Abstract
Slice-selective RF waveforms that mitigate severe B1+ inhomogeneity at 7 Tesla using parallel excitation were designed and validated in a water phantom and human studies on six subjects using a 16-element degenerate stripline array coil driven with a butler matrix to utilize the 8 most favorable birdcage modes. The parallel RF waveform design applied magnitude least squares criteria with an optimized k-space excitation trajectory to significantly improve profile uniformity compared to conventional least squares designs. Parallel excitation RF pulses designed to excite a uniform in-plane flip-angle with slice selection in the z-direction were demonstrated and compared to conventional sinc-pulse excitation and RF shimming. In all cases, the parallel RF excitation significantly mitigated the effects of inhomogeneous B1+ on the excitation flip-angle. The optimized parallel RF pulses for human B1+ mitigation were only 67% longer than a conventional sinc-based excitation, but significantly outperformed RF shimming. For example the standard deviations of the in-plane flip-angle (averaged over six human studies) were 16.7% for conventional sinc excitation, 13.3% for RF shimming, and 7.6% for parallel excitation. This work demonstrates that excitations with parallel RF systems can provide slice selection with spatially uniform flip-angles at high field strengths with only a small pulse-duration penalty.
Keywords: Parallel Excitation, Slice-selective Excitation, RF Inhomogeneity Mitigation, Multidimensional RF Pulse
INTRODUCTION
Slice selective excitation plays a crucial role in MRI. With the push towards higher magnetic field strength, dramatic B1+ inhomogeneity for human imaging has become a serious issue, causing inhomogeneous flip-angle distribution in-plane for slice-selective excitations and detrimental non-uniformity for both signal-to-noise ratio (SNR) and image contrast. Several RF design approaches have been suggested to compensate for this inhomogeneity, including adiabatic pulses (1,2), RF-shimming (3-6), and spatially tailored excitation designs (7-11).
For relatively mild B1+ inhomogeneity, using the low-flip-angle approximation (12) with appropriate echo-volumnar k-space trajectories (9-11), termed either “fast-kz” or “spokes” excitation trajectories, the within-slice flip-angle inhomogeneity can be corrected. With these pulses, slice selection is achieved with a conventional sinc-like RF pulse during each kz traversal (a spoke), and in-plane flip-angle inhomogeneity is mitigated by the appropriate choice of the complex-valued amplitude that modulates the RF waveform of each spoke. Nonetheless, if the transmit B1+ field is rapidly varying with position, a large number of spokes will be required at correspondingly high kx and ky locations, rendering the RF pulse too lengthy for practical use.
With the introduction of parallel excitation systems (13-16), the k-space trajectory can be undersampled significantly to accelerate the RF pulse and reduce its duration. A number of successful demonstrations of this concept have been reported such as in (17-20). For example, it has been demonstrated at 3T (18,21) and at 4.7T (17), that a parallel RF design method using low flip angle approximation with spoke-based excitation trajectories can produce highly uniform slice selective excitation with reasonable excitation durations.
In this work we use spoke-based excitation in combination with magnitude least square (MLS) optimization (22,23), k-space excitation trajectory optimization, and B0 fieldmap incorporation (16,24) to design parallel RF pulses that demonstrate excellent B1+ mitigation both on head-shaped water phantom and for brain imaging of six subjects at 7T.
Methods
Human subjects
Six healthy volunteers (20-35 years old, 4 males and 2 females) were recruited from the community to serve as subjects for this study. All protocols were approved by the local IRB review committee.
System Hardware
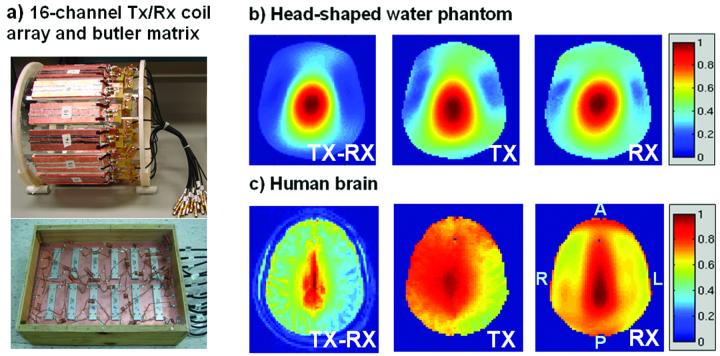
All experiments were run on a Siemens 7T Magnetom scanner (Erlangen, Germany), equipped with an 8-channel transmit RF system, with a body gradient system capable of maximum gradient amplitude of 40 mT/m and slew rate of 200 T/m/s. The two primary RF hardware components related to this study were a 16-channel stripline transmit-receive (Tx/Rx) degenerate birdcage coil array (25) and a 16-channel butler matrix (Figure 1-a). The butler matrix was used to drive the 8 optimal birdcage transmit modes from the 16-channel coil, and serves to make the best use of the RF excitation system, which has only 8 RF power amplifiers. The 16-channel RF array was built around a 28-cm diameter acrylic tube (Figure 1-a) and was driven through a transmit-receive switch at each coil. Signal was received in a birdcage mode on the 16 receive channels of the array.
Figure 1.
a) The 16-channel transmit/receive (Tx/Rx) stripline coil and the butler matrix used in this work. b) Profiles for combined Tx-Rx, along with estimates of the separate Tx (B1+) and Rx (B1−) of the circularly-polarized mode-1 birdcage of the coil for a head shaped phantom, and c) axial section in human brain (subject 1). The axes of orientation are denoted by R/L (right/left), and A/P (anterior/posterior) on the right-most image.
A head-shaped water phantom filled with doped water containing 1.25 g/L of nickel sulfate and 5 g/L of sodium chloride (T1∼500 ms), was used for initial measurements and demonstration of B1+ mitigation. Figure 1-b shows the Tx-Rx circularly-polarized (CP) mode-1 birdcage image of this water phantom. The peak-to-trough signal ratio in this image is very large, approximately 15. Also shown are the separate estimates of the Tx and Rx profiles, with the Tx magnitude signal ratio of 6.8 from maximum to minimum within the slice. This variation is more than twice the maximum range we observed for the human studies. Figure 1-c shows the relevant profiles for a human brain (subject 1).
Specific Absorption Rate Monitoring
The RF power was monitored in real time for each channel and utilized a 10 second and 6 minute average. For determining the SAR limit, a very conservative estimate was used. Based on the work by Collins et al (26) analyzing the worst case scenario for a similar 16-element stripline array, we utilized a local 10g SAR to average SAR ratio of 60. To assess this, Collins and colleagues determined the worst case ratio of local SAR to average SAR using Finite Difference Time Domain (FDTD) generated field maps in a human head model for a 16-element stripline coil. Given that the International Electrotechnical Commission (IEC) allows an average SAR of 3.2W/kg and local SAR of 10W/kg (a local to average ratio of 3.125, well below the estimated worst-case of 60) these results make it clear that local SAR is the limiting concern. Based on the worst-case local to average ratio of 60 and the IEC limit of 10W/kg for local SAR, we de-rated the allowable average SAR to 0.167W/kg. Using a 5 kg head mass, this gives an allowable average total power limit of 0.83W.
