Abstract
Although agonist-dependent endocytosis of G protein-coupled receptors (GPCRs) as a means to modulate receptor signaling has been widely studied, the constitutive endocytosis of GPCRs has received little attention. Here we show that two prototypical class I GPCRs, the β2 adrenergic and M3 muscarinic receptors, enter cells constitutively by clathrin-independent endocytosis and colocalize with markers of this endosomal pathway on recycling tubular endosomes, indicating that these receptors can subsequently recycle back to the plasma membrane (PM). This constitutive endocytosis of these receptors was not blocked by antagonists, indicating that receptor signaling was not required. Interestingly, the G proteins that these receptors couple to, Gαs and Gαq, localized together with their receptors at the plasma membrane and on tubular recycling endosomes. Upon agonist stimulation, Gαs and Gαq remained associated with the PM and these endosomal membranes, whereas β2 and M3 receptors now entered cells via clathrin-dependent endocytosis. Deletion of the third intracellular loop (i3 loop), which is thought to play a role in agonist-dependent endocytosis of the M3 receptor, had no effect on the constitutive internalization of the receptor. Surprisingly, with agonist, the mutated M3 receptor still internalized and accumulated in cells but through clathrin-independent and not clathrin-dependent endocytosis. These findings demonstrate that GPCRs are versatile PM proteins that can utilize different mechanisms of internalization depending upon ligand activation.
G protein-coupled receptors (GPCRs)2 belong to a superfamily of seven transmembrane-spanning proteins that respond to a diverse array of sensory and chemical stimuli (1–4). Activation of GPCRs through the binding of specific agonists induces conformational changes that allow activation of heterotrimeric guanine nucleotide-binding proteins (G proteins) (5, 6). To ensure that the signals are controlled in magnitude and duration, activated GPCRs are rapidly desensitized through phosphorylation carried out by G protein-coupled receptor kinases (GRKs) (7). This facilitates β-arrestin binding and promotes receptor uncoupling from the G protein (8, 9). In addition to its role in GPCRs desensitization, β-arrestins promote the translocation of the receptor to the endocytic machinery involving clathrin and adaptor protein-2 (AP-2), thereby facilitating receptor removal from the plasma membrane (10–15). Once internalized, some GPCRs may even continue to signal from endosomes (16).
Although GPCR internalization is generally considered to be an agonist-dependent phenomenon, some evidence suggests that GPCRs can be endocytosed even in the absence of agonist, a process known as constitutive internalization (17–20). The role of constitutive internalization of GPCRs is not clear. One interesting study on cannabinoid CB1 receptors in neurons has shown that constitutive internalization from the somatodendritic and not axonal membrane is responsible for the overall redistribution of receptors from the somatodentritic to the axonal membrane (17). Another study on the melanocortin MC4 receptor raised the possibility that constitutive endocytosis could be a consequence of the basal activity of the receptor (18).
Even less is known about the potential trafficking of the transducer of GPCR signaling, the G protein (21). Generally, the binding of the agonist to the GPCR promotes the exchange of GDP on the Gα protein for GTP and allows the dissociation of the trimeric G protein into Gα-GTP and Gβγ dimer subunits (5, 22). Then, the activated G proteins target different effectors (23, 24). G proteins are localized primarily to the PM where they interact with GPCRs; however, it is not known whether G proteins always remain at the PM or whether they might move into cells along endocytic pathways. Previous work showed that Gαs does not colocalize with β2 receptor on internal compartments after agonist stimulation, but the cellular distribution of Gαs was not examined (25).
In general, cargo proteins at the plasma membrane (PM) enter the cell through a variety of endocytic mechanisms that can be divided into two main groups: clathrin-dependent endocytosis (CDE) and clathrin-independent endocytosis (CIE). CDE is used by PM proteins such as the transferrin receptor (TfR) that contain specific cytoplasmic sequences recognized by adaptor proteins allowing a rapid and efficient internalization through clathrin-coated vesicles (26, 27). In contrast, CIE is used by PM proteins that lack adaptor protein binding sequences including cargo proteins such as the major histocompatibility complex class I protein (MHCI), the glycosylphosphatidylinositol-anchored protein CD59, and integrins (28–30). In HeLa cells CIE is independent of, and CDE dependent on, clathrin and dynamin and thus the two different endocytic pathways are distinct and well defined (31). After internalization in separate vesicles, MHCI-containing vesicles from CIE and transferrin receptor-containing vesicles from CDE subsequently fuse with the early endosomal compartment that is associated with Rab5 and the early endosomal antigen 1 (EEA1) (32). TfR is recycled back out to the PM in Rab4- and Rab11-dependent processes. In contrast, some MHCI is trafficked on to late endosomes and lysosomes for degradation, and some is recycled back out to the PM along tubular endosomes that lack TfR and emanate from the juxtanuclear area. Recycling of MHCI back to the PM requires the activity of Arf6, Rab22, and Rab11 (33, 34).
In this study, we analyzed the trafficking of GPCRs and their G proteins in the presence and absence of agonist in HeLa cells. We examined the trafficking of two prototypical class I GPCRs: the β2 adrenergic receptor (coupled to Gαs) and the M3 acetylcholine muscarinic receptor (coupled to Gαq). We find that β2 and M3 receptors traffic constitutively via CIE, and then, in the presence of agonist, they switch to the CDE pathway. We also examined the role of the third intracellular loop of the M3 receptor in this process. To our knowledge, this study represents the most comprehensive analysis of constitutive trafficking of class I GPCRs and related Gα proteins. We demonstrate that GPCRs are versatile PM cargos that utilize different mechanisms of internalization depending upon ligand activation. Considering the high level of homology between class I GPCRs, this evidence could be applicable to the other members of this family.
EXPERIMENTAL PROCEDURES
Materials and Antibodies—Carbamylcholine chloride (carbachol), isoproterenol hydrochloride, atropine sulfate, and propranolol hydrochloride were obtained from Sigma. N-[3H]Methylscopolamine ([3H]NMS, 79–83 Ci/mmol) was from PerkinElmer Life Sciences (Waltham, MA). The mouse monoclonal anti-HA antibody 16b12 (IgG1) was from Covance (Berkeley, CA) and a rabbit anti-HA antibody from Abgent (San Diego, CA). Mouse monoclonal antibody to human MHCI (W6/32) (IgG2a) (Naslavsky et al., Ref. 32) was described previously. The mouse anti-clathrin heavy chain was purchased from BD Biosciences (Palo Alto, CA). A mouse anti-Lamp1 antibody (H4A3) was from Developmental Studies Hybridoma Bank (Iowa City, IA). The antibodies against the endogenous Gαs and Gαq subunits were kindly provided by Dr. A. Spiegel (Albert Einstein College of Medicine, Bronx, NY) and were previously described (54). Invitrogen (Carlsbad, CA) was the source for transferrin (Tfn) conjugated to Alexa-594 and Alexa-conjugated (488, 594, and 680) fluorescent secondary goat-anti-rabbit, goat-anti-mouse (GAM), and isotype-specific GAM-IgG1 and GAM-IgG2a antibodies.
