area containing all the elements required to promote and sustain growth.
Key Words: [Ca2+]c, endocytosis, exocytosis, FM1–43, cFM4–64, secretion, tip growth
Introduction
Polar growth and reorientation are crucial properties of the pollen tube dynamic growth process, and have been used as a model to study signal transduction mechanisms and secretion.1,2 A [Ca2+]c tip-focused gradient3 has a key role in polarized growth and changes in [Ca2+]c concentration within the apical dome result in growth reorientation.4–6 This gradient is absent in non-growing pollen tubes and its disruption leads to inhibition of tube growth. Both the intracellular [Ca2+]c gradient and extracellular Ca2+ influx suffer sinusoidal oscillations which are accompanied by oscillations in growth rate7 and there is strong evidence coupling Ca2+ with the control of cytoplasmic streaming. But directional growth can be achieved by modulation of many other cellular components. Protein kinases, cAMP, GTPases, specific cytoskeleton arrangements and phosphoinositides have been documented as important players8–12 and all seem to intersect the Ca2+ signalling pathway. These observations led us to suggest the existence of an intricate signalling network where the same output can result from many different inputs providing the cell with the machinery to respond to a large multitude of signals.13
The rapid growth of pollen tubes is made possible due to a highly polarized fusion of apical vesicles, which transport cell wall components to the growing tip. This exocytic delivery of material to the extending apex is characteristic of tip growing cells such as root hairs,14 fungal hyphae15 and rhizoids.16,17 In pollen tubes, the membrane material provided by vesicles that fuse with the apex was calculated to exceed the needs to maintain growth rates suggesting an underlying recycling process1. However, the molecular and cellular mechanisms behind such process are still unclear. [Ca2+]c was implicated in the movement of vesicles towards the tip, fusion with the plasma membrane, and the state of plasticity of the membrane18 making the secretory pathway an obvious target for [Ca2+]c signalling.1,19 Evidence relating secretion with Ca2+ signalling has indeed been obtained.8,9,20 But our work has also shown that although high Ca2+ can promote exocytosis, it is not this ion the primary event in the control of growth rates.8 Work done with brefeldin-A also indicates an independence of apical vesicle oscillatory behaviour from the tip-focused Ca2+ gradient.17 Furthermore, high temporal resolution imaging studies revealed that the maximum [Ca2+]c in the tip-focused gradient occurred 1–4 sec, or ∼20°, after a peak in growth rate21 which presumably requires a higher exocytosis rate. Although these data do not contradict previous observations, it reinforces the hypothesis that Ca2+ activity is not a leader of growth but a follower. Therefore, we decided to analyse the correlation between the dynamics of apical [Ca2+]c and vesicle trafficking in tip growth and reorientation.
Material and Methods
Plant material.
Unless otherwise stated, pollen of Agapanthus umbellatus was harvested, stored, and pollen tubes were grown in vitro as described previously. Pollen tubes were germinated in semisolid growth medium containing: 0.01% H3BO3, 0.02% CaCl2, 0.02% KCl, 0.02% MgCl2, 2.5% (73 mM) sucrose and 0.8% Agarose II (Sigma), pH 6.0.
Confocal imaging of [Ca2+]c and FM dyes (1–43 and 4–64).
[Ca2+]c imaging was performed with the sensitive fluorescent dye Calcium Green-1 conjugated with a 10 KDa dextran (1 mM, Molecular Probes, Eugene, UK). The dye was loaded into pollen tubes either through pressure or ionophoretic microinjection for an approximate final concentration of 1–5 µM. Microelectrodes were pulled from borosilicate glass capillaries 120 F-10 (1.2 mm O.D. × 0.69 mm I.D., Clark Electromedical Instruments, UK), using a Sutter P-97 puller (Sutter Instrument Co., Novato, USA) to an external tip diameter of ∼0.5 µm (pressure microinjection) or ∼0.3 µm (ionophoresis). Loaded cells were allowed to recover under germination conditions for 10–20 min, prior to imaging or other treatments. Details of the experimental procedure and criteria used to establish the success of microinjection can be found in reference 22. Imaging was performed using the 488 nm line of the Kr-Ar laser of a Bio-Rad MCR-600 (Microscience Ltd, Hemel Hempstead, U.K) confocal laser scanning microscope (CLSM) in F2 scanning mode (∼1/2 sec per frame), with a 3% laser intensity, an electronic zoom of ×2 or ×3 and an optical sectioning of ∼5 µm. A ×20 Plan Apo dry objective (NA = 0.75) or a ×40 Plan dry (NA = 0.75) (Olympus) were used. Higher NA objectives could not be used due to their shorter working distance. Images were processed with the TCSM/MPL software (Bio-Rad Microscience Ltd.) and then quantified in terms of average pixel intensity (0–255 scale for 8 bit images).
