Abstract
The relationship between NF-κB and resistance to radiation treatment in many tumor cell types has been generally well recognized. However, which members of the NF-κB family contribute to radiation resistance is unclear. In the present study, we demonstrate that RelB plays an important radioprotective role in aggressive prostate cancer cells, in part by the induction of antioxidant and antiapoptotic manganese superoxide dismutase (MnSOD) gene. RelB is both constitutively present and is inducible by radiation in aggressive prostate cancer cells. Using ectopically expressed dominant negative inhibitor, p100 mutant, and the siRNA approach, we demonstrate that selective inhibition of RelB significantly decreases the levels of MnSOD resulting in a significant increase in the sensitivity of prostate cancer cells to radiation treatment. These results demonstrate that RelB plays an important role in redox regulation of the cell and protects aggressive prostate cancer cells against radiation-induced cell death. Thus, inhibition of RelB could be a novel mechanism to radiosensitize prostate cancer.
Keywords: RelB, radiation, MnSOD
Prostate cancer ranks third in cancer incidence and sixth in cancer mortality among men. Radiation is one of the modalities used to treat prostate cancer. In response to radiation, reactive oxygen species (ROS) are generated leading to the activation of redox-sensitive stress response pathways such as the NF-κB pathway (Criswell et al., 2003). NF-κB is a dimeric inducible transcription factor. NF-κB activity is modulated by its family members, consisting of five proteins: RelA, RelB, c-Rel, p50 (NF-κB1) and p52 (NF-κB2). NF-κB activates a large number of genes including manganese superoxide dismutase (MnSOD), a mitochondrial anti-oxidant enzyme that catalyses the conversions of toxic superoxide radicals to hydrogen peroxide and molecular oxygen (Weisigar and Fridovich, 1973). The MnSOD gene has NF-κB-binding sites in its promoter and enhancer which are necessary for induction by cytokines (Xu et al., 1999; Kiningham et al., 2001; Dhar et al., 2004). In response to radiation, NF-κB members activate the expression of MnSOD leading to tumor cell protection (Guo et al., 2003; Murley et al., 2004).