Cell Culture, DNA Constructs, and siRNA—HeLa and COS-7 cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 units/ml penicillin at 37 °C with 5% CO2. For transfection, cells were plated and transfected the next day by using FuGENE (Roche Applied Science, Indianapolis, IN) following the manufacturer's instructions. Experiments were performed 18–20 h after transfection. The 3HA-tagged receptor constructs, hβ2, hM3, and hM2 receptors (in the plasmid vector pcDNA 3.1 +) were purchased from UMR cDNA Resource Center (Rolla, MO). The construct β2-GFP used in live cell imaging experiments was kindly provided by Dr. J. Benovic (University of Pennsylvania, Philadelphia, PA) and was previously described (35). The 3HA-tagged hM3-short (where most of the i3 loop of M3 was deleted, from Ala-303 to Thr-499) plasmid was kindly provided by Dr. J. Wess (NIDDK, National Institutes of Health, Bethesda, MD) and it was previously described (36). To knockdown clathrin, we used the SMART pool siRNA (a mixture of 4 different siRNA) from Dharmacon. In particular, the four target sequences designed to knock down clathrin were GAGAAUGGCUGUACGUAAU, UGAGAAAUGUAAUGCGAAU, GCAGAAGAAUCAACGUUAU, and CGUAAGAAGGCUCGAGAGU. The final concentration of the pool siRNA in our experiment was 75 nm (18.7 nm for each single siRNA). For the siRNA clathrin knockdown experiments, we followed the double hit siRNA procedure of Motley et al. (55). In brief, we seeded HeLa cells at a density of 500,000 cells per 10-cm dish and after 6 h the first siRNA transfection was performed, using Oligofectamine (Invitrogen) and OPTI-MEM I (Invitrogen). Then, on Day 2, a second siRNA transfection was performed. On day 2, 6–8 h before the second treatment of siRNA, we transfected our constructs hβ2, hM3, and hM3-short receptors following our standard procedure with FuGENE. The cells were trypsinized on Day 3 and split in 2 dishes (one for immunofluorescence and one the Western blotting). On day 4, the experiment was performed.
Immunofluorescence, Antibody Internalization, and Live Cell Imaging—For immunofluorescence staining, cells were plated on to glass coverslips and transfected the following day. Eighteen hours after transfection, cells were preincubated at 4 °C for 1 h with the mouse anti-HA antibody (IgG1) to label the receptor on the plasma membrane. After washing, cells were incubated at 37 °C at different times in the presence of the mouse anti-MHCI antibody (IgG2a) or in the presence of transferrin (Alexa 594-transferrin), with or without the agonist, to allow internalization. For M3 and M2 muscarinic receptors Carbachol (1 mm) was used, while for β2 receptor isoprotenerol (1 mm) was used as agonists. The cells were fixed in 2% formaldehyde in PBS for 10 min, washed with PBS containing 10% FBS (PBS/FBS) and then incubated at room temperature for 1 h with ∼0.08 mg/ml of unlabeled goat anti-mouse (GAM) in the absence of saponin to block surface antibodies from secondary reagents. After washing, fluorescently-conjugated isotype-specific secondary antibodies (488 GAM-IgG1 and 594 GAM-IgG2a) were used in the presence of 0.2% saponin to detect the internalized receptor and MHCI, respectively. When 594-transferrin was present, we utilized only the secondary antibodies 488 GAM (IgG1). All images were obtained using a 510 LSM confocal microscope (Carl Zeiss, Thornwood, NY) with 63× 1.3 numerical aperture PlanApo objective. Unless indicated, the optical section was less than 1 μm. After acquisition, images were handled using Adobe Photoshop (Adobe Systems, San Jose, CA). All experiments were confirmed at least three times, and a representative image is shown. For live cell imaging, HeLa cells were plated onto Lab-Tek coverglass chambers (Nalge Nunc International, Rochester, NY) and transfected with β2-GFP constructs. Eighteen hours after transfection, cells were imaged on a 37 °C stage in CO2-independent media. Images were acquired every 10 s for 15 min. After 3–5 min, Alexa 594-transferrin was added to the medium with or without isoprotenerol (1 mm).
Quantification of the Internalized Receptor with the Single Cell-based Method—To determine and quantify the amount of internal cargo at different times of internalization, we used a single cell-based method. This method allows us to measure the percentage of the internal cargo compared with the total (surface and internalized) for each cell examined. After preincubation at 4 °C for 1 h with the mouse anti-HA antibody (IgG1) to label the receptor (M3 or β2) on the plasma membrane, cells were incubated at 37 °C at different times in the presence or absence of ligand. Then, the cells were fixed, washed, and incubated at room temperature for 1 h with isotype-specific secondary antibody 488 GAM-IgG1 without saponin just to label the receptor left on the PM after the internalization experiment. Next, we added ∼0.08 mg/ml of the unlabeled GAM blocking solution without saponin for 1 h to quench any remaining sites on the surface-bound mouse antibodies. Finally, we used the secondary antibody 594 GAM-IgG1 for 1 h in the presence of saponin to label the internalized receptor. In parallel to each quantification experiment, we performed a separate experiment to set up the acquisition parameters of the images so that the fluorescent signals of the two different secondary antibodies 594 GAM-IgG1 and 488 GAM-IgGI were similar. To do this, we used the two secondary antibodies, 594 GAM-IgG1 and 488 GAM-IgG1, together at the same concentration to label the primary antibody bound to the receptor in a control experiment. After this preliminary set up, all the images were taken with identical acquisition parameters and the fluorescent signals for each measurement was within the dynamic range. For these experiments, the 40× plan Apo objective was used with the pinhole completely open (optical section was about 12 μm) during image acquisition. For each treatment, we quantified the fluorescence of 50–100 cells using Metamorph 4.6. This method allowed us to measure the percentage of the internal cargo compared with the total (surface and internalized). Importantly, when we switched the secondary antibodies and used 594 GAM-IgG1 first to stain the surface receptor and then 488 GAM-IgG1 to label the internal receptor, the results obtained were similar (data not shown). In this analysis, cells expressing high levels of receptor (5-fold over that exhibited by an average cell) that gave a fluorescent signal above the dynamic range were not included (about 20–25% of the cells). Importantly, there was minimal overlap of “surface” and “internally” detected antibody when the cells were viewed by a thin confocal slice.