The dynamics of endo-exocytosis was imaged with FM1–43 as before8,19 using pollen tubes labelled with ∼0.2 µM of FM1–43 (Molecular Probes) and thin time-course optical sections (<5 µm thick) acquired with the 488 nm line of the kr-Ar CLSM and equivalent settings of the [Ca2+]c imaging. Fluorescence was quantified in terms of average pixel intensity.
For simultaneous imaging of [Ca2+]c and membrane dynamics, cells loaded with CG-1 were labelled with ∼0.2 µM of FM4–64 (Molecular Probes) and images collected in dual-channel mode (excitation: 488/568 nm; 520/630 nm double dichroic; barrier filter of 522 nm; barrier filter 2 of 585 nm).
Data analysis.
Growth rates and fluorescence intensity were measured using Image-Pro Plus 5.0 software (Media Cybernetics, Leiden, Netherlands). Fluorescence measurements presented correspond to medium fluorescence intensity in the first 0–10 µm and 10–20 µm of the pollen tube apex (apical and sub-apical region respectively).
Except if specifically mentioned, numerical data in figures correspond to single cell analysis of typical experiments and not to summary statistics. This is because there is a significant degree of variability at a biological level but also at a technical one; even minor changes in the degree of loading, disturbance on microinjection, and responsiveness of the cell can play a role in the extent of cellular response.2 Reorientation of pollen tubes was defined as a change in the growth axis higher than 5°, either to the left or right.5
For measurements on germination rate and growth rates, a one-way analysis of variance (ANOVA, p < 0.05) was applied. For analysis of fluorescence data patterns, linear regressions were applied using basic statistics software. The linear regressions were used independently in groups of points that reflect a similar pattern (judged by the correlation coefficient R2). A “collapse event” was considered to occur when a group of points changed pattern due to a rapid variation in signal intensity.
Results
The use of FM 1–43 and FM4–64 as markers for endo-exocytosis and polar growth.
We had previously optimized the use of FM 1–43 as a marker to study membrane recycling and polar growth in Agapanthus umbellatus pollen tubes.8 In this study we maintained an external dye concentration of 0.2 µM as it did not affect growth rate and yielded a good fluorescent signal. With such concentration and within a few minutes, the typical staining pattern of FM 1–43 dye was observed: a bright staining in the apical region that extends to the sub-apical region with an inverted cone shape (Fig. 1A).
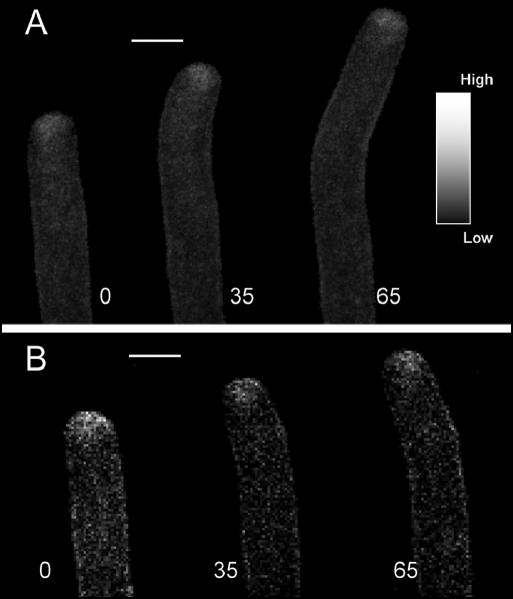
Figure 1.
Time course confocal images of A. umbellatus pollen tube labelled with FM dyes undergoing reorientation of the growth axis. (A) FM1–43. (B) FM4–64. With both dyes, the typical apical hotspot is visible. During changes in the growth axis, asymmetric distribution of fluorescence within the apical dome can be measured. The times (sec) at which the images were taken are indicated next to the images. Scale bar = 10 µm.
The emission spectra of FM1–43 overlaps with most commonly used Ca2+-sensitive dye, e.g., CG-1 thus precluding the simultaneous use of these two probes. Therefore, we tested the responses of FM4–64, an analogue of 1–43 but with a red emission. FM4–64 has been successfully used in tip-growing cells15,17,22 and we found it to produce a much stronger labelling in the plasma membrane and a weaker signal in the cytoplasm (Fig. 1B; n = 28). However, using thin-optical sectioning, we managed to obtain results similar to FM1–43. Namely, a bright smooth staining in the apex that responds to changes in the growth axis. FM4–64 was then used in subsequent experiments where co-labelling was required while FM1–43 was used in single-dye experiments. As discussed previously2,8 the FM signal is likely to be a combination of new internalysed dye and movement of previously stained vesicles from the sub-apex. In order to consider these two aspects, we measured fluorescence intensity both in the apical and sub-apical region but for matters of simplicity, we represent graphically only the ratio (A/SA) of these two FM signals.
