Abstract
Bacteria monitor their population densities using low-molecular-weight ligands in a process known as quorum sensing. At sufficient cell densities, bacteria can change their mode of growth and behave as multicellular communities that play critical roles in both beneficial symbioses and the pathogenesis of infectious disease. The development of non-native ligands that can block quorum-sensing signals has emerged as a promising new strategy to attenuate these divergent outcomes. Here, we report that N-phenylacetanoyl-l-homoserine lactones are capable of either inhibiting or, in some cases, strongly inducing quorum sensing in the bacterial symbiont Vibrio fischeri. Moreover, simple structural modifications to these ligands have remarkable effects on activity. These studies have revealed one of the first synthetic superagonists of quorum sensing, N-(3-nitro-phenylacetanoyl)-l-homoserine lactone. Together, these ligands represent a powerful new class of chemical probes with the potential to significantly expand the current understanding of quorum sensing and its role in host/bacteria interactions.
Bacteria can assess their local population densities using low-molecular- weight molecules (auto-inducers) and alter gene expression at high cell number to behave as a group. This process, termed quorum sensing, is widely used by bacteria to initiate group behaviors that have direct and often devastating impacts on human health and the environment (1, 2). For example, numerous bacterial pathogens use quorum sensing to initiate infection (3, 4). In contrast, symbiotic bacteria use these pathways to commence mutually beneficial relationships with their hosts (2, 5, 6). Because these important processes are controlled by chemical signals, there is intense and growing interest in the development of non-native ligands that can intercept these signals and attenuate or mimic quorum-sensing outcomes (7).
Quorum sensing is best characterized in Gram-negative proteobacteria, which use diffusible N-acylated-l-homoserine lactones (AHLs) and their cognate receptors (R proteins) for intercellular communication (Figure 1, panel a) (2, 8). Considerable research efforts have focused on the synthesis of ligands that can disrupt AHL-R protein binding and inhibit quorum sensing (9, 10), yet potent and general R protein antagonists remain scarce. Likewise, compounds exhibiting heightened activities relative to native AHLs (i.e., superagonists of quorum sensing) are also of significant interest, because they could potentially initiate bacterial group behaviors at lower cell numbers than those required in natural environments. For example, superagonists could be used to determine whether beneficial symbioses could be initiated earlier during colonization by a symbiont or whether a pathogen could be forced to initiate infection too early and be cleared by a host’s immune response. Such experiments, if possible, would help illuminate the relationships between bacterial group behavior and host responses. However, to our knowledge, only two superagonists of R protein activity have been reported to date, and these compounds have yet to be tested in vivo (11, 12).
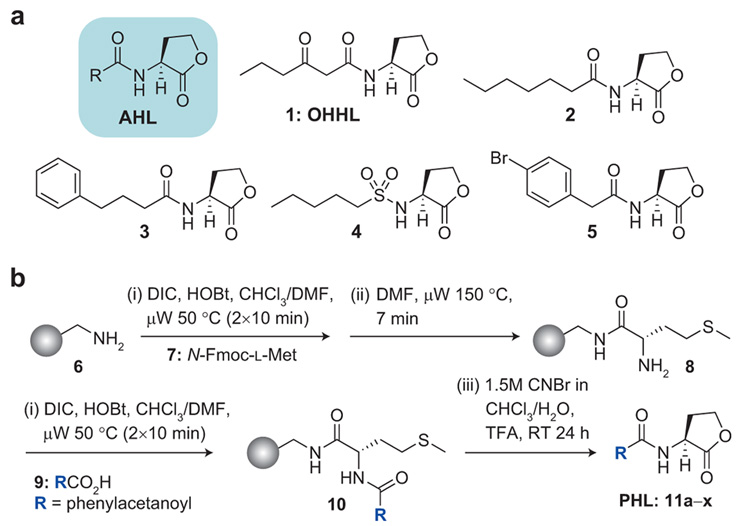
Figure 1. Ligand structures and synthesis.
a) Structures of a generic AHL, OHHL (1) (the natural autoinducer ligand of V. fischeri), and selected known synthetic inhibitors of LuxR or other R protein function (2–5). b) Solid-phase synthetic route to PHL library 11. DIC = N,N′-diisopropyl-carbodiimide. HOBt = N-hydroxybenzotriazole. µW = temperature-controlled microwave irradiation.