B1+ and B1− mapping
Central to the design of the B1+ mitigation RF pulses is the estimate of the excitation magnitude and phase profiles of the coil array for both phantom and human studies. Since the transmit and receive patterns are of opposite circular polarizations, B1+ and B1−, which in general are different at high B0 field (27), the use of a quantitative coil profile mapping technique was required.
Here, an efficient method for quantitative B1+ mapping of multiple transmission modes/coils is proposed. Instead of directly performing quantitative B1+ mapping on each of the transmit modes/coils, the first step of this B1+ mapping procedure is to estimate the density-weighted reception profile. Once this reception profile is estimated, only a single low voltage measurement will be required from each of the transmission modes/coils for the estimation of the individual B1+ transmission profiles. This novel method provides an efficient means to obtaining B1+ maps for parallel transmission systems, especially for systems with large number of transmission modes/coils.
Estimation of the density-weighted reception profile
The estimation of the density-weighted receive profile is performed in two steps. First, the mode-1 birdcage transmission profile of the coil array is estimated. This estimate is then used along with the data from an extra acquisition to estimate the receive profile.
The mode-1 birdcage transmission profile is estimated using a similar technique to the one proposed by Kerr et al in (28). The estimation technique here relies on mode-1 birdcage transmission via slice-selective excitation at a set of n voltages, v,2v,22v,.......,2n—1v , and fitting the sinusoidal signal dependence to the transmit voltages. In this method, BIR-4 magnetization saturation pulse (29) is applied after each readout period to allow for a reduced TR and shorter scan times without a detrimental T1 bias in the flip-angle estimates. Images at a set of transmission voltages are collected and the resulting image intensities are fitted to the following expression, which can be derived from e.g. (30),
| [6] |
Here, I is the image intensity, ρ is the spin density, RX is the receive coil profile (B1−), TSR is the saturation recovery time, T1 is the longitudinal relaxation time constant, θ is the flip-angle, and V is the applied transmit voltage.
With the use of BIR-4 magnetization saturation pulse, short TR acquisitions can result in high average SAR and reduced SNR (due to reduced T1 recovery time). To avoid this problem, TR = 1.2s was used in this work. A standard nonlinear search algorithm (Simplex) in Matlab was employed to perform the fitting of the image intensity to obtain the flip-angle map, . We note that the RF pulses for B1+ mapping are slice selective in the current implementation, and thus, some care must be taken to minimize the effects of imperfect slice selection at large flip angle during the fitting of Eqn. [6]. To this end, data from very large flip angles are excluded from the fitting based on a simple criterion. On a pixel by pixel basis, the signal intensity, I, was fitted only with measurements corresponding to excitations with v1, v2, …, vmax, where vmax is the voltage corresponding to the first maximum in I(x,y,vi).
From the flip-angle map, estimation of the transmission profile, (TX(x,y)), can be obtained by noting the following relationship between excitation flip-angle and transmission profile,
| [7] |
where γ is the gyromagnetic ratio and RF(t) is the RF pulse used for the transmission.
With the estimate of the birdcage transmission profile, the density-weighted reception profile, , can then be obtained by acquiring an extra slice-selective, low-flip-angle image with mode-1 birdcage transmission at a known voltage (without the magnetization saturation pulse). With the combination of a very low flip angle (we used a criterion of no more than 8° at maximum, based on quantitative flip-angle map) and a TR=1s, this image is approximately proton-density weighted
| [8] |
Again this equation can be derived from (30). By dividing out from the image, the density-weighted birdcage reception profile can be obtained.
Estimation of the individual B1+ transmission profiles
Once the density-weighted reception profile is estimated, the B1+ profile of the individual transmission modes can be obtained though a single slice-selective, low-flip-angle image acquisition of each transmission mode. According to Eqn. [8], the flip-angle profile of each transmit mode, θMode(j)(x,y,V), can be estimated via dividing the low-flip-angle image, IMode(j)(x,y,V), by density-weighted reception profile along with taking an inverse sine of the resulting image, pixel-by-pixel.
| [9] |
From this flip-angle profile, the quantitative magnitude B1+ profile (nT/V) can then be obtained via Eqn. [7].
In addition to the magnitude profile, the accompanying phase profile of B1+ is required as well. In this work, we make use of the spatial distribution of B1+ phase relative to the receive profile, ΦTXrelative,Mode(j) = ΦTX,Mode(j) + ΦRX . This phase is obtained from the phase measured in the low-flip-angle acquisition of each mode. By using this phase in the calculation of parallel excitation pulses, the receive phase variation is taken into account in the excitation design. As a result, the phase of the reconstructed image which consist of both excitation and reception phase, Φimage = ΦTX +ΦRX , should be equal to the design phase, Φdesign , with the actual excitation phase being ΦTX = Φdesign −ΦRX.
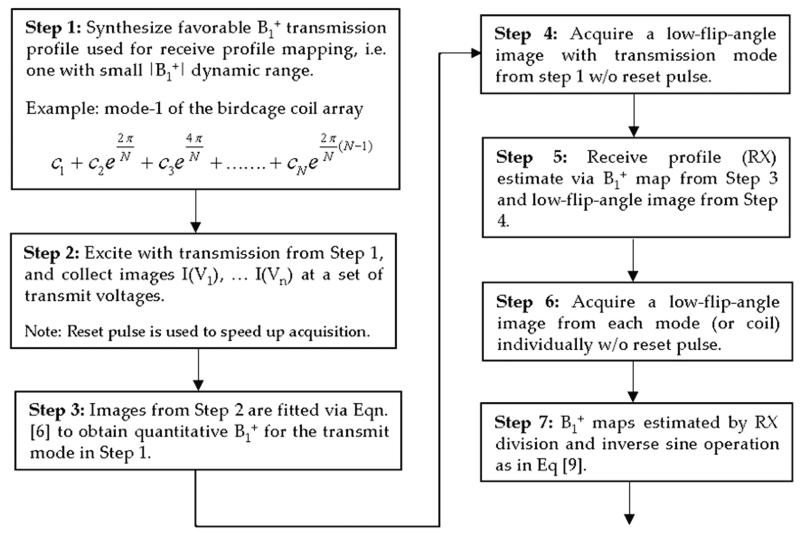
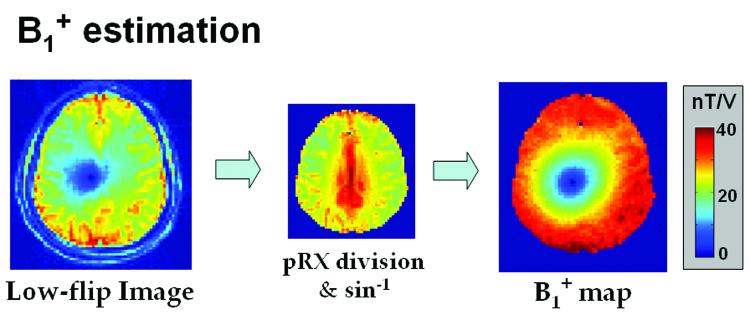
A flowchart in Figure 2 summarizes the quantitative B1+ mapping procedure, where first estimation of the receive profile is performed (steps 1-5), followed by estimation of the B1+ maps for each individual transmit coil/mode (steps 6-7). Figure 3 illustrates the estimation of the individual B1+ profiles via the use of density-weighted reception profile for in vivo B1+ mapping of the 1st gradient transmit mode in subject 1. As a side note, the use of mode-1 birdcage transmission is not required for the mapping of the receive profile. Transmission on any coil or mode can be used for this purpose. We note however, that by using a transmission mode with relatively low dynamic range of |B1+| variation, such as mode-1, only a small number of measurements are required for the receive profile estimation (4-5 voltage steps in this work for human neuroimaging at 7T).