Collapse events of FM signal precede reorientation.
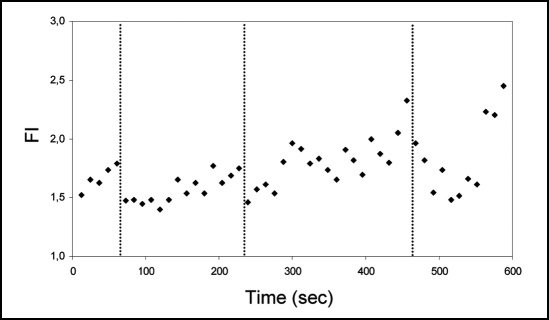
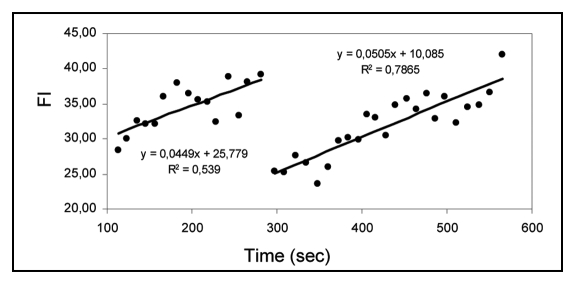
When the fluorescence dynamics of both FM dyes is followed during pollen tube growth, changes in the apical signal are clearly visible (Fig. 1A and B; n = 8). We noticed that these changes had a pattern; it consisted on a slow and steady rise of fluorescence intensity followed by a rapid and abrupt signal loss-collapse event (Fig. 2) that could reach down to 35% of the total FM apical signal. When we compared this pattern with the pollen tube morphology we observed that in over 70% of the cases the collapse event preceded a change in the growth axis. This suggests a rapid increase in fusion of apical labelled vesicles with the plasma membrane before reorientation followed by a steady reposition of newly labelled vesicles. A linear correlation analysis confirmed the significance of such events as shown in Figure 3. This tendency is to be interpreted in the data context and not as isolated data linearization; that is, the correlation factor presented (R2) serves as an illustration of the linearization method used.
Figure 2.
Typical variation of the FM4–64 fluorescent signal in a growing pollen tube. Since FM signal is a combination of new internalysed dye and movement of previously stained vesicles, fluorescence intensity (FI) was represented as the ratio apical/sub-apical signal (measured respectively in the first 10 µm and 10–20 µm of an individual A. umbellatus pollen tube apex). Equivalent data were obtained in 8 independent experiments. Associated to changes in the growth axis (indicated by vertical dashed lines), one can observe rapid decreases (“collapse event”) of apical/sub-apical FM signal followed by slower recovery periods where growth is approximately straight.
Figure 3.
Linear regression analysis (solid line) of FM4–64 apical fluorescent signal (dots) experiencing a change in growth axis at sec 300. A linear pattern can be identified during straight growth modes. The collapse event that precedes reorientation interrupts the linear pattern.
Relationship between [Ca2+]c and apical secretory events.
Considering our previous observations of a close relationship between apical [Ca2+]c and vesicle fusion and recycling, we decided to investigate their dynamics during the so-called collapse events. For that purpose, we co-loaded growing pollen tubes with CG-1 dextran and FM4–64 (n = 16). These two probes have non-overlapping emission spectra and can be simultaneously excited using the 488 and 568 nm lines of the Kr-Ar laser. CG-1, a Ca2+-specific dye, is not membrane-permeable so cells were previously microinjected with a stock solution and allowed to fully recover. Once normal growth resumed, cells were then exposed to the FM4–64 solution (membrane-permeable); imaging started ∼10 min after treatment with FM dye. The simultaneous labelling with FM4–64 precluded the use of additional volume markers for [Ca2+]c ratio imaging. However, we have previously demonstrated the usefulness of CG-1 alone to measure qualitative changes in apical [Ca2+]c5,23 and here we make use of such methodology.