Quantification of the Internalized Receptor with Radioligand Binding Studies—In the radioligand binding assays, 24 h after transfection with M3 receptor, the cells were split into 6-well plates. The next day, the cells were incubated at 37 °C with or without carbachol (1 mm) at different times (from 5 min to 60 min). After drug treatment, the cells were cooled on ice and washed three times with PBS (10-min incubation for each washing step). The loss of cell surface M3 receptor in the presence of carbachol compared with the control (without ligand) was detected by incubating the cells with the cell-impermeant muscarinic ligand [3H]NMS (2 nm) for 2 h at 4 °C. The cells were then washed three times (10-min incubation for each washing step) with ice-cold PBS and solubilized with 1% Triton X-100 in PBS for 10 min. Then, the cells were scraped, and the extracts were transferred to a vial with scintillation fluid, and the radioactivity was measured. Nonspecific binding was assessed as binding remaining in the presence of 10 mm atropine and was subtracted from all the samples. Receptor internalization was defined as the loss of binding of the cell-impermeant [3H]NMS after carbachol treatment compared with nontreated cells. Each experiment was done in triplicate and the experiment was repeated two additional times. Binding data were analyzed using the nonlinear curve-fitting program Prism 4.0b (GraphPad).
RESULTS
GPCRs Display Constitutive and Agonist-dependent Internalization through CIE and CDE, Respectively—To study both constitutive and ligand-dependent GPCR endocytosis, we expressed and examined two GPCRs in HeLa cells, the β2-adrenergic receptor, and the M3 muscarinic receptor, which couple to Gs and Gq, respectively. These receptors were tagged at their extracellular, N termini with a triple HA tag allowing us to follow the internalization of the receptor into cells. Studies of GPCR trafficking and function typically employ expression of epitope-tagged receptors in a heterologous system due to the complexity of overlapping receptor subtypes in native systems (37). To determine and quantify the amount of internal cargo at different times of internalization, we used a single cell-based method. This method allows us to measure the percentage of the internalized receptor compared with the total receptor (surface and internalized) for each cell examined (we measured about 50–100 cells for each treatment; see “Experimental Procedures” for details).
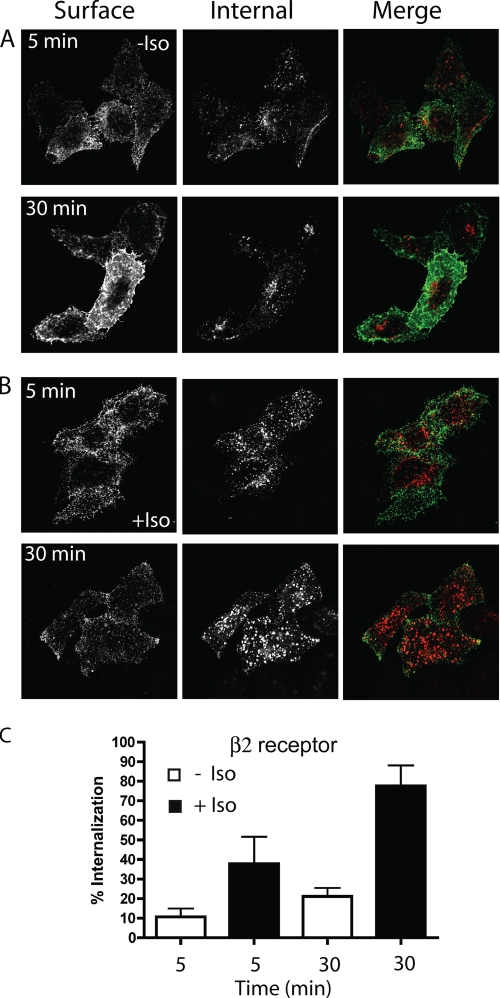
When we examined the trafficking of the β2 receptor using antibody internalization, β2 was internalized into cells in the absence of agonist (Fig. 1A). This constitutive internalization was apparent at 5 min and increased further at 30 min. Quantification of the internalized receptor revealed that about 10% of the initially bound material was inside the cell at 5 min and 20% at 30 min (Fig. 1C). The addition of agonist (1 mm Iso) increased the amount of β2 receptor internalized and this could be observed in fluorescence images (Fig. 1B). About 36% of surface β2 receptor was internalized in 5 min and 75% at 30 min (Fig. 1, B and C). Similar observations were made with the M3 receptor by imaging (not shown) and quantification of antibody internalization (see below). The amount of the GPCR internalized with ligand measured with the single cell-based method was more compared with the amount obtained using biochemical methods (supplemental Fig. S1 and see “Experimental Procedures” for details) or reported by other groups (38). One explanation for this is that biochemical assays measure total receptor internalization of all cells whereas our method of quantification excludes cells expressing very high levels of receptor. Thus biochemical methods could under estimate receptor internalization compared with the cell-based method we employed. To investigate if the constitutive internalization of the β2 and M3 receptors was a consequence of their basal activity or the presence of low concentration of ligand in the culture medium, we used the antagonists (inverse agonists) propranolol and atropine, respectively, to block this activity. Neither propranolol nor atropine affected the constitutive receptor internalization of β2 or M3 at 30 min (supplemental Fig. S2).
FIGURE 1.
β2 receptor internalization in the absence and the presence of ligand. A and B, HeLa cells expressing HA-tagged β2 receptors were preincubated at 4 °C for 1 h with mouse anti-HA antibody (IgG1) to allow binding of antibody to the cell surface. The cells were then incubated at 37 °C in the absence (A) or in the presence of ligand (Iso, 1 mm)(B) to allow antibody internalization for 5 or 30 min. The cells were fixed and then incubated with 488-goat anti mouse (GAM) antibody (green in the merge image) without detergent to label the remaining surface receptor (β2R surface), followed by 594-GAM (red in the merge image) in the presence of saponin to label the internalized HA antibody (see “Experimental Procedures” for details). The images were taken with the pinhole completely open (the optical section was about 12 μm), and images shown are representative of experiments that were repeated three times. C, quantification of theβ2 receptor internalization at 5 and 30 min, with or without ligand, using a single-cell based method (see “Experimental Procedures” for details). The bar graph shows the percentage of the internalized cargo compared with the total (surface and internalized) as the average ± S.D. of a representative experiment where between 50 and 100 cells were measured for each treatment. This experiment was repeated two additional times.