Figure 2.
Flowchart outlining the proposed quantitative B1+ mapping technique, where, first a receive profile of the reception coil is estimated in steps 1-5, after which B1+ maps of the transmit modes/coils can then be obtained via steps 6-7.
Figure 3.
In vivo quantitative B1+ mapping of the 1st gradient transmit mode using a single low-flip-angle acquisition (subject 1). The B1+ map is obtained by dividing the low-flip-angle image with the density-weighted receive profile estimate along with applying a sine inverse operation.
We also note that once was estimated as a by-product of obtaining mode-1 birdcage transmission profile via Eq. [6], the reset pulse sequence could have been used in estimating the B1+ profiles of the individual modes without having to estimate . Nonetheless, estimating only requires one extra low flip angle acquisition, and allows us to leave out the use of reset pulse in estimating the B1+ profiles of the individual modes. This significantly reduces the SAR requirement for the mapping, which is important for in-vivo imaging application. Furthermore, the estimation of the density-weighted receive profile is needed in dividing the reconstructed image in the parallel excitation experiments to obtain the excitation profiles.
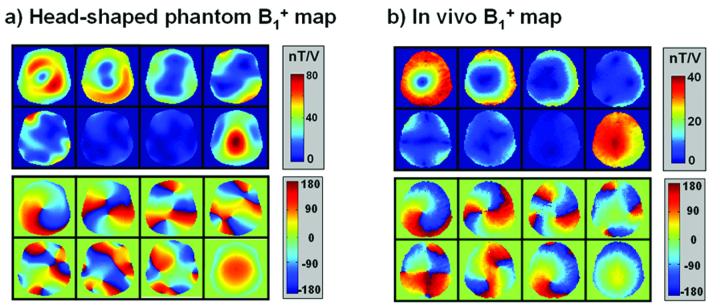
Examples of transmit profiles for the head-shaped phantom and a human subject are shown in Figure 4. It is noted that the head-shaped phantom experiment was performed prior to the full development of the presented B1+ mapping technique. The mapping technique used for the head-shape phantom differs slightly to the one presented here in that it does not utilize the BIR-4 reset pulse. Instead, the method relies on long TR acquisition to minimize T1 dependence. On the other hand, for the in vivo B1+ mapping of all human subjects, the presented method was used. In all acquisitions for B1+ mapping, a 2D gradient-recalled echo sequence was used with 80×80 pixel grid in x,y at 2.5 × 2.5 mm2 resolution over a 20-cm FOV in-plane (TE/BW = 5ms, 260 Hz/pixel).
Figure 4.
Magnitude (top) and phase (bottom) B1+ maps of the 8 optimal modes for a) the head-shaped phantom, and b) an axial section in human brain (subject 1).
B0 Maps
B0 maps were estimated from two gradient-echo acquisitions at TE1/TE2=5ms/6ms, and were incorporated into the pulse design (16,24) to improve the robustness of the desired excitation in the presence of unavoidable B0 inhomogeneity. The spoke-based excitation trajectory was shown to be sensitive to off-resonance effects (24) present in vivo at 3T, causing deterioration in performance. With the incorporation of the measured field map, these undesirable effects of B0 inhomogeneity are minimized.
RF Design
We based our parallel RF pulse design on the spatial domain method introduced by Grissom et al (16). In addition, we applied MLS optimization (22,23) to improve the excitation magnitude profile and reduce the required RF power at a cost of only a small spatial phase variation in the excitation. Here the method presented in (23) was used. Furthermore, the spoke placement in the k-space excitation trajectory was optimized to provide additional improvement to the excitation performance. For this optimization, the spokes were constrained to be symmetric around the origin in the (kx,ky) plane, and were optimized over a grid defined by 0°, 45°, 90°, and 135° angles, and different separation values (Δk = 1/FOV), with the FOV value ranging from 16 to 36 cm in incremental step of 2 cm. Examples of the optimized spoke-based k-space excitation trajectory for the phantom and the in vivo studies are shown in Figure 5.
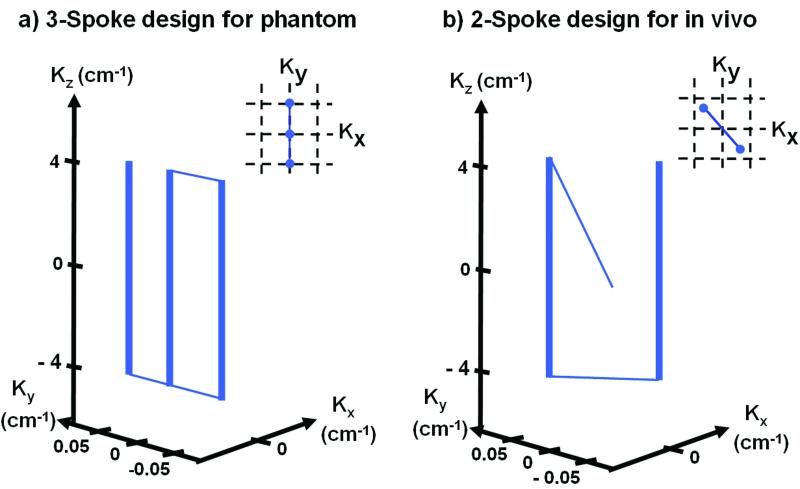
Figure 5.
The optimized three- (a) and two-spoke (b) k-space excitation trajectories for the pulse design in the head-shaped phantom and human excitation experiment respectively. The optimized two-spoke placements in (kx,ky) for the in the in vivo experiments varied from subject to subject, but in all cases were two-spoke designs.
In the RF design, a slice-selective spoke excitation with time-bandwidth-product equal to 4 was used for the sinc sub-pulses. For our initial phantom work where B1+ inhomogeneity was very severe, a three-spoke excitation trajectory was employed. Slice-selective specifications of 1-cm thickness, along with maximum gradient amplitude constrain of 20 mT/m, and slew rate constrain of 150 T/m/s were used. For the in vivo studies, a two-spoke excitation trajectory was used with a slice-selective specification of 0.5-cm thickness, maximum gradient amplitude constrain of 30 mT/m, and slew rate constrain of 150 T/m/s.
RF shimming design used for comparison in both the phantom and in vivo experiments was cast as a single-spoke RF design at the (kx,ky)-space origin with an MLS optimality criterion. The pulse duration quoted in this work includes the duration of the gradient rephaser applied after the excitation to properly return excitation trajectory to k-space origin. The predicted magnetization patterns were calculated using a Bloch equation simulation of the parallel RF excitation.
Experiments and Comparisons
For experimental verification, the spoke excitations were designed and tested on both a head-shaped phantom with severe B1+ inhomogeneity and on six human subjects with axial slice selection at six different superior/inferior (S/I) axial positions. In addition, for all experiments, excitation via mode-1 birdcage transmission and RF shimming were also acquired for comparison. For the head-shaped-phantom experiment, data were also collected to compare the performance of the conventional least-squares (LS) vs. the MLS design of the spoke-based RF pulses. The RF excitation pulses were adjusted so that for all the experiments the target flip angle was 5°.