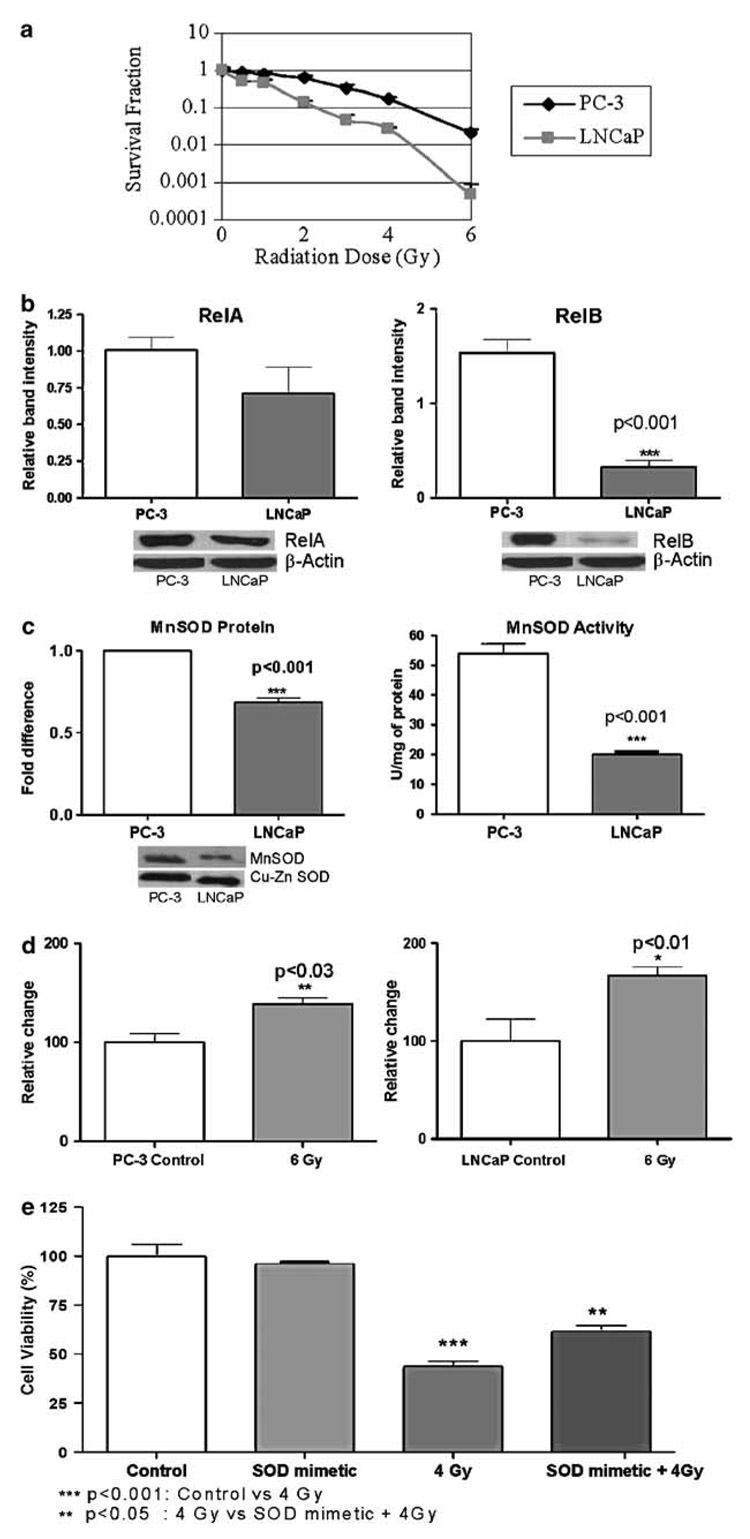
Although it is well established that NF-κB activity confers radiation resistance, which member of the NF-κB family contributes to radiation resistance is unclear. Using immunohistochemical studies, it has been demonstrated that all the members of the NF-κB family were expressed by normal prostate tissues, prostatic intraepithelial neoplasia and prostate cancer. However, only the nuclear localization of RelB correlated with the prostate cancer patient’s Gleason scores (Lessard et al., 2005), suggesting that the level of RelB is associated with prostate cancer progression. In this study, we examined the role of RelB in prostate cancer cells in response to radiation. We used an aggressive human metastatic prostate cancer cell line, PC-3, to represent the radiation-resistant type, and a nonaggressive prostate cancer cell line, LNCaP, to represent the radiation-sensitive cell type. We found that PC-3 cells were more resistant to radiation-induced cell death compared to LNCaP cells using the clonogenic survival assay (Figure 1a). The constitutive nuclear level of RelB was significantly higher in PC-3 compared to LNCaP cells. Whereas RelA was slightly increased, it was not judged to be statistically significant (Figure 1b). Consistent with the nuclear levels of RelB, the basal levels of MnSOD protein and MnSOD activity (Figure 1c) were significantly higher in PC-3 cells compared to LNCaP cells. Radiation caused a significant increase in superoxide levels in both cell lines (Figure 1d). However, a proportionately higher increase in superoxide radicals was detected in LNCaP cells after radiation, consistent with the intrinsically low levels of MnSOD protein. To verify that a low level of MnSOD is the cause of a high radiation sensitivity of LNCaP cells, a catalytic SOD mimetic (MnTE-2-PyP5+) was added to LNCaP cells before exposure to radiation. Using cell viability (MTT) assay, we observed a significant decrease in radiation sensitivity of LNCaP cells in the presence of the SOD mimetic (Figure 1e). These results suggest that high levels of nuclear RelB and MnSOD protein may be responsible for the intrinsic radiation resistance of PC-3 cells.