New synthesis and design strategies are needed to expand the current set of quorum-sensing modulators active in Gram-negative bacteria. Unfortunately, the structures of known antagonists and agonists vary widely, and their mechanisms of action are unclear; thus, no obvious rationales have emerged for new ligand design. To address this problem, we have been engaged in the design of focused combinatorial libraries of ligands for the modulation of quorum sensing (13–15). Here, we report the discovery of a family of non-native AHLs capable of either inhibiting or, in some cases, strongly inducing quorum sensing in the marine symbiont Vibrio fischeri. In addition, we report the first superagonist of quorum sensing in V. fischeri. These ligands provide a new blueprint for the design of both quorum-sensing agonists and antagonists and represent powerful new chemical probes to investigate the mechanisms of bacterial communication.
V. fischeri colonizes the light-producing organs of certain marine fish and squids and uses quorum sensing to initiate bioluminescence at high cell densities (6, 8). Quorum sensing is mediated in part by an AHL signal, N-(3-oxo-hexanoyl)-l-homoserine lactone (OHHL, 1), and its cytoplasmic receptor, LuxR (Figure 1, panel a) (16). OHHL is synthesized by the LuxI protein at low basal levels, and high cell densities are required to achieve a sufficient concentration of OHHL for LuxR binding (≥100 nM in vivo (17)); thereafter, the OHHL–LuxR complex activates the transcription of luminescence genes and other genes involved in symbiosis and illuminates the fish or squid light organ. Quorum sensing in V. fischeri represents the best-characterized quorum-sensing signaling pathway to date (2); however, the role of quorum sensing in host–V. fischeri symbiosis is complex and remains poorly understood (6, 8, 16). As a first step toward studying the interplay between quorum sensing and bacterial symbioses, we sought to identify non-native signal molecules that could intercept quorum sensing in V. fischeri.
Certain AHLs with non-native acyl chains have been reported to inhibit LuxR protein function in reporter gene assays. These antagonists include N-heptanoyl-l-homoserine lactone (2) reported by Eberhard et al. (18) and Schaefer et al. (19) and N-(4-phenylbutanoyl)-l-homoserine lactone (3) and N-pentylsulfonyl-l-homoserine lactone (4) reported by Castang et al. (20) (Figure 1, panel a). Recent studies in our laboratory have shown that N-(4-bromo-phenylacetanoyl)-l-homoserine lactone (4-bromo-PHL, 5) is a potent antagonist of LuxR homologues in several other Gram-negative bacteria (Figure 1, panel a). For example, 4-bromo-PHL, 5, inhibits R protein function in Agrobacterium tumefaciens at a 1:1 ratio against native AHL ligand, as determined by reporter gene assays (13). Because the putative ligand binding sites of the known R proteins have considerable sequence homology (70–80%) (8, 21), we hypothesized that PHLs might also modulate LuxR activity in V. fischeri and, if so, they represented a promising ligand class with which to initiate this study.
PHLs can be readily synthesized using a microwave-assisted, solid-phase route previously reported by our laboratory (Figure 1, panel b) (13). Using this method, we synthesized a small focused library of 24 PHLs to systematically examine the effects of different phenylacetanoyl moieties on ligand activity. This route gave PHL products 11a–x (Table 1) in excellent purities (~95%), good isolated yields (>65%), and sufficient quantities (i.e., 30 mg per compound) for multiple biological experiments.
TABLE 1.