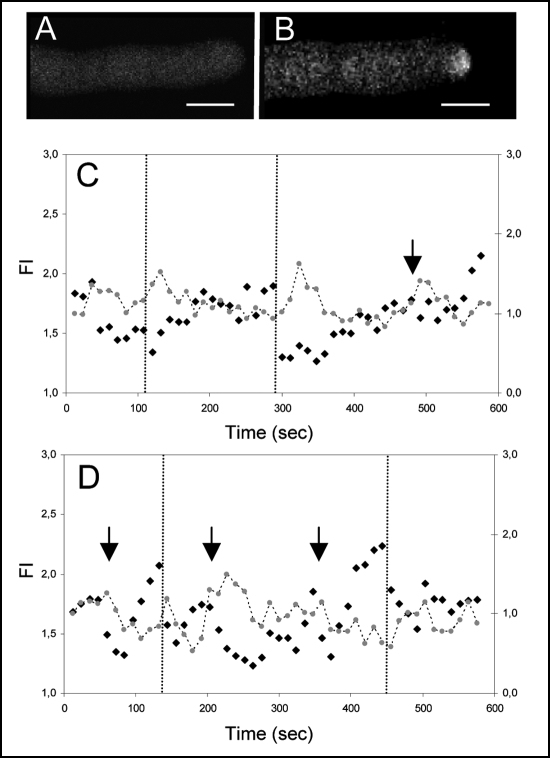
Figure 4 shows the results of such co-labelling experiments. The collapse events that precede reorientation of the tube growth axis are marked (vertical dashed lines) and interestingly, we found that the drop in the FM signal is not preceded by any significant increase in [Ca2+]c (Fig. 4C). In fact, a clear rise in [Ca2+]c is only visible upon reorientation, reaching its maximum once the change in the growth axis occurs. This observation suggests that an increase in [Ca2+]c is not the first signal to trigger apical secretion but that it is involved in sustaining (and perhaps enforcing) such signal. We had reported previously5 that discrete increases in [Ca2+]c confined to a sub-domain of the apical region (induced by photorelease of caged-Ca2+) could promote a predictable change in the pollen tube growth axis. Although the photorelease experiments induced a much higher Ca2+ transient, we looked for spontaneous [Ca2+]c increases not coupled to collapse events and compared them to the apical morphology. Figure 4C shows one of such peaks at ∼500 sec (arrow). Noteworthy, to such peaks there was no reorientation associated. In the specific case of the pollen tube represented, and when compared to the two prior reorientation events, one can note the following: (1) the intensity and decrease in the FM signal is of smaller magnitude and (2) the magnitude of the [Ca2+]c peak is also smaller. An identical situation is reported in Figure 4D. This suggests that for the reorientation process to be triggered, a threshold in either (or both) apical secretion and [Ca2+]c increases must be achieved thus reinforcing the idea of a close link between these two aspects.
Figure 4.
Fluorescence imaging of the FM4–64 and Calcium Green-1 apical staining in a pollen tube experiencing changes in growth direction. (A) Confocal image of the CG-1 distribution. (B) Confocal image of the FM4–64 distribution. Scale bar = 10 µm. (C) Numerical representation of FM 4–64 (black dots, Y1 axis) and CG-1 (Grey dots, Y2 axis) apical fluorescence over time. Vertical lines represent changes in the pollen tube growth axis. The collapse events of FM signal precede such changes but distinct elevations of [Ca2+]c are only visible afterwards. Fluorescence intensity (FI) was measured as in Figure 2. Equivalent data were obtained in 16 independent experiments.(D) Numerical representation of FM 4–64 (black dots) and CG-1 (Grey dots) apical fluorescence over time. Measurements and symbols as in C. At ∼500 sec, a peak in [Ca2+]c could be observed without a correspondent collapse event of the FM signal; in such cases, reorientation of the growth axis was not evident.
Discussion
Pollen tube growth in vivo through the style is guided by molecular cues but also constricted by physical structures.24 Although it is not clear at present which molecular mechanism is more important for directing growth (if any), fast reorientation capabilities are required to achieve fertilization. These transient changes in the polarity axis are known to involve structural elements (e.g., secretory vesicles) and signalling molecules (Ca2+, calmodulin, protein kinases, etc.13) but we know little about the order upon which they are called to act. Here we discuss the correlation between apical secretion and [Ca2+]c during growth and reorientation events.
The regulation of endo- and exocytosis by Ca2+ ions.
[Ca2+]c is a key element in the regulation of pollen tube elongation and guidance. A tip focused [Ca2+]c gradient has been imaged with a high 1–3 µM Ca2+ concentration in the tip region and a low 0.2–0.3µM Ca2+ concentration in the sub apical and basal part of the tube.7 At basal levels streaming occurs normally; when the concentration is elevated to 1 µM or higher, streaming is rapidly but reversibly inhibited.25 High Ca2+ is known to fragment F-actin26 and to inhibit myosin motor activity thus apical [Ca2+]c is likely to have an important role in the local regulation of vesicle docking and fusion with the plasma membrane.27 Indeed, we have demonstrated using caged-Ca2+ and the FM1–43 dye8 that localized increases in Ca2+ modify apical secretion within the apical dome and are able to trigger reorientation. This does not necessarily mean that Ca2+ is always the primary event triggering reorientation. The photolysis of caged-Ca2+ releases high amounts of ion which is unlikely to occur in non-stimulated cells. Such experiments can be regarded as mimicking a Ca2+ increase triggered by an extracellular stimulus but it has been shown that other molecules are also able to induce reorientation. This supports our hypothesis that transduction of guidance signals depends not of one, but of several signalling pathways that crosstalk.