The excitations were imaged with a 2D gradient-recalled echo sequence with matrix = 80×80, FOV = 20 × 20 cm2, voxel resolution = 2.5 × 2.5mm2, TR/TE/BW = 1s/5ms/260 Hz/pixel, where the combination of relatively long TR and low-flip-angle excitations resulted in proton-density weighted images. The flip-angle maps of the excitations were inferred from the proton-density images using the same method as in the individual mode B1+ profile estimation i.e. by dividing out the estimated density-weighted receive profile, , and taking inverse sine.
Standard deviation and uniformity threshold levels were used for quantitative comparison between mode-1 birdcage, RF shimming and spoke excitation. For a fair comparison of the standard deviation, the excitation flip-angle profiles were all scaled to have a mean value of 1.0 before the standard deviation was calculated (i.e. normalized standard deviation). The uniformity threshold levels used in the comparisons, ‘<10% dev’ and ‘<20% dev’, are defined as the percentage of the excitation flip-angle profile that deviate less than +/− 10% and +/−20% from the mean flip-angle value. The pixel set used in these comparisons is defined by the mask used in the generation of the B1+ maps. For the water phantom, this mask is generated by applying intensity thresholding to the birdcage Tx-Rx image to exclude the region beyond the phantom edge as well as partial-volume pixels in the periphery of the phantom. For the in vivo cases, the mask is also generated using the birdcage Tx-Rx image. However, an additional refinement step of manual “skull stripping” was applied before the mask generation via intensity thresholding. Figures 1-b and 1-c show the birdcage Tx-Rx image without masking and the Tx and Rx profiles with masking for both phantom and in vivo cases.
To demonstrate slice selection fidelity of the spoke excitation, a 3D readout was also acquired in one of the in vivo study (TE/TR/flip = 5ms/100ms/5°, 26 slices, FOV = 20 × 20 × 2.6 cm3, resolution = 2.5 × 2.5 × 1 mm3).
Results
Head-shaped water phantom
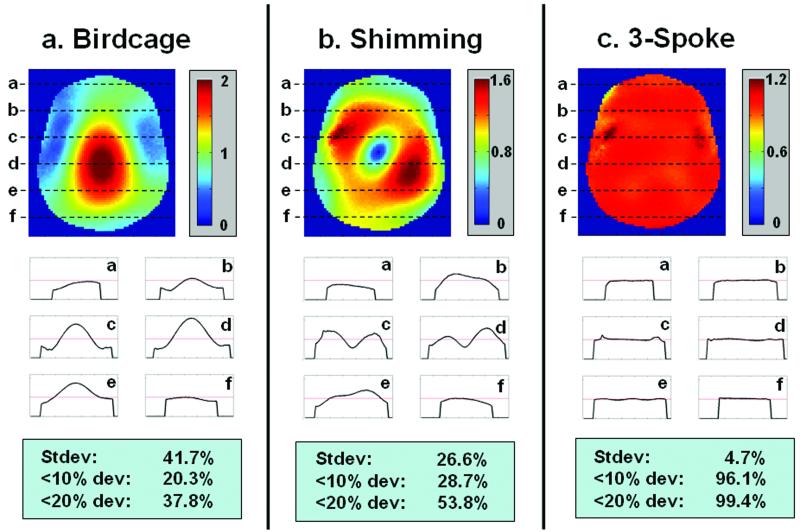
The head-shaped water phantom used for these experiments poses a very challenging B1+ mitigation task due to the severity of the transmit profile variation with a peak-to-valley magnitude variation of 6.8-to-1 in B1+ at an approximately central S/I axial slice through the phantom. The conventional sinc-based mode-1 birdcage excitation demonstrates the consequences of this severe B1+ inhomogeneity as a highly non-uniform in-plane flip-angle (Figure 6-a), while RF shimming (single-spoke at DC) is able to partly mitigate the B1+ variation (Figure 6-b). We note that the mode selected by the RF shimming optimization looks like the 1st gradient mode (top left of Figure 4-a), with some additional improvements by mixing in contributions from other modes. It is interesting to note that the mode-1 (the last mode in Figure 4-a) in this case is more inhomogeneous than the 1st gradient mode as measured by the standard deviation of the magnitude across the FOX.
Figure 6.
Head-shaped water phantom B1+ mitigation. Flip-angle maps and line profiles for: a) birdcage mode with conventional 1-ms long sinc slice-selective excitation, demonstrating a 1:6.8 magnitude variation within the field-of-excitation (FOX); b) RF shimming, 1-ms long pulse, demonstrating a substantial residual flip-angle inhomogeneity as measured by the standard deviation and threshold metrics; and, c) three-spoke MLS, slice-selective 2.4-ms long pulse, demonstrating excellent mitigation of the B1+ inhomogeneity.
The partial mitigation of the RF shimming is dramatically improved by adding another two spokes to the excitation as is clear from Figure 6-c. Visually, based on the images and line profiles, the B1+ mitigation of the spokes is excellent. Also quantitatively, based on the standard deviation across the FOX and the 10% and 20% deviation brackets, the three-spoke mitigation significantly outperforms RF shimming. The tradeoff in pulse duration is from 1ms for RF shimming to 2.4 ms for the 3-spoke design. The optimized placement of the spokes yielded a separation of 1/20 cm-1, at an angle of 90° (i.e. along ky), as shown in Figure 4-a.