Figure 1. Increased nuclear protein levels of RelB correlate with intrinsic radiation resistance.
(a) Radiation resistances of PC-3 and LNCaP cells were determined by clonogenic survival assay. LNCaP cells were used from passages 1–31 and PC-3 from 76–90. A 130 kV X-ray machine (Faxitron X-ray corporation) was used and the dose rate was 89.7 cGy/min. Colony survival assay was performed as previously mentioned (Inayat et al., 2002). PC-3 cells took 12 days and LNCaP took 21 days to form colonies. (b) Nuclear protein levels of RelA and RelB in PC-3 and LNCaP cells were determined by Western analysis. Nuclear extract preparation and Western analysis were performed as previously described (Dhar et al., 2004). All antibodies, if not otherwise mentioned, were purchased from Santa Cruz Biotechnology. Protein bands were normalized to β-actin (Sigma). (c) Protein level of NF-κB target, MnSOD (Upstate Biotechnologies), was determined using Western analysis. Protein bands were normalized to cupper zinc superoxide dismutase (Cu-Zn SOD, Calbiochem) as their levels were not different in the cell lines used. Representative Western blots are attached below the graphs. MnSOD enzyme activity was measured using the modified nitroblue tetrazolium method (Spitz and Oberley, 1989). (d) Superoxide levels were measured using the oxidation of dihydroethidium dye (5 µM) (Molecular probes), immediately after radiation treatment (6 Gy). The excitation and emission wavelengths were 488 and 530 nm, respectively. (e) LNCaP cells were treated with SOD mimetic (MnTE-2-PyP5+ −80 pg/ml) and were used to confer protection in response to radiation treatment. These were demonstrated using a cell cytotoxicity assay (MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)) (Alley et al., 1988). Each experiment was repeated independently two to three times and statistically analysed by t-test or analysis of variance (ANOVA)– Tukey’s multiple comparisons test.
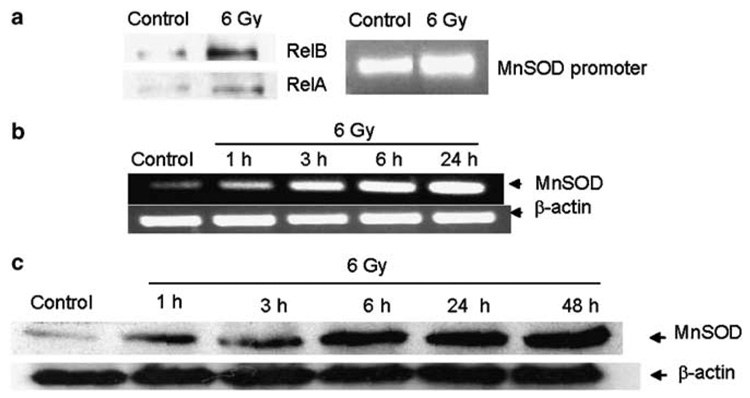
Using chromatin immunoprecipitation assay, we demonstrate that RelB was constitutively bound to MnSOD promoter in PC-3 cells and the binding was further increased in response to radiation (Figure 2a). The observed increase in PC-3 cells of RelB in response to radiation is consistent with the results from a study using microarray analysis of radiation-treated cancer cells (Amundson et al., 1999). To further elucidate the role of RelB in the response of PC-3 cells to radiation, the levels of specific sequences of sod2 promoter region after radiation were determined. The results indicate that RelB complexes were constitutively present on the MnSOD promoter and were increased upon radiation (Figure 2a). To determine whether these complexes are transcriptionally active, the levels of MnSOD mRNA after radiation were determined. The mRNA levels increased as early as 1 h and continued to increase with time after radiation (Figure 2b). This result is consistent with a previous study of MnSOD promoter activation, which characterized MnSOD promoter as a constitutively and immediately accessible promoter (Saccani et al., 2001). The MnSOD promoter has high basal histone H4 acetylation and undergoes further acetylation in an NF-κB-dependent manner allowing for immediate gene transcription (Saccani et al., 2001). Thus, RelB binding to the MnSOD promoter may allow for more accessibility for binding of other transcription factors including Sp1 (Xu et al., 2002) and coactivators (nucleophosmin) (Dhar et al., 2004). RelB itself could activate its own gene (Bren et al., 2001) and additional transcription factors to activate the MnSOD gene in response to radiation. Consistent with the induction of MnSOD, a time-dependent increase in MnSOD protein was observed paralleling the MnSOD mRNA levels (Figure 2c). The sustained increase in the MnSOD protein may be a protective adaptive response to radiation-initiated ROS generation. In the presence of oxygen, radiation generates large amounts of superoxide radicals within the cell. These radicals can be further propagated via radical chain reactions to activate transcription of antioxidant enzymes, such as MnSOD. These results suggest that RelB-mediated MnSOD induction is an early event in responding to radiation treatment.