Structures and primary antagonism and agonism screening data for PHL library 11 in V. fischeri (Δ-luxI)
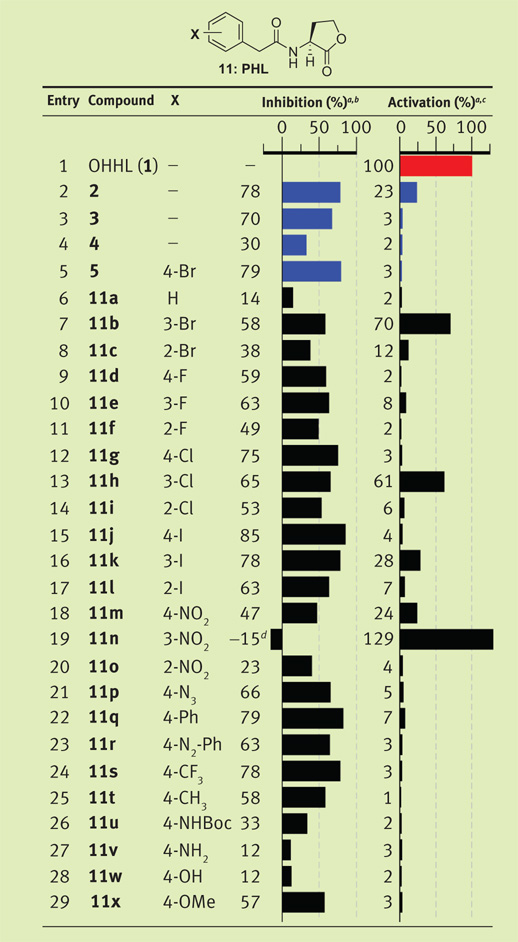 |
Strain: V. fischeri ES114 (Δ-luxI). Luminescence data measured in relative light units and normalized to OHHL (1, red). Control compounds shown in blue. All assays performed in triplicate; error did not exceed ±10% of the mean.
Screen performed using 5 µM synthetic ligand against 5 µM OHHL (1).
Screen performed using 200 µM ligand.
PHL 11n displayed 115% activation in this assay.
Non-native ligands are commonly assessed for R protein agonism and antagonism using bacterial reporter strains (7, 9, 10). These strains lack AHL synthase genes, but retain their native R genes. Exogenous AHL ligand is therefore required for R protein activation, which can be measured by reporter gene read-outs based on luminescence or fluorescence. The majority of synthetic LuxR modulators have been evaluated using the luxR plasmid pSB401 in various Escherichia coli strains (19, 20). We therefore began our biological evaluation of PHL library 11 using the E. coli strain JM109 (pSB401) (22). Competitive inhibition assays were performed in the presence of both OHHL and PHLs 11, while agonism assays were performed with PHLs alone. The known LuxR protein inhibitors 2–4 and 4-bromo-PHL 5 served as important controls for these studies (Figure 1, panel a). However, unacceptably large error values in the luminescence data (see Supplementary Figure 1) forced us to seek an alternative strain. We found that a Δ-luxI derivative of V. fischeri ES114 (16), in which the native lux operon behaves as the bioluminescent reporter, gave highly reproducible luminescence data in these assays. This nonstandard reporter strain was used for the subsequent primary antagonism and agonism assays in this study.
The antagonism assays in V. fischeri revealed several active PHL ligands and a number of striking structure–activity relationships (SARs) (Table 1). First, the control compound 4-bromo-PHL (5) showed 79% inhibition at a 1:1 ratio with native ligand 1 (Table 1, entry 5; both ligands at 5 µM). This result supported our hypothesis that PHLs could modulate LuxR function. Indeed, 50% of the PHL library 11 exhibited ≥50% inhibition in this assay. Inhibitory activity was extremely dependent on the substituents and their locations on the phenyl-acetanoyl group. For example, replacement of the 4-bromo substituent with a hydrogen in PHL 11a abolished inhibitory activity (Table 1, entry 6). PHLs with bromo (5, 11a,b), chloro (11g–i), and iodo substituents (11j–l) exhibited a ~10% increase in antagonism as the halogen was moved from the 2- to the 3- to the 4-position on the phenyl ring. Antagonistic activity also increased slightly with increasing halogen size, with 4-iodo-PHL (11j) exhibiting the highest activity (Table 1, entry 15; 85%) for the halogen series.