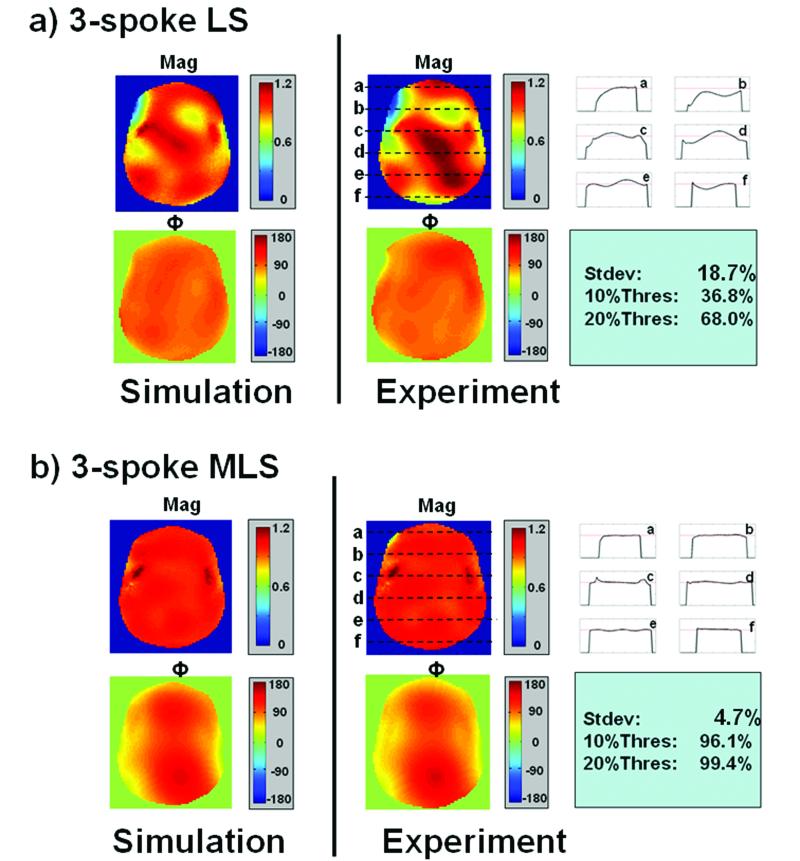
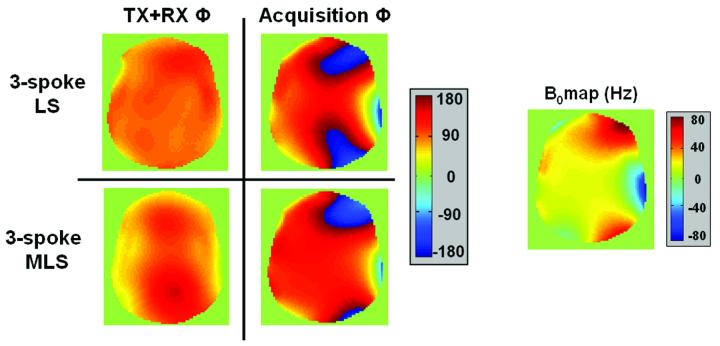
The same head-shaped phantom was used to compare the performance of three-spoke B1+ mitigation designed with conventional LS and MLS. The LS design strives to create an image with a uniform phase and magnitude, while the MLS allows slowly-varying spatial phase as a tradeoff in order to improve on the magnitude profile uniformity. Clearly, as seen in Figure 7, this relaxation of the phase constraint by the MLS design yields a very favorable tradeoff for the |B1+| mitigation. Further, as seen in Figure 8, the image phase variation, Φimage = ΦTX +ΦRX , which resulted from the MLS excitation is negligible compared to the B0 inhomogeneity-induced phase accrual at gradient-recalled echo time of 5ms for this phantom at 7T. The image spatial phase (left) is very slowly varying, is far from introducing intra-voxel dephasing, and is much smaller than the accrued B0 inhomogeneity-induced phase in the acquisition phase image (right). The image phases of the experimental results in both Figure 7 and 8 are calculated from the acquisition phases at TE=5ms by unwinding the effect due to B0 inhomogeneity, Φunwind = -B0(x,y)×TEeff , where TEeff is the time from center of excitation k-space to center of readout k-space.
Figure 7.
Comparison of B1+ mitigation by a) a least-squares, and b) a magnitude-least-squares three-spoke RF design with the same k-space trajectory (2.4 ms) and pulse shape (sinc, time-bandwidth product=4) as demonstrated on a head-shaped water phantom with substantial transmit inhomogeneity. On the left is a Bloch simulation of the magnitude and phase profiles, on the right are experimental results with line profiles through the magnitude image.
Figure 8.
Comparison between the image phase due to the combined excitation and reception phase (left) and the acquisition phase measured at TE=5ms (right), which also includes the phase accrual due to B0 inhomogeneity. Also shown on the far right is the estimated B0 fieldmap. Clearly, the combined excitation and reception phase variation resulting from the MLS design is very small compared to the accrued phase due to B0 inhomogeneity at TE=5ms. The phase resulting from the MLS design is slowly varying over the FOX, and thus does not introduce any intra-voxel dephasing.
B1+ mitigation in vivo
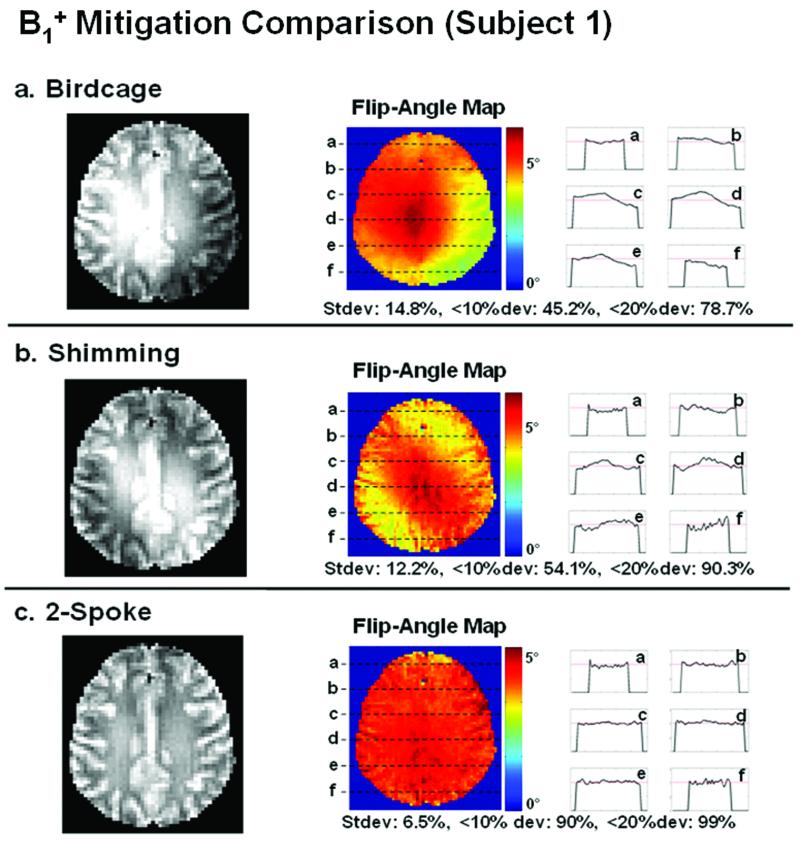
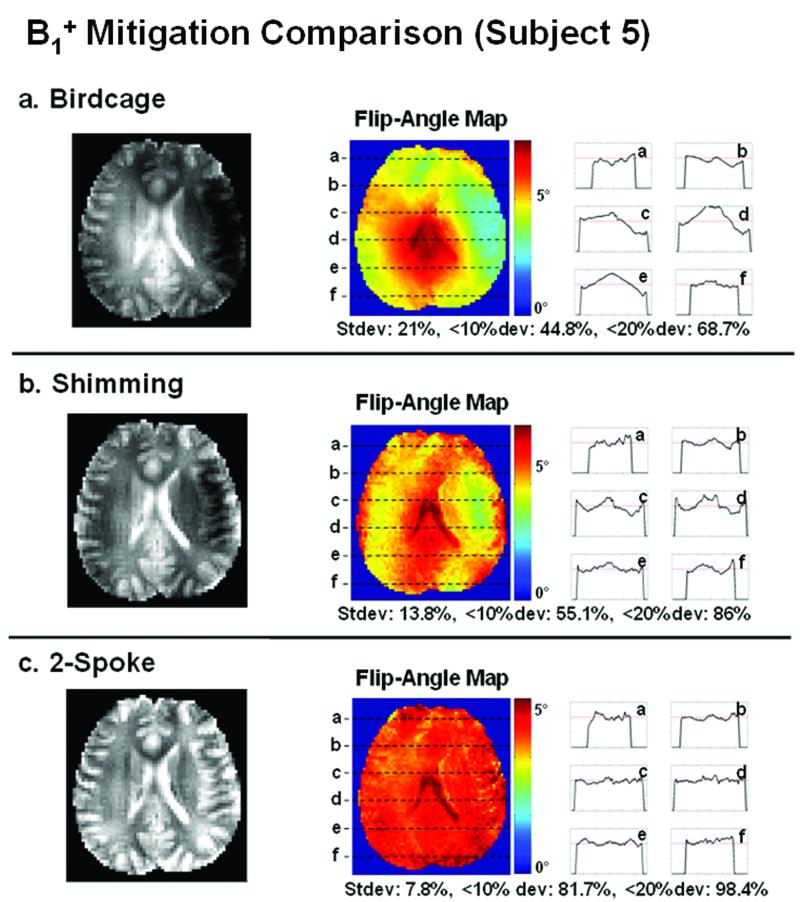
Based on the successful mitigation of the significant B1+ inhomogeneity in the water phantom, we ran in vivo experiments on six human subjects to demonstrate flip-angle correction for brain imaging in the presence of inhomogeneous B1+ and B0. Figures 9 and 10 compare the excitation performance of mode-1 birdcage (top row), RF shimming (center row), and two-spoke excitation pulses (bottom row) for two of the six subjects. The results in Figure 9 are for subject 1 who exhibits an average amount of B1+ variation in the birdcage excitation, as measured by the standard deviation (σ), when compared to the other subjects. The results in Figure 10 are for subject 5 who exhibits the largest amount of B1+ variation in the birdcage excitation. In each figure, on the left of each row is the in-plane image of the excited slice after the removal of the received profile (which is estimated here as the 10th-order polynomial fit of the density-weighted receive profile). On the right of each row is the flip-angle map estimate, along with line profile plots. In both Figures 9 and 10, RF shimming is more homogenous than the birdcage excitation, but still suffers from significant residual flip-angle inhomogeneity, whereas the two-spoke excitation provides excellent mitigation. It is noted that small amount of residual anatomy exists in the flip-angle map estimates, which most likely arises from small subject movement between the time of density-weighted receive profile estimation and the imaging of the mitigated excitation. Nonetheless, given the considerable improvement in the mitigation performance by the spoke excitation, this minor estimation artifact does not affect the overall conclusion that the two-spoke, slice-selective parallel RF excitation yields very effective flip-angle mitigation.