It then remains to be elucidated what is the normal sequence of events in non-stimulated cells. For that purpose we resorted to in vitro growth systems. Aside from technical simplicity, pollen tubes grown in an homogenous simple culture medium are not exposed to guidance signals as those likely to be found in the female tissue (e.g. ,physical constraints, gradients, complex molecules).28
Increases in [Ca2+]c follow peaks of apical secretion.
The mechanisms which enforce and regulate the [Ca2+]c gradient at the tube apex are still controversial. Apical influx of extracellular Ca2+ is required but there is an apparent discrepancy between internal Ca2+ measurements and external Ca2+ fluxes7 that suggest the existence of primary mechanisms to regulate the ion dynamics. The cell wall and/or internal stores13 were suggested to play an important role and at the molecular level, GTPases, phosphoinositides and signalling phospholipids have been proposed to act in this process.9,29,30 Furthermore, during oscillatory growth [Ca2+]c peaks after growth rate21 and that led us to investigate a possible correlation between [Ca2+]c and secretory events (upon which cell wall assembly depends).
We had previously observed2 that in A. umbellatus pollen tubes the apical FM fluorescent signal exhibited significant variations. Usually, a rapid decrease in apical signal was followed by a steady increase/recovery. We designated this rapid decrease as “collapse event” (in analogy to the catastrophic events of microtubule polymerization) and found that in more than 70% of the cases it was associated with a subsequent change in growth axis. A linear regression analysis shows indeed a strong link between reorientation and the collapse events. We interpret this as the required fusion of vesicles to promote reorientation. When we simultaneously imaged [Ca2+]c and secretory events, we observed that the collapse event was not preceded by a distinguishable rise in apical [Ca2+]c which only occurred concomitantly with reorientation. Furthermore, the highest [Ca2+]c value was often observed after the change in growth axis becomes visible which is in agreement with our previous data.5 These data suggest that in non-stimulated cells, reorientation is not initially triggered by [Ca2+]c but changes in this ion are required for the transduction of the signal. Such role of [Ca2+]c as a second messenger reinforces our hypothesis of signalling loops controlling reorientation.
If distinct raises in [Ca2+]c do not precede the collapse event, what is then the initial mechanism triggering fusion? Yang and co-workers30,31 have suggested that active ROP1 protein defines the active growth domain by spatially regulating vesicle delivery and fusion. The activity of ROP1 would, in turn, be controlled by effectors such as RIC3 and RIC4. A similar role was suggested for another small GTPase, Rab11.32 Altering Rab11 activity by expressing either a constitutive active or a dominant negative variant of Rab11b in pollen resulted in reduced tube growth rate and meandering pollen tubes. These mutant GTPases also inhibited targeting of exocytic and recycled vesicles to the pollen tube inverted cone region and compromised the delivery of secretory and cell wall proteins to the extracellular matrix.32 Signalling phospholipids (e.g., phosphatidic acid2) and fusogenic proteins27 might also be involved again arguing strongly for an intricate signalling network. We cannot rule out the hypothesis of discrete domains of high Ca2+ initiating the process and leading to a positive feed-back influx of this ion; the methodology used in this work does not have the spatial resolution to validate or discard such hypothesis. But the data acquired with high temporal resolution of extracellular Ca2+ fluxes21,33 indicates that influx peaks after growth pulses thus favoring a secondary messenger role for Ca2+ ions.
What model for apical secretion?
We have recently suggested that fusion of apical vesicles could occur in one of two modes, depending on the circumstances. A rapid endocytosis mechanism might occur in the apex of rapidly straight growing pollen tubes.1,2 This mechanism is compatible with extremely fast delivery of wall material and is a Ca2+-dependent process thus fitting our observations during straight growth phases. A second mode, where endo- and exocytosis are uncoupled, would be favoured in situations where the cell needs to interpret extracellular cues and reorient the growth axis. In such case, Ca2+ acts mainly as a second messenger transducing reorientation signals and primary control of membrane recycling would rely on other signalling components (e.g., GTPases). Our previous8 and present data support such hypothesis and in barley protoplasts, Homann and Tester34 have reported the existence of two exocytotic modes, a Ca2+-dependent and a GTP-binding-dependent mode. Roy et al.,20 using the Yariv reagent, concluded that a Ca2+-dependent exocytosis served mainly to secrete cell wall components. It is likely that other signalling components such as small GTPases play an active role in endo/exocytosis by coupling the actin cytoskeleton to the sequential steps underlying membrane trafficking at the site of exocytosis. This hypothesis is supported by the recent discovery of Rho and Rab effectors of the exocyst complex and their importance for pollen tube growth.35 Changes in the intracellular levels of phosphoinositides, CaM and cAMP also modify growth rate and orientation through modulation of apical secretion.9,19
Functional zonation in the pollen tube: The role of the apical and sub-apical regions.