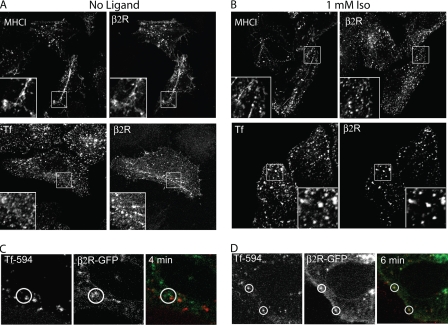
To determine the pathway of constitutive internalization of β2 and M3 receptors, we compared the endocytosis of these receptors with two endogenous PM proteins, MHCI and TfR, that are internalized by CIE and CDE, respectively, using antibodies to MHCI and Alexa594-conjugated transferrin (Tf). At 5 min of internalization in the absence of agonist, β2 (Fig. 2A) and M3 (supplemental Fig. S3A) colocalized with MHCI but not with Tf on peripheral endosomal structures and on recycling tubular endosomes. The presence of M3 and β2 receptors on recycling tubular endosomes that colocalized with MHC I was especially pronounced in cells treated with inhibitors of actin polymerization (data not shown); such treatments block recycling of CIE cargo back to the PM (33, 34). By contrast, in the presence of agonist for 5 min, internalized β2 (Fig. 2B) and M3 (supplemental Fig. S3B) receptors colocalized with Tf and not with MHCI. Even at longer times with agonist (30 min), β2 and M3 did not localize to the recycling tubular endosomes (data not shown). We also found that constitutive internalization of β2 and M3 receptors was not inhibited by the inhibitory mutant of dynamin 2, K44A, whereas ligand-activated internalization was impaired (data not shown). Taken together, these observations suggest that in the absence of ligand, both β2 and M3 internalized and colocalized with proteins that enter cells via a CIE pathway and then upon agonist stimulation, these receptors were found in compartments containing CDE cargo.
FIGURE 2.
β2 receptor internalization compared with MHCI and transferrin. A and B, HeLa cells were preincubated with anti-HA antibody (IgG1), and then incubated at 37 °C for 5 min without (A) or with ligand (B) in the presence of mouse anti-MHCI antibody (IgG2a) or Alexa 594-conjugated Tf. After fixation, unlabeled GAM antibody without saponin was used to block the remaining surface antibodies, and then isotype-specific antibodies 488-GAM-IgG1 and 594-GAM-IgG2a were used, in the presence of saponin to detect the internalized β2 receptor and MHCI, respectively. When Alexa 594-transferrin was present, we utilized only the secondary antibody 488-GAM (IgG1). Paired insets show magnified views and indicate the presence of the β2 receptor, without ligand, on tubular recycling endosomes. C, internalization of β2-GFP and Tf 594 in living HeLa cells in the absence of ligand. Still images (taken 4 min after Tf addition) from supplemental Movie S1. Without agonist, β2R-GFP and Tf 594 (red) were not observed together during the 15 min of incubation (see circled regions). D, internalization of β2-GFP compared with Tf 594 in living HeLa cells in the presence of (Iso, 1 mm). Still images (taken 6 min after addition of Tf and Iso) from supplemental Movie S2. Isoproterenol and Tf 594 were added at the same time. In the presence of isoproterenol, β2R-GFP, and Tf594 were present together in the same endosome (see circled regions). Images shown are representative of experiments that were repeated three times.
Having observed a shift in endocytic pathways used by these receptors upon addition of ligand in fixed cells, we wished to examine this in living cells. β2R-GFP expressed in HeLa cells mostly localized to the PM, on internal vesicles and on endosomal recycling compartments of the CIE pathway (supplemental Fig. S4). To study the mechanism of internalization of this receptor, we imaged the internalization of Tf-594 in cells expressing a GFP-tagged version of the β2 receptor. Without agonist, β2R-GFP and Tf were not observed together during the 15-min incubation (Fig. 2C, and supplemental Movie S1). By contrast, in the presence of ligand, β2R-GFP, and endocytosed Tf were present together in the same endosome (Fig. 2D, and supplemental Movie S2). These results demonstrate that β2 and M3 traffic constitutively in the CIE pathway, and then, in the presence of agonist switch to the CDE pathway. Similar results were obtained in COS-7 cells (data not shown).
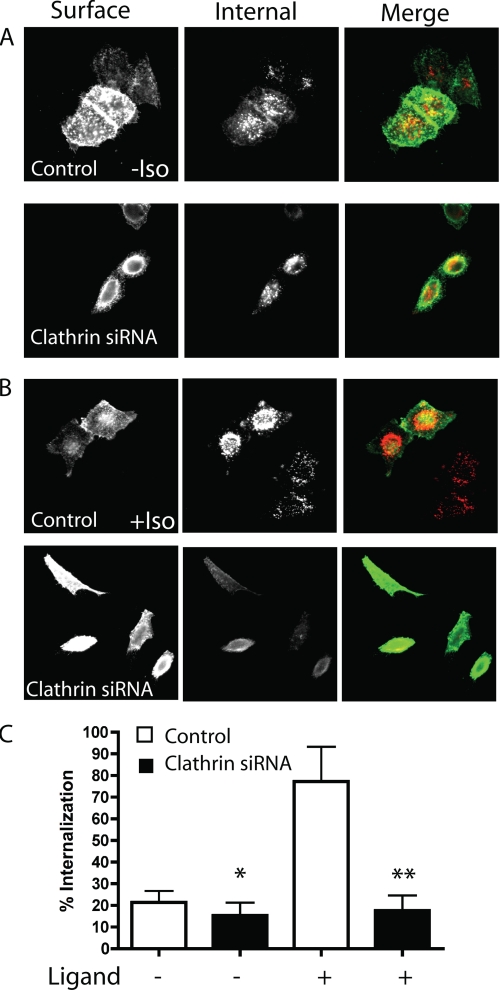
To confirm the differences between the constitutive and agonist-dependent GPCR endocytosis, we depleted clathrin from HeLa cells using siRNA. As shown by immunoblot and by immunofluorescence, clathrin was 85–90% depleted (supplemental Fig. S5) and we observed that endocytosis of Tf was blocked in cells depleted of clathrin (data not shown). Under these conditions, the constitutive internalization of β2 receptors was slightly inhibited by the depletion of clathrin (Fig. 3, A and C). By contrast, the agonist-dependent endocytosis of β2 was strongly affected by the knock-down of clathrin (Fig. 3, B and C). Endocytosis in the presence of the agonist was reduced in cells depleted of clathrin to the level observed for endocytosis in the absence of ligand. Similar to the β2 receptor, the constitutive internalization of the M3 receptor was largely unaffected by clathrin depletion whereas ligand stimulated internalization was inhibited (see below). These data show that GPCRs display constitutive and agonist-dependent internalization through clathrin-independent (CIE) and clathrin-dependent (CDE) endocytosis, respectively.
FIGURE 3.
β2 receptor internalization in cells depleted of clathrin. A, after preincubation at 4 °C for 1 h with the mouse anti-HA antibody (IgG1) to label the β2 receptor on the plasma membrane, HeLa cells, mock-treated (Control) or depleted of clathrin (Clathrin siRNA), were incubated at 37 °C for 30 min in the absence of the ligand. After fixation, the cells were incubated with 488-GAM to label the remaining surface receptor followed by 594-GAM with saponin to label the internalized receptor. The images were taken with the pinhole completely open (the optical section was about 12 μm), and images shown are representative of experiments that were repeated three times. B, same as A but in the presence of ligand (Iso, 1 mm). C, quantification of the β2 receptor internalization at 30 min, with or without depletion of clathrin, in the absence or in the presence of ligand, using a single cell-based method (see “Experimental Procedures” for details). The bar graph shows the percentage of the internalized cargo compared with the total (surface and internalized) of between 50 and 100 cells ± S.D. for each treatment of a representative experiment that was repeated two additional times. The difference between β2 receptor internalization in cells, with or without depletion of clathrin, was significant in both cases, in the absence and in the presence of ligand (*, p < 0.01, and **, p < 0.001).