Figure 9.
B1+ mitigation comparison for subject 1. The comparison includes slice selection based on mode-1 birdcage (top row), RF shimming (center row), and two-spoke (bottom row) excitation pulses. On the left of each row is the in-plane image of the excited slice after the removal of the receive profile. On the right is the flip-angle map estimate, along with the line profile plots.
Figure 10.
B1+ mitigation comparison for subject 5, who has the most severe B1+ variation (in the mode-1 birdcage excitation) out of all the six subjects.
Table 1 tabulates the standard deviation and pixel fractions for 10% and 20% deviation of the transmit profile for birdcage, RF shimming, and two-spoke excitation for all 6 subjects. The trend across the data is very clear. The birdcage excitation is by far the most inhomogeneous, the RF shimming provides some improvement, and the two-spoke excitation is the most homogenous and reliable excitation for slice-selective B1+ mitigation. The associated tradeoff in pulse duration is 2.29 ms for two-spoke excitation vs. 1.37 ms for birdcage and RF shimming excitation. Note that the increase in pulse duration of the two-spoke excitation over the birdcage and RF shimming excitation is 67% rather than 100%, since the two-spoke excitation utilizes a single gradient rephasing lobe identical to one used in the birdcage and RF shimming excitation.
Table1.
Standard deviation and pixel fractions for 10% and 20% deviation of the flip-angle map of birdcage, RF Shimming, and two-spoke excitation for all six subjects.
| σ(%) |
<10% |
<20% |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BC | Shim | Spokes | BC | Shim | Spokes | BC | Shim | Spokes | |
| Subject 1 | 14.8 | 12.2 | 6.5 | 45.2 | 54.1 | 90.1 | 78.7 | 90.3 | 99 |
| Subject 2 | 12.4 | 11.4 | 6.6 | 54.8 | 60.9 | 91.3 | 88.9 | 93.1 | 98.8 |
| Subject 3 | 19.8 | 17.5 | 9.3 | 39.1 | 38.6 | 77.7 | 66.8 | 84.5 | 96 |
| Subject 4 | 15.1 | 12.9 | 7.3 | 52.2 | 59.4 | 87.1 | 80.6 | 88.5 | 98.2 |
| Subject 5 | 21 | 13.8 | 7.8 | 44.8 | 55.1 | 81.7 | 68.7 | 86 | 98.4 |
| Subject 6 | 16.9 | 11.7 | 8.1 | 42.3 | 63.4 | 83 | 76.4 | 91 | 96.9 |
|
| |||||||||
| Avg | 16.7 | 13.3 | 7.6 | 46.4 | 55.3 | 85.2 | 76.7 | 88.9 | 97.9 |
| ± Stdev | ± 3.2 | ± 2.3 | ± 1.0 | ± 6.0 | ± 8.9 | ± 5.3 | ± 8.1 | ± 3.2 | ± 1.2 |
For the in vivo parallel RF pulse design, the optimal spoke placement varies dramatically between subjects and does not seem to be intuitive. When compared to the default placement (assumed here to be at Δk = 1/20 cm-1, along the kx-axis), the optimal spoke placement provides significant improvement in excitation performance. Based on simulation results obtained for the six human subjects, the average reduction in excitation error (measured here as the standard deviation) is 10.5%, and the average reduction in RF pulse energy and peak power are 9% and 3.4% respectively.
Table 2 tabulates the total energy and peak power of RF pulses used for birdcage, RF shimming, and two-spoke excitation for all 6 subjects. To provide a fairer comparison, the longer pulse duration of the two-spoke excitation is accounted for. Namely, the RF energy and peak power calculation for the birdcage and RF shimming excitation are performed for an excitation that concatenate two consecutive, identical sinc RF excitations at half the RF amplitude of the actual single sinc RF pulse used in the experiments. This procedure reduces the RF energy of the birdcage and RF shimming by a factor of 2 and the peak power by a factor of 4, and yields a normalized comparison in terms of pulse duration. Based on this normalization, the RF pulse energy of the 2-spoke excitation is approximately double that of the birdcage excitation and is slightly lower than that of the RF shimming. On the other hand, the peak-power values of all the three excitations are similar.
Table2.
Total RF energy and RF peak power for birdcage, RF Shimming, and two-spoke excitation pulses for all six subjects. The reported energy and power are for excitation pulses of normalized duration.