Underlying the structural zonation described for pollen tubes is the idea of a continuous flux of components towards the apex (vesicles, proteins, wall precursors). However, technological advances and re-evaluation of older data revealed that this is a too simplistic view. For example, it is now generally accepted that early mapping of actin, myosin and calmodulin distribution were flawed by experimental artefacts.
The observations of vesicle dynamics we report in this work suggest that most of the secretory vesicle processing (movement, fusion, recycling) is confined to the sub-apical and apical region. Intracellularly, FM labeling is restricted to these regions and vacuolar regions do not stain (within the time course of our experiments). Fusion will take place mainly at the apex and recycling at the sub-apex forming a sort of spatially restricted loop (Fig. 5). Similar observations have been made in root hairs36,37 where the endocytic markers were shown to rapidly recycle between the endosomes, the Trans Golgi Network (TGN) and the plasma membrane thus resembling synaptic vesicles. Budding profiles of the TGN have been suggested to invade the root hair apex38 and similar structures were observed in pollen tubes1. Dettmer and co-workers further showed that endocytosed material is redirected into the secretory flow after reaching the TGN.39 Interestingly, such fusion-recycling loop resembles the electric dipole described for these areas,40 two events that can enforce a positive feed-back and thus maintain a highly polarized domain. In the absence of chemical cues and physical constraints, it is possible that stochastic reorientation of the growth axis occur as a consequence of membrane fusion and recycling events that modify the spatial positioning of membrane machinery and/or the tension applied to the membrane (resulting in activation of stretch-activated channels5). [Ca2+]c oscillations are also confined to this area7 and recent findings on GTPase localization,30,32 the presence of an exocyst complex35 and actin dynamics25 further suggest that the apical and sub-apical regions contain all the necessary enzymatic machinery to maintain growth (protein synthesis and turn-over, lipid metabolism, assembly of complexes, etc.). This challenges the idea of a continuous flux of components from older parts of the tube and raises new questions about the physiology and biochemistry of the pollen tube.
Figure 5.
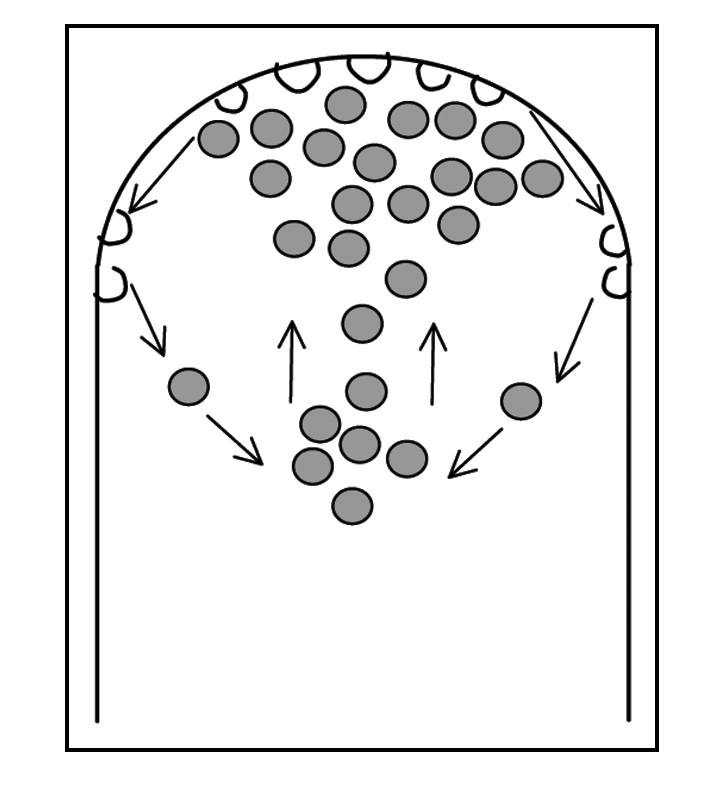
Diagram representing the movement of labelled vesicles (grey) towards the apical region, subsequent fusion with the apical plasma membrane and membrane recycling. This cycle of secretion and recycling gives rise to the typical inverted cone shape visible with FM dyes.