Having demonstrated that β2 and M3 receptors constitutively internalize and recycle back to the PM along the CIE pathway, we decided to investigate if, over time, these receptors could reach late endosomal and lysosomal compartments for degradation. After 8 h of incubation in either the absence or presence of ligand at 37°, the β2 receptor localized to structures that labeled with Lamp1, a marker for lysosomes, indicating that this receptor could reach degradative compartments (supplemental Fig. S6). Similar observations were made for the M3 receptor (not shown).
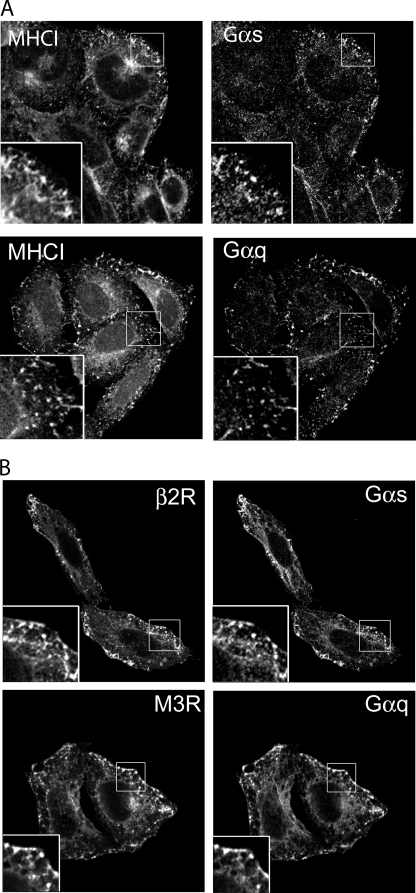
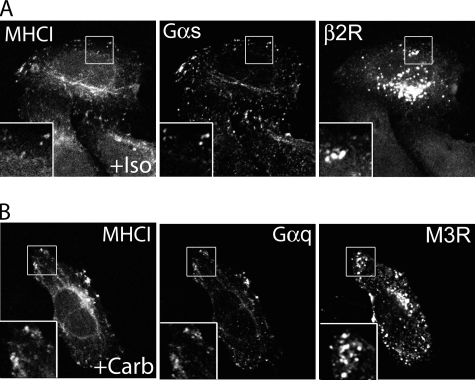
G Proteins (Gαs and Gαq) Associate with CIE Before and After Activation of the Cognate GPCR (β2R and M3R)—Most of the GPCR functions depend on the activation of specific heterotrimeric G proteins. Although GPCR ligand-dependent endocytosis has been studied extensively, much less is understood about the trafficking of G proteins. We analyzed the localization in HeLa cells of the endogenous G proteins Gαs and Gαq because they are activated by β2 and M3 receptors, respectively. It was shown previously that HeLa cells express Gαs and Gαq (39). Both Gαs and Gαq localized together with MHCI at the PM and on internal cellular compartments (Fig. 4A). They also were present on tubular recycling endosomes indicating the capability of the G proteins to traffic from the PM to internal endosomal structures and then move back to the PM under basal conditions. Furthermore, we found that the Gαq protein colocalized with M3 and Gαs with β2 receptor (Fig. 4B). This indicates that the G proteins and their cognate receptors are localized together on the PM, where they might form a platform to initiate the signaling cascade, and, also on endosomal recycling membranes that comprise the CIE endocytic pathway. Both of these receptors and their cognate G proteins could also be trapped, like MHCI and other CIE cargo proteins, in the vacuoles that form in cells expressing the constitutively active mutant of Arf6, Q67L (data not shown) (28, 32), confirming that these proteins travel along this CIE pathway.
FIGURE 4.
Endogenous Gαs and Gαq colocalize with MHCI and their unstimulated cognate receptors (β2R and M3R). After fixation, cells were double-labeled with rabbit anti-Gαs or Gαq, and with mouse anti-MHCI antibody (A) or mouse anti-HA to detect β2 and M3 receptors (B) in the presence of saponin. This was followed by fluorescently conjugated secondary antibodies. Paired insets show areas of colocalization. Images shown are representative of experiments that were repeated three times.
With agonist stimulation for 30 min, however, there was a separation between the receptor and its cognate G protein. Both the β2 and M3 receptors entered cells via CDE mechanisms, as we saw above, whereas the Gα proteins remained associated with the PM and the CIE endosomal membranes where they co-localized with MHCI (Fig. 5, A and B). This clear separation of receptor from G protein was also observed at shorter times after agonist addition (not shown). This suggests that the Gα proteins and their cognate receptors are localized at steady state at the PM but, after agonist activation, they separate and traffic through different pathways, CIE and CDE, respectively.
FIGURE 5.
Endogenous Gαs and Gαq separate from their cognate receptors (β2R and M3R) after activation. Surface β2 or M3 receptors were pre-labeled with anti-HA antibodies (IgG1) as before. The cells were then incubated at 37 °C for 30 min with mouse anti-MHCI antibody (IgG2a) and isoproterenol 1 mm (A) or carbachol 1 mm (B). After fixation, the cells were labeled, in the presence of saponin, with rabbit anti-Gαs (A) or anti-Gαq (B) antibodies, followed by 488-conjugated goat anti rabbit antibody. Internalized MHCI and HA-tagged receptors were visualized with isotype-specific antibodies 594-GAM-IgG2a and 633-GAM-IgG1. Insets show enlarged views and colocalization of G proteins with MHCI on endosomes. Images shown are representative of experiments that were repeated three times.