Figure 2. Radiation treatment activates RelB leading to the induction of MnSOD in PC-3 cells.
(a) RelB DNA binding and MnSOD promoter occupancy in response to radiation were detected by chromatin immunoprecipitation (ChIP) assay. Cells were treated with 6Gy radiation and processed using the ChIP-IT kit (Active Motif, CA, USA). Protein/DNA interactions were fixed, and DNA was sheared and precipitated using p50 antibody. Immunocomplexes were detected for RelB and RelA by Western analysis. The DNA was reversed crosslinked, purified and then analysed by quantitative PCR. For amplifying the GC-rich MnSOD promoter region (fragment −154 to −6), an AccuPrimer GC-rich DNA polymerase (Invitrogen) was used. The polymerase chain reaction (PCR) conditions were 28 cycles of 95°C for 45 s, 60°C for 30 s and 72°C for 30 s. (b) MnSOD mRNA levels in response to 6Gy radiation were detected at different time points by reverse transcriptase (RT)–PCR reaction kit (SuperScript™ First-Strand Synthesis System for RT–PCR) (Invitrogen). β-Actin mRNA was used to normalize the bands. (c) NF-κB target gene product, MnSOD protein, was detected by Western analysis in response to 6Gy radiation at different time points in PC-3 cells. All experiments were repeated independently two to three times.
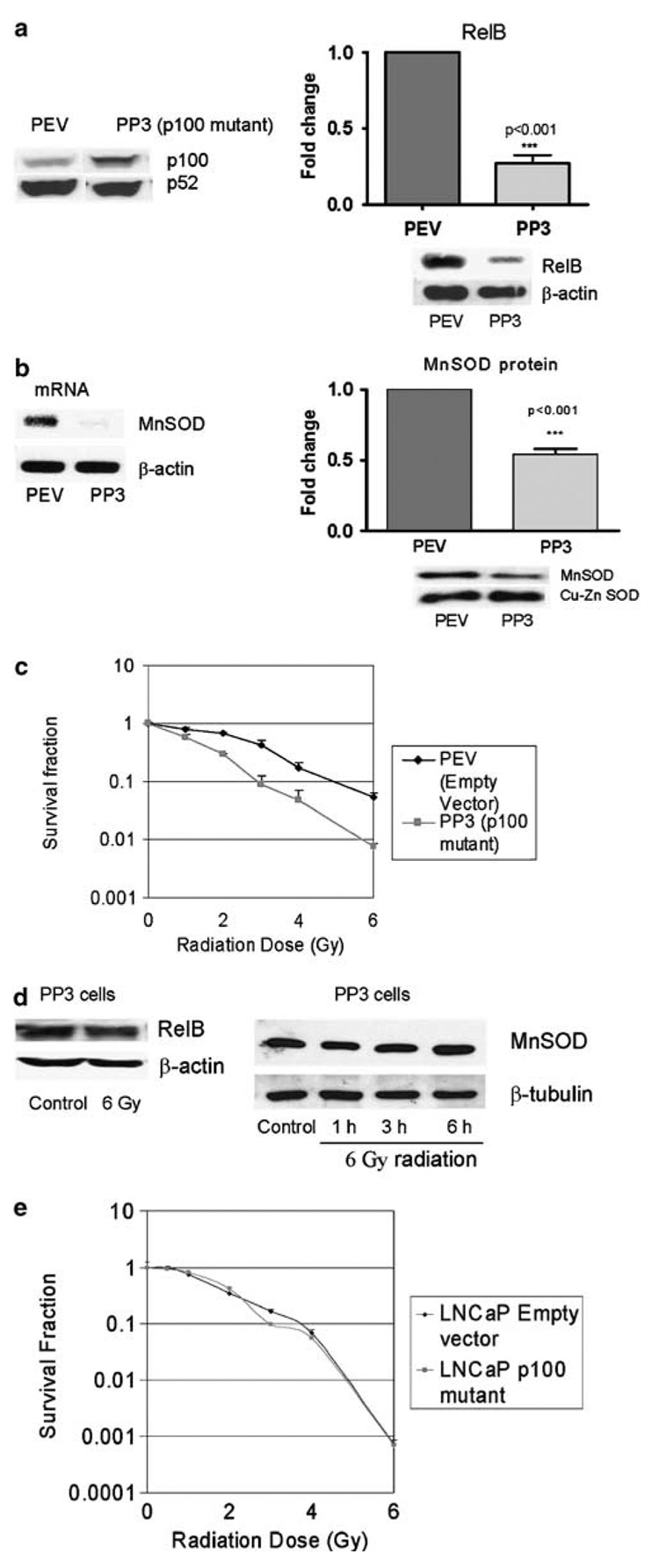
RelB is normally bound to p100, an inhibitor protein, which prevents its translocation to the nucleus (Solan et al., 2002). Upon cell stimulation, p100 is degraded resulting in the release of RelB and p52. RelB dimerizes with either p50 or p52 and translocates to the nucleus to transactivate genes (Ryseck et al., 1992). To further elucidate the direct role of RelB in radiation response, RelB function was inhibited by two