Acknowledgements
The work was supported by Fundação Ciência e Tecnologia, Lisboa, Portugal (Grant No BIA-BCM/56997/2004; FEDER).
Abbreviations
- [Ca2+]c
cytosolic free calcium
- CG-1
calcium green dextran
- TGN
trans golgi network
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2999
References
- 1.Malhó R, Castanho Coelho P, Pierson E, Derksen J. Endocytosis and membrane recycling in pollen tubes. In: Samaj J, Baluska F, Menzel D, editors. The Plant Endocytosis. Germany: Springer-Verlag; 2005. pp. 277–291. [Google Scholar]
- 2.Monteiro D, Liu Q, Lisboa S, Scherer GEF, Quader H, Malhó R. Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J Exp Bot. 2005;56:1665–1674. doi: 10.1093/jxb/eri163. [DOI] [PubMed] [Google Scholar]
- 3.Pierson ES, Miller DD, Callaham DA, Shipley AM, Rivers BA, Cresti M, Hepler PK. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell. 1994;6:1815–1828. doi: 10.1105/tpc.6.12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathore KS, Cork RJ, Robinson KR. A cytoplasmic gradient of Ca2+ is correlated with the growth of Lily pollen tubes. Develop Biol. 1991;148:612–619. doi: 10.1016/0012-1606(91)90278-b. [DOI] [PubMed] [Google Scholar]
- 5.Malhó R, Trewavas A. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell. 1996;8:1935–1949. doi: 10.1105/tpc.8.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierson E, Miller D, Callaham D, van Aken J, Hackett G, Hepler PK. Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 7.Holdaway-Clarke TL, Hepler PK. Control of pollen tube growth: role of ion gradients and fluxes. New Phytol. 2003;159:539–563. doi: 10.1046/j.1469-8137.2003.00847.x. [DOI] [PubMed] [Google Scholar]
- 8.Camacho L, Malhó R. Endo-exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J Exptl Bot. 2003;54:83–92. doi: 10.1093/jxb/54.380.83. [DOI] [PubMed] [Google Scholar]
- 9.Monteiro D, Castanho Coelho P, Rodrigues C, Camacho L, Quader H, Malhó R. Modulation of endocytosis in pollen tube growth by phosphoinositides and phospholipids. Protoplasma. 2005;226:31–38. doi: 10.1007/s00709-005-0102-x. [DOI] [PubMed] [Google Scholar]
- 10.Moutinho A, Hussey PJ, Trewavas AJ, Malhó R. cAMP acts as a second messenger in pollen tube growth and reorientation. Proc Natl Acad Sci USA. 2001;98:10481–10486. doi: 10.1073/pnas.171104598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidali L, Hepler PK. Actin and the pollen tube growth. Protoplasma. 2001;215:64–76. doi: 10.1007/BF01280304. [DOI] [PubMed] [Google Scholar]
- 12.Yoon GM, Dowd PE, Gilroy S, McCubbin AG. Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell. 2006;18:867–878. doi: 10.1105/tpc.105.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhó R, Camacho L. Signalling the cytoskeleton in pollen tube germination and growth. In: Hussey PJ, editor. The Plant Cytoskeleton in Cell Differentiation and Development. UK: Blackwell Publishers; 2004. pp. 240–264. (Ann Plant Review Series). [Google Scholar]
- 14.Samaj J, Baluska F, Voigt B, Schlicht M, Volkmann D, Menzel D. Endocytosis, actin cytoskeleton, and signaling. Plant Physiol. 2004;135:1150–1161. doi: 10.1104/pp.104.040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer-Parton S, Parton R, Hickey P, Dijksterhuis J, Atkinson H, Read N. Confocal microscopy of FM4–64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J Microsc. 2000;198:246–259. doi: 10.1046/j.1365-2818.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 16.Braun M, Sievers A. Role of the microtubule cytoskeleton in gravisensing Chara rhizoids. Eur J Cell Biol. 1994;63:289–298. [PubMed] [Google Scholar]
- 17.Parton RM, Fischer-Parton S, Trewavas AJ, Watahiki MK. Pollen tubes exhibit regular periodic membrane trafficking events in the absence of apical extension. J Cell Sci. 2003;116:2707–2719. doi: 10.1242/jcs.00468. [DOI] [PubMed] [Google Scholar]
- 18.Picton JM, Steer MW. The effects of ruthenium red, lanthanum, fluorescein isothiocyanate and trifluoperazine on vesicle transport, vesicle fusion and tip extension in pollen tubes. Planta. 1985;163:20–26. doi: 10.1007/BF00395892. [DOI] [PubMed] [Google Scholar]