| Total Energy (mJ) |
Peak Power (W) |
|||||
|---|---|---|---|---|---|---|
| BC | Shim | Spokes | BC | Shim | Spokes | |
| Subject 1 | 7.68 | 13.8 | 13.3 | 30.2 | 25.9 | 30.4 |
| Subject 2 | 7.35 | 12 | 10.4 | 28.8 | 26.3 | 23.9 |
| Subject 3 | 10.8 | 29.7 | 18 | 42.3 | 39.6 | 38.7 |
| Subject 4 | 7.79 | 17.4 | 16.2 | 30.4 | 25.9 | 24.5 |
| Subject 5 | 10.7 | 24.8 | 21.8 | 42.1 | 47.4 | 32 |
| Subject 6 | 8.65 | 14.7 | 15.8 | 33.6 | 30.8 | 46.1 |
|
| ||||||
| Avg | 8.83 | 18.7 | 15.9 | 34.6 | 32.7 | 32.6 |
| ± Stdev | ± 1.5 | ± 7.0 | ± 3.9 | ± 6.1 | ± 9.0 | ± 8.6 |
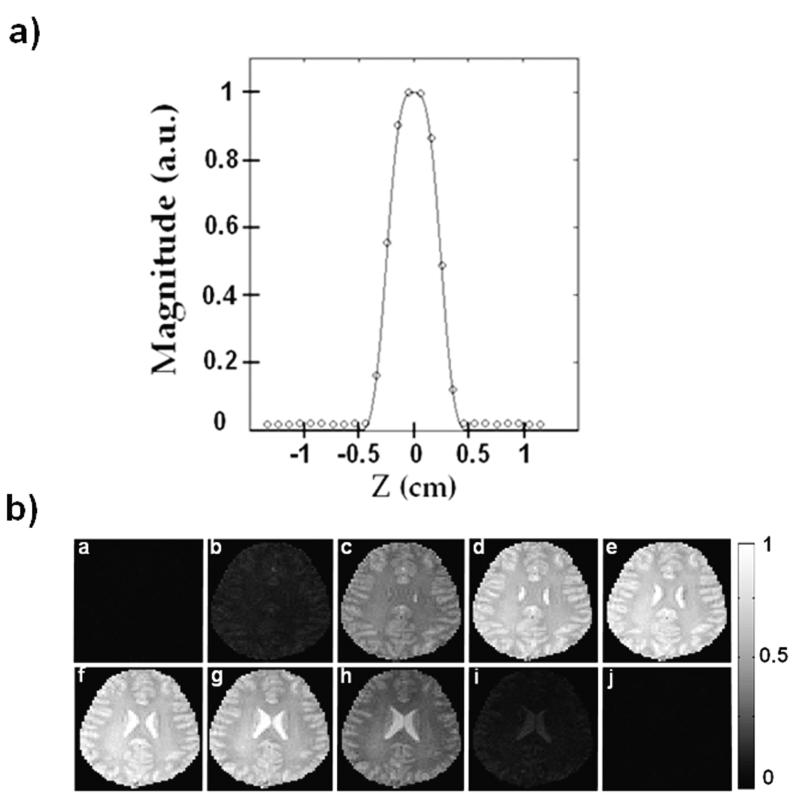
Figure 11 shows the slice profile performance of the two-spoke excitation on a human subject (subject 4). In Figure 11-a, the experimental slice profile is plotted as circles, along with the predicted profile by simulation which is shown as a solid line. Each data point along the profile represents the average in-plane intensity at that particular z-location. Good agreement between experiment and prediction can be observed, with excellent slice selection behavior. In Figure 11-b are the in-plane images (after compensating for the effect of the receive profile) of 1-mm separation along z, over a 1-cm range around the 0.5 cm excited slice. Good slice selection and B1+ mitigation performance can be observed.
Figure 11.
Two-spoke excitation for subject 4 with a 3D readout. a) Slice profile plot, where the solid line represents the predicted profile and the circles represent the experimental data. Each data point along the slice profile represents the average in-plane intensity at that particular z-location. b) In-plane images (a)-(j), at 1-mm separation along z, over a 1-cm range around the 0.5-cm excited slice.
Discussion
In this work we have successfully demonstrated mitigation of severe B1+ inhomogeneity at 7T, for a head-shaped water phantom and on six human subjects. The phantom result is interesting in that the B1+ inhomogeneity is more than twice as severe as we observed in any of the six subjects. Correspondingly, a three-spoke excitation was used for the phantom excitation, while a two-spoke design was adequate in vivo. The phantom finding suggests that at even higher fields, where the B1+ inhomogeneity in vivo becomes more pronounced than at 7T, spoke-based RF design is viable with a very high degree of flip-angle uniformity and a short pulse duration.
The novel B1+ mapping technique used in this work allow for fast B1+ estimation, requiring only a single low-flip angle image acquisition from each transmission mode/coil after the initial density-weighted receive profile estimation. With this method, the use of the reset pulse is limited to the density-weighted reception profile mapping, resulting in a much lower required SAR, which is particularly relevant for in vivo applications. Future work includes combining this B1+ mapping technique with more efficient readout methods such as ones based on spiral, echo-planar, or echo-volumnar trajectories.
A comparison of peak power and total energy of the RF pulses used for birdcage, RF shimming, and spoke excitation was provided in Table 1. This comparison points to similarities in peak-power and significant increases in pulse energy for RF shimming and spokes excitation when compared to conventional birdcage transmission. However, this work does not address a direct SAR comparison between the three methods. SAR calculation is a topic of an ongoing work, which will be keyed to clinical application of parallel transmission.
A limitation of the current transmit coil array, which is built around a cylindrical ring with local coil elements placed horizontally adjacent to each other, is that it is primarily fit for transversal acceleration and hence mitigation in the axial plane. Future work on coil development will address this point and extend the coil design to allow for longitudinal profile variation between the coil elements, and therefore longitudinal acceleration. We expect that the presented methodology for slice-selective excitation with B1+ mitigation will hold for sagittal, coronal and generally oblique scan planes beyond the currently presented axial demonstrations.
As part of the RF design method used in this work, a simple and fast k-space excitation trajectory optimization was employd to provide significant improvement in the excitation performance. Based on the performance of a Linux Intel® Xeon 3 GHz server, in Matlab, this optimization process can be performed in ∼50 seconds. We note that the optimization procedure can easily be made more sophisticated by evaluating the optimal spoke placement on a finer grid, but we suggest limiting the search to symmetric placement of spokes in (kx,ky) for these simple designs to maintain them within a regime compatible with the linear class of large tip angle pulses proposed by Pauly et al (31) and used for parallel transmit work by Xu et al (19). Such an approach would simplify the extension to larger flip angles.
Conclusion
Slice-selective RF waveforms that mitigate severe B1+ inhomogeneity at 7 Tesla were designed and demonstrated for parallel excitation in phantom and in six human subjects. This work demonstrates that slice-selective excitations with parallel RF systems offer the means to implement slice selection with spatially uniform flip-angle at high field strengths with only a small pulse duration penalty.
Acknowledgements
This work was supported by National Institutes of Health, NIBIB grants R01EB006847, R01EB007942, R01EB000790, and NCRR grant P41RR14075, Siemens Medical Solutions, R.J. Shillman Career Development Award, and the MIND Institute.