different approaches, a dominant negative form of p100 and RelB-specific siRNA. The dominant negative p100 has its serine 866/870 mutated to alanine 866/870 (p100 mutant), preventing RelB translocation to the nucleus (Xiao et al., 2001). PC-3 cells were cotransfected with p100 dominant negative plasmid and a geneticin selectable marker plasmid or selectable marker plasmid alone. Single clones were selected from pooled clones. PEV represents an empty vector control-transfected clone and PP3 represents a p100 mutant-transfected clone, both of which are stable transfected clones. The presence of dominant negative p100 in PP3 cells was detected by Western analysis (Figure 3a). A correspondingly significant decrease in RelB levels was observed in the nucleus of the PP3 cells (Figure 3a). To further confirm if MnSOD, a target of RelB, is also reduced, the mRNA and protein levels of MnSOD in PP3 cells were determined. Inhibition of RelB nuclear translocation correlated with a significant decrease in MnSOD mRNA and protein levels (Figure 3b). Importantly, inhibition of RelB significantly sensitized the PP3 cells to radiation treatment, confirming the hypothesis that RelB has a protective role against radiation treatment in prostate cancer cells (Figure 3c). To determine if radiation induced levels of RelB in PP3 cells, the nuclear levels of RelB protein and its transcriptional product MnSOD were evaluated. No increase in RelB or MnSOD protein levels was observed (Figure 3d). These data indicate that inhibition of RelB and its antioxidant target sensitized the prostate cancer cells to ionizing radiation.
Figure 3. Inhibition of RelB translocation using p100 dominant negative in PC-3 cells resulted in suppression of radiation resistance.