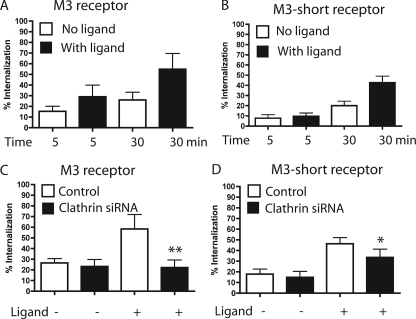
M3 Short Receptor Traffics through the CIE Pathway—To further examine the molecular mechanism of GPCR internalization, we decided to analyze receptor motifs that are required for receptor endocytosis. It is known that the third intracellular loop (i3 loop) and the carboxyl tail of the receptors play a major role in internalization of GPCRs in the presence of agonist (38, 40, 41). In particular for the M3 receptor, it was previously demonstrated that mutations of particular residues within the i3 loop strongly affected agonist-dependent receptor endocytosis (41). Conversely, there is no information regarding receptor motifs that might affect constitutive receptor internalization. For this reason, we analyzed a receptor mutant of M3 where most of the i3 loop has been deleted (M3-short). Others had demonstrated that deletion of the i3 loop does not affect the functional properties of the M3 receptor, at least in terms of its ability to activate phospholipase Cβ (36). We analyzed the internalization of M3-short and compared it to that of the wild type receptor in the presence and in the absence of ligand. The constitutive internalization of M3-short appeared similar to that of the wild type M3 receptor by immunofluorescence (not shown). The amount constitutively internalized at 30 min (about 20%) was reduced somewhat over that of the wild-type receptor (26%) (Fig. 6, A and B). Surprisingly, however, deletion of the i3 loop did not alter the extent of agonist-stimulated internalization of the receptor. Although, the internalization of M3-short in the presence of agonist was delayed at early time points, at 30 min the amount of receptor internalized for both receptors was over 2-fold that of the internalization in the absence of ligand (Fig. 6, A and B).
FIGURE 6.
M3 and M3-short receptor internalization in cells depleted of clathrin. A, after preincubation at 4 °C for 1 h with mouse anti-HA antibody to label the M3 receptor on the plasma membrane, HeLa cells were incubated at 37 °C for 5 and 30 min, in the absence or in the presence of the ligand (carbachol 1 mm). To detect and to quantify the amount of the cargo internalized we followed the same single cell-based procedure used for β2 receptor (see Fig. 1 and “Experimental Procedures”). B, same experiment performed in A for M3 receptor was made for M3-short. C, following the same procedure performed for β2 receptor (See Fig. 3C and “Experimental Procedures”), we determined the amount of M3 receptor internalized in HeLa cells, with or without depletion of clathrin. The difference between M3 receptor internalization, in the presence of ligand, in cells with or without depletion of clathrin was significant (**, p < 0.001). In contrast, the difference was not significant in the experiment without ligand. D, the same experiment performed in C for M3 receptor was performed for M3-short. The difference between M3-short receptor internalization, in presence of ligand, in cells with or without depletion of clathrin was significant (*, p < 0.05). In contrast, it was not significant in the experiment without ligand. For A–D, the bar graphs show the percentage of the internalized cargo compared with the total (surface and internalized) as the average ± S.D. of a representative experiment where between 50 and 100 cells for each treatment were measured. Each experiment was repeated two additional times.
To determine the mechanism of internalization of the M3-short with or without ligand, we examined the endocytosis of the receptor in cells depleted of clathrin. In cells depleted of clathrin, agonist-dependent internalization of M3-short receptor was slightly reduced compared with that in cells containing clathrin (Fig. 6D). However, agonist-stimulated internalization of M3-short was still about 2-fold more than that observed for constitutive internalization (Fig. 6D). This contrasts with the complete loss of agonist-stimulated endocytosis of wild-type M3 in clathrin-depleted cells (Fig. 6C), which was reduced to that observed in the absence of ligand. Additionally, as was observed with the β2 receptor (Fig. 3C) and the wild-type M3 receptor (Fig. 6C), the constitutive internalization of M3-short was not affected by the depletion of clathrin in cells (Fig. 6D). These data suggest that the M3-short receptor enters the cell constitutively similar to wild type M3 and β2 receptors, independently of clathrin. Furthermore, unlike wild type, in the presence of ligand, M3-short can still exhibit enhanced internalization under conditions where clathrin-dependent endocytosis is blocked.
DISCUSSION
The rapid internalization of GPCRs after agonist activation is important for the regulation of receptor signaling. Although much is known about agonist-dependent endocytosis of GPCRs, little is known about constitutive endocytosis of GPCRs and the role it plays in GPCR function. In this study, we found that both the β2 adrenergic and the M3 acetylcholine muscarinic receptors and their cognate G proteins were internalized constitutively in cells by CIE and colocalized with MHCI on tubular endosomes, which recycle endosomal membrane back to the PM. In addition to constitutively cycling into and out of the cells along this CIE pathway, internalized receptors could also reach late endosomal and lysosomal compartments for degradation, following a route taken by MHCI and CD59 (30, 32). The constitutive internalization of β2 and M3 receptors did not require receptor activation since the antagonists did not block their constitutive internalization. However, this does not rule out the possibility that the receptors might be capable of constitutive activity on CIE endosomes. Also, the constitutive internalization of the M3 receptor did not require the third intracellular loop.
In the presence of specific agonist, the β2 and M3 receptors switch their route of internalization from CIE to that of CDE and are removed from the PM more rapidly. Now the receptors colocalize with TfR, a marker of the CDE pathway, and no longer are observed in tubular recycling endosomes that contain MHCI. Depletion of clathrin in cells did not block constitutive CIE but blocked agonist stimulated CDE. This shift in pathways was also observed in living cells; β2GFP and TfR were not together in the absence of ligand but with ligand were observed entering the cell together in the same vesicle. In cells depleted of clathrin, agonist-stimulated internalization was reduced to the level of constitutive endocytosis for both β2 and M3 receptors.
Intriguingly, we found the endogenous G proteins, Gαs and Gαq, on the same endosomal membranes that traffic their cognate receptors constitutively. Previous studies have shown that GPCRs and their cognate G proteins are recruited to microdomains, such as lipid rafts or caveolae, to organize these signaling molecules (42, 43) and that stable complexes are formed between some inactive GPCRs and their cognate G proteins (44, 45). The CIE pathway followed by GPCRs and G proteins described here also contains GPI-anchored proteins such as CD59, which partition into “raft”-like microdomains at the PM (30). In addition to the Gα, there are also other signaling molecules associated with CIE endosomes including H-Ras, Rac, and Src, which could also provide alternative environments for signaling on endosomes (31). Upon receptor activation, the two signaling molecules, the GPCR and the cognate Gα protein, then traffic through different pathways, the CDE and the CIE, respectively. Consistent with this, a previous study reported on the separation of Gαs and the β2 receptor upon agonist activation and suggested that they used different endocytic pathways (42).
The extent to which other GPCRs will exhibit constitutive endocytosis is not known. When we examined endocytosis of the M2 receptor, we did not detect significant internalization during 30 min in the absence of ligand.3 This is in agreement with an earlier study that found very slow constitutive endocytosis of the M2 receptor (46). Interestingly, the M2 receptor has been reported to enter cells during ligand stimulation by a CIE pathway (47) and thus its behavior contrasts with the B2 and M3 receptors, whose ligand-stimulated endocytosis is clathrin-, dynamin-, and β-arrestin-dependent and sensitive to the overexpression of Git1, a GTPase-activating protein (GAP) for Arf6 (48). The M2 receptor may not engage in constitutive trafficking because during agonist stimulation it then enters cells by CIE. Given the similarity between the M2 and M3 receptors, it will be interesting to identify what sequences in the receptors are responsible for their alternative behaviors in the absence and presence of ligand. Nevertheless, Houndolo et al. (49) have found that most GPCRs, including M2, are dependent upon the activity of Arf6 for ligand-dependent endocytosis. Although Arf6 is associated with the CIE endosomal pathway that β2 and M3 receptors traffic through constitutively, Arf6 also functions at the PM through activation of phosphatidylinositol 4-phosphate 5-kinase and this activity may be critical for ligand-stimulated endocytosis.