- 19.Rato C, Monteiro D, Hepler PK, Malhó R. Calmodulin activity and cAMP signalling modulate growth and apical secretion in pollen tubes. Plant J. 2004;38:887–897. doi: 10.1111/j.1365-313X.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- 20.Roy SJ, Holdaway-Clarke TL, Hackett GR, Kunkel JG, Lord EM, Hepler PK. Uncoupling secretion and tip growth in lily pollen tubes: evidence for the role of calcium in exocytosis. Plant J. 1999;19:379–386. doi: 10.1046/j.1365-313x.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- 21.Messerli MA, Creton R, Jaffe LF, Robinson KR. Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev Biol. 2000;222:84–98. doi: 10.1006/dbio.2000.9709. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Kong L, Hao H, Wang X, Lin JJ, Samaj J, Baluska F. Effects of Brefeldin A on pollen germination and tube growth. Antagonistic effects on endocytosis and secretion. Plant Physiol. 2005;139:1692–1703. doi: 10.1104/pp.105.069765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camacho L, Parton R, Trewavas AJ, Malhó R. Imaging cytosolic free-calcium distribution and oscillations in pollen tubes with confocal microscopy: a comparison of different dyes and loading methods. Protoplasma. 2000;212:162–173. [Google Scholar]
- 24.Sanchez A, Bosch M, Bots M, Nieuwland J, Feron R, Mariani C. Pistil factors controlling pollination. Plant Cell. 2004;16:98–106. doi: 10.1105/tpc.017806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokota E, Shimmen T. The actin cytoskeleton in pollen tubes: actin and actin binding proteins. In: Malhó R, editor. The Pollen tube—A cellular and molecular perspective. Germany: Springer-Verlag; 2006. pp. 139–155. [Google Scholar]
- 26.Yokota E, Takahara K, Shimmen T. Actin-bundling protein isolated from pollen tubes of lily: biochemical and immunocytochemical characterization. Plant Physiol. 1998;116:1421–1429. doi: 10.1104/pp.116.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battey NH, James NC, Greenland AJ, Brownlee C. Exocytosis and endocytosis. Plant Cell. 1999;11:643–659. doi: 10.1105/tpc.11.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lord EM. Adhesion and guidance in compatible pollination. J Exptl Bot. 2003;54:47–54. doi: 10.1093/jxb/erg015. [DOI] [PubMed] [Google Scholar]
- 29.Potocky M, Elias M, Profotova B, Novotna Z, Valentova O, Zarsky V. Phosphatididc acid produced by phospholipase D is required for tobacco pollen tube growth. Planta. 2003;217:122–130. doi: 10.1007/s00425-002-0965-4. [DOI] [PubMed] [Google Scholar]
- 30.Hwang JU, Lee Y, Ying G, Yang Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell. 2005;16:5385–5399. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang JU, Yang Z. Small GTPases and spatiotemporal regulation of pollen tube growth. In: Malhó R, editor. The Pollen tube - A cellular and molecular perspective. Germany: Springer-Verlag; 2006. pp. 95–116. [Google Scholar]
- 32.De Graaf BHJ, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu H-m. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell. 2005;17:2564–2579. doi: 10.1105/tpc.105.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holdaway-Clarke TL, Feijó JA, Hackett GR, Kunkel JG, Hepler PK. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 1997;9:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homann U, Tester M. Ca2+-independent and Ca2+/GTP-binding protein-controlled exocytosis in a plant cell. Proc Natl Acad Sci USA. 1997;94:6565–6570. doi: 10.1073/pnas.94.12.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole R, Synek L, Zarsky V, Fowler J. SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiol. 2005;138:2005–2018. doi: 10.1104/pp.105.062273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ovecka M, Lang I, Baluska F, Ismail A, Illes P, Lichtscheidl I. Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma. 2005;226:39–54. doi: 10.1007/s00709-005-0103-9. [DOI] [PubMed] [Google Scholar]
- 37.Voigt B, Timmers ACJ, _amaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H, Nakano A, Baluska F, Menzel D. Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur J Cell Biol. 2005;84:609–621. doi: 10.1016/j.ejcb.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 38.Preuss ML, Schmitz AJ, Thole JM, Bonner HKS, Otegui MS, Nielsen E. A role for the RabA4b effector protein PI-4Kβ1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol. 2006;172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. Vacuolar H+-ATPase activity Is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feijó JA, Shipley AM, Jaffe LF. Spatial and temporal patterns of electric and ionic currents around in vitro germinating pollen of lily. In: Heberle-Bors E, Hesse M, Vicente O, editors. Frontiers in Sexual Plant Reproduction. Austria: University of Vienna; 1994. p. 40. [Google Scholar]