(a) Expression of p100/p52 and nuclear RelB in PC-3 clones, PEV (PC-3 empty vector) and PP3 (PC-3 p100 mutant, p100 SS866/870AA) as determined by Western analysis. For the generation of stable clones, the plasmids were linearized with ScaI and transfected into PC-3 cells using Lipofectamine (Invitrogen) and were selected using 400 µg/ml geneticin (Life Technologies). Single clones were isolated from pooled transfected cells. These clones were used between passages 2 and 6. (b) MnSOD mRNA was detected by RT–PCR in PEV and PP3 cells. MnSOD protein was detected by Western analysis in PEV and PP3 cells. (c) Radiation sensitivity of PEV and PP3 cells was determined by clonogenic survival assay. (d) The expression levels in PP3 cells of nuclear RelB protein and its target MnSOD in response to 6Gy radiation were determined by Western analysis. (e) Radiation sensitivity of LNCaP p100 mutant clone was determined by clonogenic survival assay. Each experiment was repeated independently two to three times. Statistical analysis was performed using t-test.
It has been reported that the p100 protein can have a p53-dependent proapoptotic function (Wang et al., 2002). However, this is not likely to be the case in our study for several reasons. First, PC-3 cells are p53 deficient, excluding the possibility of p53-dependent proapoptotic pathway of p100. Second, no toxicity of LNCaP cells expressing p100 mutant was observed. As opposed to PC-3, LNCaP cells possess functional p53. Finally, LNCaP cells have a low intrinsic level of RelB and inhibition of RelB using dominant negative p100 did not further alter their radiation sensitivity (Figure 3e). These results demonstrate that mere overexpression of dominant negative p100 alone in cancer cells had no direct effect on their radiation sensitivity.
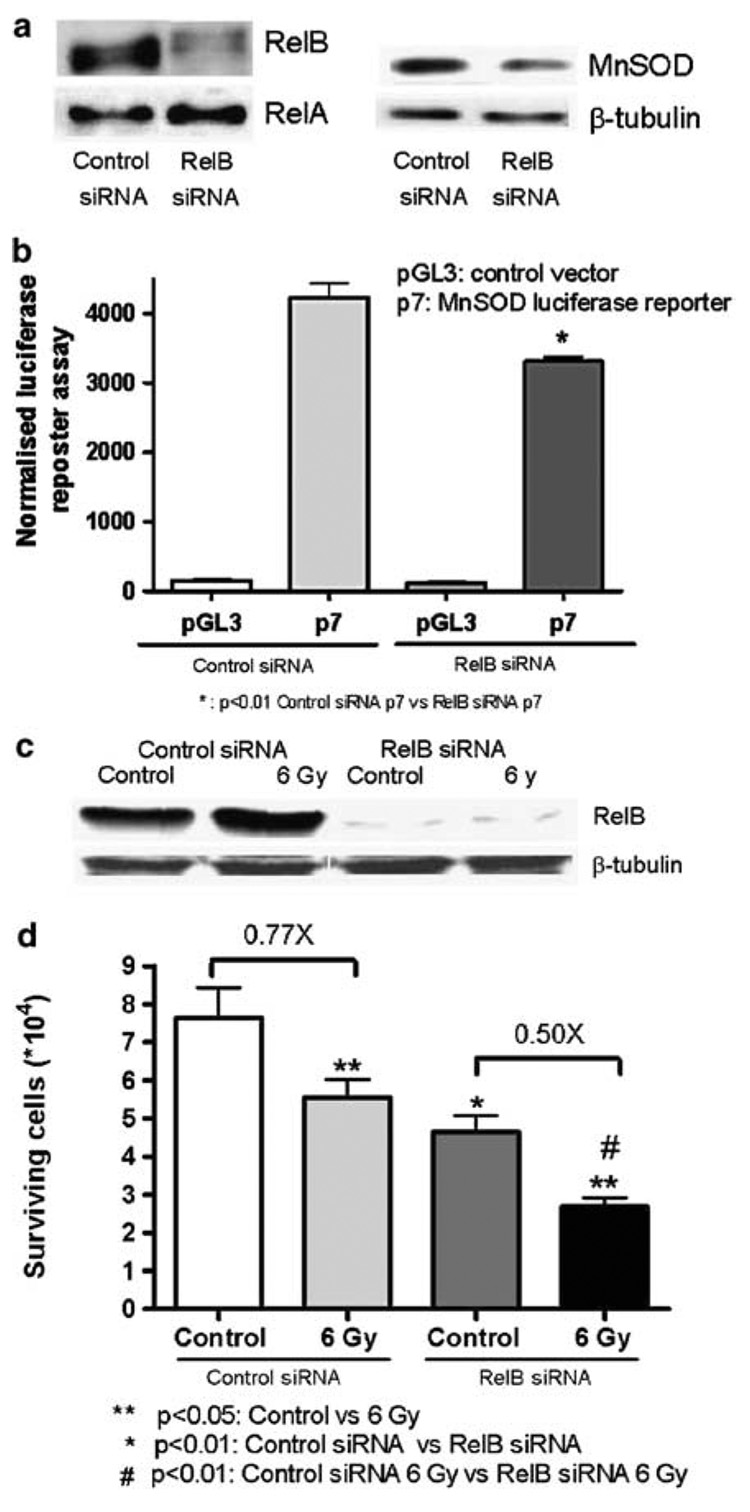
As an alternate approach to inhibit RelB, siRNA specific for RelB were used to further verify its role in the intrinsic radiation resistance of PC-3 cells. PC-3 cells were transfected with control or RelB siRNA oligonucleotides. Significant reductions in the levels of RelB protein and its target gene product MnSOD were observed in cells treated with RelB siRNA, but not control siRNA (Figure 