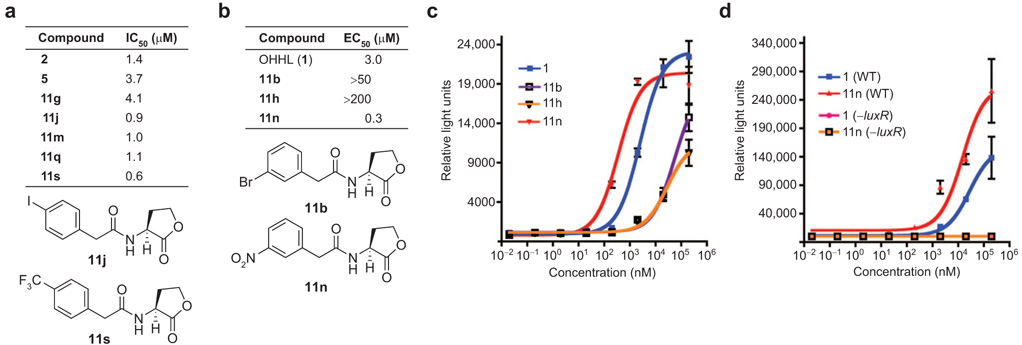
In general, sterically large and lipophilic groups in the 4-position enhanced PHL (11) antagonism in V. fischeri (Δ-luxI). This is exemplified by the high activities of 4-phenyl-PHL (11q) and 4-trifluoromethyl-PHL (11s) (~80% inhibition; Table 1). In contrast, hydrogen-bond-donating substituents in the 4-position engendered the lowest inhibitory activities (i.e., 4-amino-PHL, 11v, and 4-hydroxy-PHL, 11w). The nitro-PHL series (11m–o), however, showed a more complicated activity trend, with 3-nitro-PHL (11n) showing no inhibitory activity relative to the 2- and 4-nitro-PHLs (entries 18–20; see below). We determined IC50 values for the most potent PHL inhibitors identified in this assay, along with the most potent controls (2 and 5) for comparison. The 4-iodo-PHL (11j) and 4-trifluoromethyl-PHL (11s) exhibited the lowest IC50 values in this study, with PHL 11s at least two-fold more active than control compound 2 (0.6 µM vs 1.4 µM, respectively; Figure 2, panel a).
Figure 2. New synthetic modulators of LuxR.
a) IC50 table of most potent inhibitors and structures of selected compounds. b) EC50 table of most potent activators and structures of selected compounds. See Supporting Information for dose–response curves. c) Agonism dose–response curves for OHHL (1) and synthetic ligands 11b, 11h, and 11n in V. fischeri ES114 (Δ-luxI). d) Agonism dose–response curves for OHHL (1) and 11n in wildtype (WT) V. fischeri ES114 and V. fischeri ES114 (Δ-luxR). (Note, we were unable to achieve a maximum level of luminescence over the concentrations tested and therefore could not determine accurate EC50 values in WT V. fischeri. Experiments at higher concentrations were precluded by the diminished solubilities of both OHHL (1) and PHL 11n at concentrations >500 µM.) Luminescence measured in relative light units. Error bars, standard deviation of the means of triplicate samples.
Similar assays were performed on PHL library 11 to screen for agonistic activity in V. fischeri (Δ-luxI). Again, we observed striking trends in the activities for PHLs with halogen and nitro groups (at 200 µM compound; Table 1). In contrast to the antagonism data, the 3-substituted compound in each of these PHL families showed the strongest activity relative to the 2- and 4-substituted derivatives, with the 3-bromo-(11b), 3-chloro- (11h), and 3-nitro-PHLs (11n) exhibiting at least 60% luminescence induction relative to native OHHL at the same concentration. Remarkably, simply shifting substituents on the PHL phenyl ring by a single carbon converted these ligands from LuxR antagonists to LuxR agonists. Moreover, 3-nitro-PHL (11n) was able to induce 29% higher luminescence than OHHL in this primary assay (Table 1, entry 19). This result was extraordinary and explained the unusual inhibition trends for the nitro-PHL series (11m–o; see above).
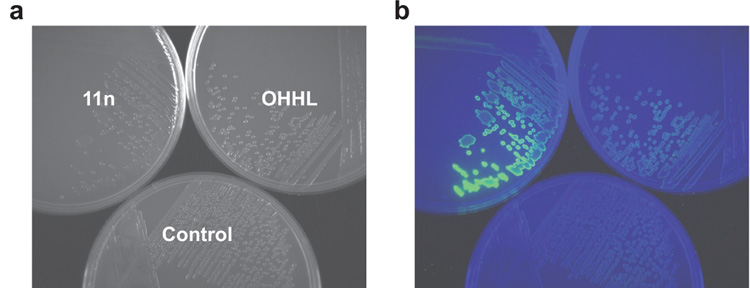
For a more quantitative comparison of ligand activity, we determined EC50 values for our most active PHL agonists (11b, 11h, and 11n) and OHHL in V. fischeri (Δ-luxI) (Figure 2, panels b and c). These studies identified 3-nitro-PHL (11n) as the most active LuxR agonist, exhibiting a 10-fold lower EC50 than OHHL (0.3 µM vs 3 µM, respectively). We performed analogous dose–response studies with 11n and OHHL in wild-type V. fischeri ES114 (23) and observed similarly heightened activity for PHL 11n relative to OHHL (Figure 2, panel d). The superagonistic activity of PHL 11n in V. fischeri relative to OHHL could be easily visualized by luminescence imaging with a CCD camera; Figure 3 shows a representative image of these experiments. We also examined the activity of 11n and OHHL in a Δ-luxR derivative of V. fischeri ES114. Neither 11n nor OHHL induced any detectable luminescence in this strain (Figure 2, panel d), which suggests that 11n, like the native ligand OHHL, exerts its activity through the LuxR protein (see below). The discovery of compound 11n is significant, because it is one of the first synthetic superagonists of quorum sensing reported (11, 12) and to our knowledge the first superagonist in V. fischeri.