We do not know what the exact physiologic role is for constitutive GPCR trafficking. There are a number of possible functions that this constitutive endocytosis and recycling pathway. It could be used for repositioning the receptor to specific regions of the PM. Support for this possibility comes from work in neurons where CB1 receptors accumulate on axons due to the constitutive internalization from the somatodendritic plasma membranes but not from the axonal membrane (17, 50). The CIE pathway could also be used to regulate the level of receptors at the PM and for receptor turnover. We show here that in the absence of ligand, this CIE pathway in addition to recycling back to the PM can lead to trafficking to late endosomes and lysosomes for degradation.
It is not known whether there are specific requirements or signals for proteins that are internalized by CIE. For GPCR internalization in the presence of agonist, it is known that the third intracellular loop (i3 loop) and the carboxyl tail of the receptors are important because mutations within the i3 loop inhibit agonist-dependent receptor endocytosis (41). We found that the M3 receptor lacking the i3 loop was able to be internalized by CIE suggesting that the i3 loop is not required for constitutive endocytosis. The i3 loop of M3 receptor is phosphorylated by different kinases, such as GPCR kinases and casein kinases (7), and it interacts with adaptor proteins, such as β-arrestins and others (51, 52). Both events are important for the regulation of the signaling and for the agonist-dependent internalization of M3 receptor. Our findings reveal that GPCR kinases (GRKs) and β-arrestins are probably not involved in the constitutive internalization of M3 receptor.
Surprisingly, the deletion of the i3 loop did not block, although it did delay, M3 receptor internalization in the presence of agonist. But, in the presence of ligand, internalization of M3-short was mostly independent of clathrin, as the receptor colocalized with MHCI3 and its internalization was not blocked by clathrin depletion. These data are supported by a study where it was shown that M3-short loses the capability to recruit β-arrestin in the presence of carbachol (53). These findings show that the i3 loop is required for agonist induced rapid endocytosis of the M3 receptor by clathrin. However, the receptor M3-short can still enter cells by CIE and possibly accumulate inside the cell when agonist is present as opposed to recycling back out to the PM. Previous reports have demonstrated that modifications of particular residues in the middle of the i3 loop of the M3 receptor strongly affected the agonist-dependent internalization in HEK and CHO cell lines (41). These mutated receptors may get trapped at the PM through some interactions that are still allowed between the i3 loop and some components, but not able to enter cells in clathrin structures.
This study demonstrates that GPCRs are versatile PM proteins that utilize different mechanisms of internalization depending upon ligand activation and receptor modifications. Considering the high level of homology between class I GPCRs, this finding could be applicable to the other members of this family.
Supplementary Material
Acknowledgments
We thank Drs. J. Wess, E. Korn, L. Cohen, C. Eyster, and R. Weigert for suggestions and critical reading of the manuscript. We also thank Drs. J. Benovic and E. Rosemond for plasmids and A. Spiegel for antibodies.
This work was supported, in whole or in part, by the Intramural Research Program of the NHLBI, National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contain supplemental Figs. S1–S6 and Movies S1 and S2.
Footnotes
The abbreviations used are: GPCR, G protein-coupled receptor; β2R, β2 adrenergic receptor; Carb, carbachol; CDE, clathrin-dependent endocytosis; CIE, clathrin-independent endocytosis; Gαq, α-subunit of the heterotrimeric Gq protein; Gαs, α-subunit of the heterotrimeric Gs protein; G protein, heterotrimeric guanine nucleotide-binding protein; HA, hemagglutinin; [3H]NMS, N-[3H]methylscopolamine; i3 loop, the third intracellular loop of G protein-coupled receptors; Iso, isoproterenol; MHCI, major histocompatibility complex class I protein; M3R, M3 muscarinic receptor; M3-short, M3R where most of the i3 loop was deleted, from Ala-303 to Thr-499; PM, plasma membrane; Tf, transferrin; TfR, transferrin receptor; PBS, phosphate-buffered saline; GFP, green fluorescent protein.
J. G. Donaldson, unpublished observations.
References
- 1.Bockaert, J., and Pin, J. P. (1999) EMBO J. 18 1723–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce, K. L., Premont, R. T., and Lefkowitz, R. J. (2002) Nat. Rev. Mol. Cell Biol. 3 639–650 [DOI] [PubMed] [Google Scholar]
- 3.Kristiansen, K. (2004) Pharmacol. Ther. 103 21–80 [DOI] [PubMed] [Google Scholar]
- 4.Foord, S. M., Bonner, T. I., Neubig, R. R., Rosser, E. M., Pin, J. P., Davenport, A. P., Spedding, M., and Harmar, A. J. (2005) Pharmacol. Rev. 57 279–288 [DOI] [PubMed] [Google Scholar]
- 5.Oldham, W. M., and Hamm, H. E. (2008) Nat. Rev. Mol. Cell Biol. 9 60–71 [DOI] [PubMed] [Google Scholar]
- 6.Deupi, X., and Kobilka, B. (2007) Adv. Protein Chem. 74 137–166 [DOI] [PubMed] [Google Scholar]
- 7.Tobin, A. B., Butcher, A. J., and Kong, K. C. (2008) Trends Pharmacol. Sci. 29 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter, E., and Lefkowitz, R. J. (2006) Trends Endocrinol. Metab. 17 159–165 [DOI] [PubMed] [Google Scholar]
- 9.Moore, C. A., Milano, S. K., and Benovic, J. L. (2007) Annu. Rev. Physiol. 69 451–482 [DOI] [PubMed] [Google Scholar]
- 10.Tsao, P., Cao, T., and von Zastrow, M. (2001) Trends Pharmacol. Sci. 22 91–96 [DOI] [PubMed] [Google Scholar]