4a). Moreover, MnSOD promoter- driven luciferase transcriptional activity was reduced in RelB siRNA-treated cells (Figure 4b). In response to radiation, a significant increase in RelB levels was observed in cells transfected with control siRNA as opposed to a near absence of constitutive and inducible RelB protein in RelB siRNA-treated cells (Figure 4c). To determine if inhibition of RelB by siRNA enhanced the cytotoxic effect of radiation treatment in PC-3 cells, a cytotoxicity assay was performed. Consistent with the observations when using the p100 mutant, suppression of RelB significantly decreased cell survival of RelB siRNA radiation-treated cells (Figure 4d). These experiments reinforce the protective role of RelB in the intrinsic radiation resistance of aggressive prostate cancer cells.
Figure 4. Inhibition of RelB using siRNA in PC-3 cells sensitized PC-3 cells to radiation.
(a) Detection of RelB protein by Western analysis, after transfection with siRNA. RelA protein is used as control. MnSOD was detected after transfection of RelB siRNA and normalized to β-tubulin. Cells were plated at 40% confluence at the time of transfection and transfected with control siRNA or RelB siRNA (Santa Cruz Biotechnology) using Lipofectamine 2000 for 5 h in a serum-free medum. After 36 h, the cells were radiated, and 24 h later collected for Western analysis or cell toxicity assay. (b) Detection of transcriptional activity in MnSOD promoter-driven luciferase in RelB treated siRNA. Cells were transfected with siRNA, MnSOD promoter-driven luciferase reporter plasmid (p7) or control plasmid (pGL3) and β-galactosidase (used as internal control). Luciferase activity was detected 24 h after transfection. (c) Western analysis of RelB was detected in control siRNA and RelB siRNA after radiation treatment. β- Tubulin was used for normalization. (d) Trypan blue exclusion assay was used for the detection of cytotoxicity to radiation treatment in control siRNA and RelB siRNA. Cells were stained with 0.4% Trypan blue dye and counted 24 h following radiation. Each experiment was repeated independently two to three times. Statistical analysis was performed using analysis of variance (ANOVA)–Tukey’s multiple comparisons test.