Figure 3. Bioluminescence induction by OHHL and quorum-sensing superagonist 11n in V. fischeri.
a) V. fischeri ES114 (Δ-luxI) streaked onto agar plates containing either no compound (control), 0.5 µM OHHL (1), or 0.5 µM superagonist 11n and grown for 8 h and imaged. b) Identical plates from panel a imaged for luminescence using a CCD imager.
In view of the structural similarity of PHLs 11 to native AHLs and the assay data and subtle SAR described above, we hypothesize that these ligands target the LuxR ligand-binding site and inhibition or activation is based on the specific binding mode and thus resulting affinity of the ligand. Further, we do not believe that these changes in activity simply reflect the different chemical properties of the PHLs (11) (10). This view is supported by several observations. First, the percentage of PHL 11 lactone hydrolysis (which abolishes activity for native AHLs (24)) is minimal and identical to that of OHHL over the time course of these luminescence assays. Second, the functionalities on PHLs 11 are unreactive under the assay conditions tested (see Methods). Third, higher lipophilicity within the PHL series, and therefore higher potential cell permeability, does not correlate with enhanced antagonistic or agonistic activity (Table 1). This is further exemplified by the d-enantiomers of control antagonists 3 and 5, which have identical lipophilicities as 3 and 5 yet exhibit markedly reduced activities (~20% inhibition; see Supplementary Figure 6). We have performed molecular modeling studies of several PHLs and OHHL docked into the putative ligand binding site of LuxR (built in silico from the one known structure of a complete R protein, TraR (20, 21, 25, 26); see Supplementary Figure 7–Supplementary Figure 13) to further test this hypothesis. The results of these studies suggest that the LuxR binding site can readily accommodate PHLs yet is better able to accommodate 3-substitutued PHLs (agonists) relative to 4-substititued PHLs (antagonists) and that activation or inhibition of LuxR may depend on the balance of favorable hydrogen bonding and unfavorable steric interactions within the binding pocket. While additional biochemical and structural studies will be required to fully elucidate PHL function in V. fischeri, these initial calculations provide support that PHLs 11 target LuxR.
In summary, we have discovered that PHLs 11 elicit remarkable and varied quorum-sensing responses in the bacterial symbiont V. fischeri. This family of ligands includes some of the most active antagonists and agonists of Gram-negative bacterial quorum sensing reported to date. One significant outcome of this work is the observation that subtle alteration of substituents and their placement on the phenylacetanoyl moiety dramatically influence ligand activity. These changes do not simply abolish activity but rather convert potent antagonists into agonists. A second major outcome of this investigation is the discovery of the first synthetic superagonist of quorum sensing in V. fischeri, PHL 11n. This ligand displays 10-fold higher activity relative to native autoinducer OHHL and is one of the first known superagonists of quorum sensing in Gram-negative bacteria (11, 12). Collectively, PHLs represent a new and valuable set of chemical tools for the study of quorum sensing in V. fischeri and could, with further development, provide broad insights into the roles of quorum sensing in bacterial symbioses. Preliminary experiments indicate superagonist 11n is well tolerated by the main symbiotic partner of V. fischeri, the Euprymna scolopes squid (6), and is active in vivo; these studies will be reported in due course.
METHODS
Ligand Synthesis
PHL library 11, OHHL (1), and control compounds 2, 3, and 5 were prepared using reported methods (13) (Figure 1, panel b), except the final cyclization–cleavage step was performed at RT for 24 h. Control 4 was synthesized in an analogous fashion except 4-dimethylaminopyridine and 1-pentanesulfonyl chloride replaced reagents i and 9 (Figure 1, panel b). d-AHLs were accessible using N-Fmoc-d-methionine in place of 7. Compounds were submitted to biological assays following cleavage and an aqueous work-up without further purification. See Supporting Information for compound characterization data.