References
- 1.Silver MS, Joseph RI, Hoult DI. Highly selective 90° and 180° pulse generation. J Magn Reson. 1984;59:347–351. [Google Scholar]
- 2.Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153(2):155–177. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim TS, Lee R, Baertlein BA, Abduljalil AM, Zhu H, Robitaille PM. Effect of RF coil excitation on field inhomogeneity at ultra high fields: a field optimized TEM resonator. Magn Reson Imaging. 2001;19(10):1339–1347. doi: 10.1016/s0730-725x(01)00404-0. [DOI] [PubMed] [Google Scholar]
- 4.Adriany G, Van de Moortele PF, Wiesinger F, Moeller S, Strupp JP, Andersen P, Snyder C, Zhang X, Chen W, Pruessmann KP, Boesiger P, Vaughan T, Ugurbil K. Transmit and receive transmission line arrays for 7 Tesla parallel imaging. Magn Reson Med. 2005;53(2):434–445. doi: 10.1002/mrm.20321. [DOI] [PubMed] [Google Scholar]
- 5.Mao W, Smith MB, Collins CM. Exploring the limits of RF shimming for high-field MRI of the human head. Magn Reson Med. 2006;56(4):918–922. doi: 10.1002/mrm.21013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughan T, DelaBarre L, Snyder C, Tian J, Akgun C, Shrivastava D, Liu W, Olson C, Adriany G, Strupp J, Andersen P, Gopinath A, van de Moortele PF, Garwood M, Ugurbil K. 9.4T human MRI: preliminary results. Magn Reson Med. 2006;56(6):1274–1282. doi: 10.1002/mrm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenger VA, Saekho S, Zhang Z, Yu S, Boada FE. B1 Inhomogeneity Reduction with Transmit SENSE. Proceedings of the 2nd international workshop on parallel MRI; Zurich, Switzerland. 2004.p. 94. [Google Scholar]
- 8.Saekho S, Boada FE, Noll DC, Stenger VA. Small tip angle three-dimensional tailored radiofrequency slab-select pulse for reduced B1 inhomogeneity at 3 T. Magn Reson Med. 2005;53(2):479–484. doi: 10.1002/mrm.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saekho S, Yip CY, Noll DC, Boada FE, Stenger VA. Fast-kz three-dimensional tailored radiofrequency pulse for reduced B1 inhomogeneity. Magn Reson Med. 2006;55(4):719–724. doi: 10.1002/mrm.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulloa JL, Irarrazaval P, Hajnal JV. Exploring 3D RF shimming for slice selective imaging. Proceedings of the 13th Annual Meeting of ISMRM; Miami Beach, Florida, USA. 2005.p. 21. [Google Scholar]
- 11.Sung K, Cunningham CH, Nayak KS. Validation of B1+ Non-uniformity Correction in the Chest at 3T using TIP-COMP. Proceedings of the 14th Annual Meeting of ISMRM; Seattle, Washington, USA. 2006.p. 597. [Google Scholar]
- 12.Pauly J, Nishimura D, Macovski A. A k-space analysis of small-tip angle excitation. J Magn Reson. 1989;81:43–56. doi: 10.1016/j.jmr.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y. Parallel excitation with an array of transmit coils. Magn Reson Med. 2004;51(4):775–784. doi: 10.1002/mrm.20011. [DOI] [PubMed] [Google Scholar]
- 14.Katscher U, Bornert P, Leussler C, van den Brink JS. Transmit SENSE. Magn Reson Med. 2003;49(1):144–150. doi: 10.1002/mrm.10353. [DOI] [PubMed] [Google Scholar]
- 15.Griswold M, Kannengiesser S, Muller M, Jakob P. Autocalibrated accelerated parallel excitation (transmit-GRAPPA). Proceedings of the 13th Annual Meeting of ISMRM; Miami Beach, FL, USA. 2005.p. 2435. [Google Scholar]
- 16.Grissom W, Yip CY, Zhang Z, Stenger VA, Fessler JA, Noll DC. Spatial domain method for the design of RF pulses in multicoil parallel excitation. Magn Reson Med. 2006;56(3):620–629. doi: 10.1002/mrm.20978. [DOI] [PubMed] [Google Scholar]
- 17.Ullmann P, Junge S, Wick M, Seifert F, Ruhm W, Hennig J. Experimental analysis of parallel excitation using dedicated coil setups and simultaneous RF transmission on multiple channels. Magn Reson Med. 2005;54(4):994–1001. doi: 10.1002/mrm.20646. [DOI] [PubMed] [Google Scholar]
- 18.Setsompop K, Wald LL, Alagappan V, Gagoski B, Hebrank F, Fontius U, Schmitt F, Adalsteinsson E. Parallel RF transmission with eight channels at 3 Tesla. Magn Reson Med. 2006;56(5):1163–1171. doi: 10.1002/mrm.21042. [DOI] [PubMed] [Google Scholar]
- 19.Xu D, King KF, Zhu Y, McKinnon GC, Liang ZP. A noniterative method to design large-tip-angle multidimensional spatially-selective radio frequency pulses for parallel transmission. Magn Reson Med. 2007;58(2):326–334. doi: 10.1002/mrm.21314. [DOI] [PubMed] [Google Scholar]
- 20.Vernickel P, Roschmann P, Findeklee C, Ludeke KM, Leussler C, Overweg J, Katscher U, Grasslin I, Schunemann K. Eight-channel transmit/receive body MRI coil at 3T. Magn Reson Med. 2007;58(2):381–389. doi: 10.1002/mrm.21294. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Yip CY, Grissom W, Noll DC, Boada FE, Stenger VA. Reduction of transmitter B1 inhomogeneity with transmit SENSE slice-select pulses. Magn Reson Med. 2007;57(5):842–847. doi: 10.1002/mrm.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr AB, Zhu Y, Pauly JM. Phase Constraint Relaxation in Parallel Excitation Pulse Design. Proceedings of the 15th Annual Meeting of ISMRM; Berlin, Germany. 2007.p. 1694. [Google Scholar]
- 23.Setsompop K, Wald LL, Alagappan V, Gagoski BA, Adalsteinsson E. Magnitude Least Squares Optimization for Parallel RF Excitation Design Demonstrated at 7 Tesla with 8 Channels. Magn Reson Med. 2008;59(4):908–915. doi: 10.1002/mrm.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Setsompop K, Zelinski AC, Alagappan V, Nistler J, Hebrank F, Fontius U, Schmitt F, Wald LL, Adalsteinsson E. In vivo Parallel RF Excitation with B0 correction. Proceedings of the 15th Annual Meeting of ISMRM; Berlin, Germany. 2007.p. 671. [Google Scholar]
- 25.Alagappan V, Nistler J, Adalsteinsson E, Setsompop K, Fontius U, Zelinski A, Vester M, Wiggins GC, Hebrank F, Renz W, Schmitt F, Wald LL. A Degenerate Mode Band-Pass Birdcage for Accelerated Parallel Excitation. Magn Reson Med. 2007;57(6):1148–1158. doi: 10.1002/mrm.21247. [DOI] [PubMed] [Google Scholar]
- 26.Collins CM, Wang Z, Smith MB. A Conservative Method for Ensuring Safety within Transmit Arrays. Proceedings of the 15th Annual Meeting of ISMRM; Berlin, Germany. 2007.p. 1092. [Google Scholar]
- 27.Tropp J. Reciprocity and gyrotropism in magnetic resonance transduction. Phys Rev A. 2006;74(6):062103. [Google Scholar]
- 28.Kerr AB, Cunningham CH, Pauly JM, Piel JE, Giaquinto RO, Watkins RD, Zhu Y. Accelerated B1 Mapping for Parallel Excitation. Proceedings of the 15th Annual Meeting of ISMRM; Berlin, Germany. 2007.p. 80. [Google Scholar]
- 29.Cunningham CH, Pauly JM, Krishna NS. Saturated Double-Angle Method for Rapid B1+ Mapping. Magn Reson Med. 2006;55:1326–1333. doi: 10.1002/mrm.20896. [DOI] [PubMed] [Google Scholar]
- 30.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical Principles and Sequence Design. Wiley-Liss: 1999. [Google Scholar]
- 31.Pauly J, Nishimura D, Macovski A. A linear class of large-tip-angle selective excitation pulses. J Magn Reson. 1989;82:571–587. [Google Scholar]