In summary, we demonstrate for the first time that RelB plays an important transcriptional role in the activation of genes involved in protection against oxidative stress resulting in intrinsic radiation resistance. As high levels of RelB correlated with prostate cancer aggressiveness, inhibition of RelB could be a novel mechanism to radiosensitize the more aggressive prostate cancers. Thus, targeting RelB might have some potential therapeutic benefits.
Acknowledgements
We thank Dr Shao-Cong Sun for the p100 mutant plasmid. We are grateful to Dr Ali Meigooni, Professor of Physics in the Department of Radiation Medicine, for the dosimetry and calibration of the X-ray machine. This work was supported by the University of Kentucky Research Foundation and NIH grant CA 49797.
References
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- Amundson SA, Bittner M, Chen Y, Trent J, Meltzer P, Fornace AJ., Jr Oncogene. 1999;18:3666–3672. doi: 10.1038/sj.onc.1202676. [DOI] [PubMed] [Google Scholar]
- Bren GD, Solan NJ, Miyoshi H, Pennington KN, Pobst LJ, Paya CV. Oncogene. 2001;20:7722–7733. doi: 10.1038/sj.onc.1204868. [DOI] [PubMed] [Google Scholar]
- Criswell T, Leskov K, Miyamoto S, Luo G, Boothman DA. Oncogene. 2003;22:5813–5827. doi: 10.1038/sj.onc.1206680. [DOI] [PubMed] [Google Scholar]
- Dhar SK, Lynn BC, Daosukho C, St Clair DK. J Biol Chem. 2004;279:28209–28219. doi: 10.1074/jbc.M403553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, et al. Mol Cell Biol. 2003;23:2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inayat MS, Chendil D, Mohiuddin M, Elford HL, Gallicchio VS, Ahmed MM. Cancer Biol Ther. 2002;1:539–545. doi: 10.4161/cbt.1.5.174. [DOI] [PubMed] [Google Scholar]
- Kiningham KK, Xu Y, Daosukho C, Popova B, St Clair DK. Biochem J. 2001;353:147–156. [PMC free article] [PubMed] [Google Scholar]
- Lessard L, Begin LR, Page CL, Gleave ME, Hurtado-Coll A, Mes-Masson AM, Saad F. Proc Am Assoc Cancer Res. 2005;46:3160. [Google Scholar]
- Murley JS, Kataoka Y, Cao D, Li JJ, Oberley LW, Grdina DJ. Radiat Res. 2004;162:536–546. doi: 10.1667/rr3256. [DOI] [PubMed] [Google Scholar]
- Ryseck RP, Bull P, Takamiya M, Bours V, Siebenlist U, Dobrzanski P, et al. Mol Cell Biol. 1992;12:674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. J Exp Med. 2001;193:1351–1359. doi: 10.1084/jem.193.12.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan NJ, Miyoshi H, Carmona EM, Bren GD, Paya CV. J Biol Chem. 2002;277:1405–1418. doi: 10.1074/jbc.M109619200. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Oberley LW. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cui H, Schroering A, Ding JL, Lane WS, McGill G, et al. Nat Cell Biol. 2002;4:888–893. doi: 10.1038/ncb872. [DOI] [PubMed] [Google Scholar]
- Weisigar RA, Fridovich I. J Biol Chem. 1973;248:3582–3592. [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Xu Y, Kiningham KK, Devalaraja MN, Yeh CC, Majima H, Kasarskis EJ, et al. DNA Cell Biol. 1999;18:709–722. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- Xu Y, Porntadavity S, St Clair DK. Biochem J. 2002;362:401–412. doi: 10.1042/0264-6021:3620401. [DOI] [PMC free article] [PubMed] [Google Scholar]