Compound Handling and Reagents
Stock solutions of synthetic compounds (10 mM) were prepared in DMSO. The structures of PHLs 11 and the control compounds remained unchanged after prolonged storage in DMSO (~4–6 months) and over the course of the biological assays (as determined by GC/MS, NMR, or both). Nitro-PHL derivatives (11m–o) were stored in the dark and not observed to degrade over the course of these studies. All biological reagents were purchased from Fisher Scientific. Luria–Bertani (LB) and LB salt media (LBS) were prepared as instructed with pH = 7.5 (LBS contained an additional 1.5% (w/v) NaCl, 0.3% (w/v) glycerol, and 50 nM Tris-HCl (16)).
Bacterial Strains
The E. coli strain used for these studies was JM109 (pSB401) (22). The V. fischeri strains were ES114 (23), ES114 (Δ-luxI), and ES114 (Δ-luxR) (16).
Luminescence Assays
For agonism assays, an appropriate amount of AHL stock solution was added into a 96-well plate. An overnight culture of E. coli or V. fischeri was diluted 1:10 with appropriate media (LB plus 10 µg mL−1 tetracycline for E. coli; LBS for V. fischeri). A 200-µL portion of the diluted culture was added to each well of the plate. Plates were grown for 4–8 h with shaking (200 rpm; 30 °C for E. coli and RT for V. fischeri). Luminescence was measured using a multilabel plate reader and normalized to cell density. Antagonism screens were performed in an analogous manner against OHHL at its approximate EC50 value (10 nM in E. coli; 5 µM in V. fischeri). Similar methods were used for dose–response assays, except the concentrations of PHL (11) or control varied between 2 × 10−2 and 2 × 105 nM. None of the PHLs (11) or control compounds in this study inhibited bacterial growth or displayed insolubility over the range of concentrations tested in the luminescence assays. All assays were performed in triplicate. Graphpad Prism software (version 5.0) was used to calculate IC50 and EC50 values. See Supporting Information for detailed assay protocols.
Supplementary Material
Supporting Information Available: This material is free of charge via the Internet.
Acknowledgment
We thank the National Institutes of Health (Grant AI063326-01), Research Corp., Burroughs Welcome Fund, Greater Milwaukee Foundation Shaw Scientist Program, Johnson & Johnson, and DuPont for financial support of this work. H.E.B. is an Alfred P. Sloan Foundation Fellow. G.D.G and J.C.O. were supported by an ACS Division of Medicinal Chemistry Graduate Fellowship and a Novartis Graduate Fellowship in Organic Chemistry, respectively. We thank E. Ruby for donations of bacterial strains and contributive discussions. S. Ho is acknowledged for luminescence imaging.
Footnotes
Note added after print publication: Because of a production error, the DOI was incorrectly listed. This error does not affect the scientific integrity of the article. This paper was originally posted May 4, 2007, and the electronic version was corrected and reposted to the web on May 22, 2007. An Addition and Correction appears in ACS Chem. Biol. 2 (6).
REFERENCES
- 1.Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 3.de Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg EP. Quorum sensing in Gram-negative bacteria: an important signaling mechanism in symbiosis and disease. In: Rosenberg E, editor. Microbial Ecology and Infectious Disease. Washington, DC: American Society for Microbiology; 1999. pp. 112–122. [Google Scholar]
- 6.Ruby EG. Lessons from a cooperative, bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu. Rev. Microbiol. 1996;50:591–624. doi: 10.1146/annurev.micro.50.1.591. [DOI] [PubMed] [Google Scholar]
- 7.Lyon GJ, Muir TW. Chemical signaling among bacteria and its inhibition. Chem. Biol. 2003;10:1007–1021. doi: 10.1016/j.chembiol.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Fuqua C, Greenberg EP. Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen TB, Givskov M. Quorum sensing inhibitors: a bargain of effects. Microbiology. 2006;152:895–904. doi: 10.1099/mic.0.28601-0. [DOI] [PubMed] [Google Scholar]
- 10.Welch M, Mikkelsen H, Swatton JE, Smith D, Thomas GL, Glansdorp FG, Spring DR. Cell-cell communication in Gram-negative bacteria. Mol. Biosyst. 2005;1:196–202. doi: 10.1039/b505796p. [DOI] [PubMed] [Google Scholar]
- 11.Muh U, Hare BJ, Duerkop BA, Schuster M, Hanzelka BL, Heim R, Olson ER, Greenberg EP. A structurally unrelated mimic of a Pseudomonas aeruginosa acyl-homoserine lactone quorum-sensing signal. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16948–16952. doi: 10.1073/pnas.0608348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssens JC, Metzger K, Daniels R, Ptacek D, Verhoeven T, Habel LW, Vanderleyden J, De Vos DE, De Keersmaecker SC. Synthesis of N-acyl homoserine lactone analogues reveals strong activators of SdiA, the Salmonella typhimurium LuxR homologue. Appl. Environ. Microbiol. 2007;73:535–544. doi: 10.1128/AEM.01451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geske GD, Wezeman RJ, Siegel AP, Blackwell HE. Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J. Am. Chem. Soc. 2005;127:12762–12763. doi: 10.1021/ja0530321. [DOI] [PubMed] [Google Scholar]
- 14.Lin Q, Blackwell HE. Rapid synthesis of diketopiperazine macroarrays via Ugi four-component reactions on planar solid supports. Chem. Commun. 2006:2884–2886. doi: 10.1039/b604329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorske BC, Blackwell HE. Interception of quorum sensing in Staphylococcus aureus: a new niche for peptidomimetics. Org. Biomol. Chem. 2006;4:1441–1445. doi: 10.1039/b517681f. [DOI] [PubMed] [Google Scholar]
- 16.Lupp C, Urbanowski M, Greenberg EP, Ruby EG. The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol. Microbiol. 2003;50:319–331. doi: 10.1046/j.1365-2958.2003.t01-1-03585.x. [DOI] [PubMed] [Google Scholar]
- 17.Boettcher KJ, Ruby EG. Detection and quantification of Vibrio fischeri autoinducer from symbiotic squid light organs. J. Bacteriol. 1995;177:1053–1058. doi: 10.1128/jb.177.4.1053-1058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eberhard A, Widrig CA, McBath P, Schineller JB. Analogs of the autoinducer of bioluminescence in Vibrio fischeri. Arch. Microbiol. 1986;146:35–40. doi: 10.1007/BF00690155. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer AL, Hanzelka BL, Eberhard A, Greenberg EP. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J. Bacteriol. 1996;178:2897–2901. doi: 10.1128/jb.178.10.2897-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castang S, Chantegrel B, Deshayes C, Dolmazon R, Gouet P, Haser R, Reverchon S, Nasser W, Hugouvieux-Cotte-Pattat N, Doutheau A. N-Sulfonyl homoserine lactones as antagonists of bacterial quorum sensing. Bioorg. Med. Chem. Lett. 2004;14:5145–5149. doi: 10.1016/j.bmcl.2004.07.088. [DOI] [PubMed] [Google Scholar]
- 21.Zhang RG, Pappas T, Brace JL, Miller PC, Oulmassov T, Molyneaux JM, Anderson JC, Bashkin JK, Winans SC, Joachimiak A. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature. 2002;417:971–974. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 22.Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR, Bycroft BW, Williams P, Stewart GS. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 1998;163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- 23.Boettcher KJ, Ruby EG. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glansdorp FG, Thomas GL, Lee JJK, Dutton JM, Salmond GPC, Welch M, Spring DR. Synthesis and stability of small molecule probes for Pseudomonas aeruginosa quorum sensing modulation. Org. Biomol. Chem. 2004;2:3329–3336. doi: 10.1039/B412802H. [DOI] [PubMed] [Google Scholar]
- 25.Persson T, Hansen TH, Rasmussen TB, Skinderso ME, Givskov M, Nielsen J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 2005;3:253–262. doi: 10.1039/b415761c. [DOI] [PubMed] [Google Scholar]
- 26.Frezza M, Castang S, Estephane J, Soulere L, Deshayes C, Chantegrel B, Nasser W, Queneau Y, Reverchon S, Doutheau A. Synthesis and biological evaluation of homoserine lactone derived ureas as antagonists of bacterial quorum sensing. Bioorg. Med. Chem. 2006;14:4781–4791. doi: 10.1016/j.bmc.2006.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: This material is free of charge via the Internet.