- 11.Ferguson, S. S. (2001) Pharmacol. Rev. 53 1–24 [PubMed] [Google Scholar]
- 12.Hanyaloglu, A. C., and von Zastrow, M. (2008) Annu. Rev. Pharmacol. Toxicol. 48 537–568 [DOI] [PubMed] [Google Scholar]
- 13.Marchese, A., Paing, M. M., Temple, B. R., and Trejo, J. (2008) Annu. Rev. Pharmacol. Toxicol. 48 601–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamdan, F. F., Rochdi, M. D., Breton, B., Fessart, D., Michaud, D. E., Charest, P. G., Laporte, S. A., and Bouvier, M. (2007) J. Biol. Chem. 282 29089–29100 [DOI] [PubMed] [Google Scholar]
- 15.Mundell, S. J., Luo, J., Benovic, J. L., Conley, P. B., and Poole, A. W. (2006) Traffic 7 1420–1431 [DOI] [PubMed] [Google Scholar]
- 16.Shenoy, S. K., Drake, M. T., Nelson, C. D., Houtz, D. A., Xiao, K., Madabushi, S., Reiter, E., Premont, R. T., Lichtarge, O., and Lefkowitz, R. J. (2006) J. Biol. Chem. 281 1261–1273 [DOI] [PubMed] [Google Scholar]
- 17.McDonald, N. A., Henstridge, C. M., Connolly, C. N., and Irving, A. J. (2007) Mol. Pharmacol. 71 976–984 [DOI] [PubMed] [Google Scholar]
- 18.Mohammad, S., Baldini, G., Granell, S., Narducci, P., Martelli, A. M., and Baldini, G. (2007) J. Biol. Chem. 282 4963–4974 [DOI] [PubMed] [Google Scholar]
- 19.Wolfe, B. L., Marchese, A., and Trejo, J. (2007) J. Cell Biol. 177 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanasila, L., Abuin, L., Dey, J., and Cotecchia, S. (2008) Mol. Pharmacol. 74 562–573 [DOI] [PubMed] [Google Scholar]
- 21.Marrari, Y., Crouthamel, M., Irannejad, R., and Wedegaertner, P. B. (2007) Biochemistry 46 7665–7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wess, J. (1998) Pharmacol. Ther. 80 231–264 [DOI] [PubMed] [Google Scholar]
- 23.Cabrera-Vera, T. M., Vanhauwe, J., Thomas, T. O., Medkova, M., Preininger, A., Mazzoni, M. R., and Hamm, H. E. (2003) Endocr. Rev. 24 765–781 [DOI] [PubMed] [Google Scholar]
- 24.Johnston, C. A., and Siderovski, D. P. (2007) Mol. Pharmacol. 72 219–230 [DOI] [PubMed] [Google Scholar]
- 25.Allen, J. A., Yu, J. Z., Donati, R. J., and Rasenick, M. M. (2005) Mol. Pharmacol. 67 1493–1504 [DOI] [PubMed] [Google Scholar]
- 26.Conner, S. D., and Schmid, S. L. (2003) Nature 422 37–44 [DOI] [PubMed] [Google Scholar]
- 27.Le Roy, C., and Wrana, J. L. (2005) Nat. Rev. Mol. Cell Biol. 6 112–126 [DOI] [PubMed] [Google Scholar]
- 28.Brown, F. D., Rozelle, A. L., Yin, H. L., Balla, T., and Donaldson, J. G. (2001) J. Cell Biol. 154 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayor, S., and Pagano, R. E. (2007) Nat. Rev. Mol. Cell Biol. 8 603–612 [DOI] [PubMed] [Google Scholar]
- 30.Naslavsky, N., Weigert, R., and Donaldson, J. G. (2004) Mol. Biol. Cell 15 3542–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donaldson, J. G., Porat-Shliom, N., and Cohen, L. A. (2009) Cell Signal. 21 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naslavsky, N., Weigert, R., and Donaldson, J. G. (2003) Mol. Biol. Cell 14 417–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radhakrishna, H., and Donaldson, J. G. (1997) J. Cell Biol. 139 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weigert, R., Yeung, A. C., Li, J., and Donaldson, J. G. (2004) Mol. Biol. Cell 15 3758–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallal, L., Gagnon, A. W., Penn, R. B., and Benovic, J. L. (1998) J. Biol. Chem. 273 322–328 [DOI] [PubMed] [Google Scholar]
- 36.Maggio, R., Barbier, P., Fornai, F., and Corsini, G. U. (1996) J. Biol. Chem. 271 31055–31060 [DOI] [PubMed] [Google Scholar]
- 37.Nathanson, N. M. (2008) Pharmacol. Ther. 119 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanyaloglu, A. C., and von Zastrow, M. (2007) J. Biol. Chem. 282 3095–3104 [DOI] [PubMed] [Google Scholar]
- 39.Krumins, A. M., and Gilman, A. G. (2006) J. Biol. Chem. 281 10250–10262 [DOI] [PubMed] [Google Scholar]
- 40.Lameh, J., Philip, M., Sharma, Y. K., Moro, O., Ramachandran, J., and Sadee, W. (1992) J. Biol. Chem. 267 13406–13412 [PubMed] [Google Scholar]
- 41.Moro, O., Lameh, J., and Sadee, W. (1993) J. Biol. Chem. 268 6862–6865 [PubMed] [Google Scholar]
- 42.Allen, J. A., Halverson-Tamboli, R. A., and Rasenick, M. M. (2007) Nat. Rev. Neurosci. 8 128–140 [DOI] [PubMed] [Google Scholar]
- 43.Patel, H. H., Murray, F., and Insel, P. A. (2008) Annu. Rev. Pharmacol. Toxicol. 48 359–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nobles, M., Benians, A., and Tinker, A. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 18706–18711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin, K., Sethi, P. R., and Lambert, N. A. (2008) Faseb J. 22 2920–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roseberry, A. G., and Hosey, M. M. (1999) J. Biol. Chem. 274 33671–33676 [DOI] [PubMed] [Google Scholar]
- 47.Delaney, K. A., Murph, M. M., Brown, L. M., and Radhakrishna, H. (2002) J. Biol. Chem. 277 33439–33446 [DOI] [PubMed] [Google Scholar]
- 48.Claing, A., Perry, S. J., Achiriloaie, M., Walker, J. K., Albanesi, J. P., Lefkowitz, R. J., and Premont, R. T. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Houndolo, T., Boulay, P. L., and Claing, A. (2005) J. Biol. Chem. 280 5598–5604 [DOI] [PubMed] [Google Scholar]
- 50.Bohn, L. M. (2007) Mol. Pharmacol. 71 957–958 [DOI] [PubMed] [Google Scholar]
- 51.Vogler, O., Nolte, B., Voss, M., Schmidt, M., Jakobs, K. H., and van Koppen, C. J. (1999) J. Biol. Chem. 274 12333–12338 [DOI] [PubMed] [Google Scholar]
- 52.Popova, J. S., and Rasenick, M. M. (2004) J. Biol. Chem. 279 30410–30418 [DOI] [PubMed] [Google Scholar]
- 53.Novi, F., Stanasila, L., Giorgi, F., Corsini, G. U., Cotecchia, S., and Maggio, R. (2005) J. Biol. Chem. 280 19768–19776 [DOI] [PubMed] [Google Scholar]
- 54.Goldsmith, P., Gierschik, P., Milligan, G., Unson, C. G., Vinitsky, R., Malech, H. L., and Spiegel, A. M. (1987) J. Biol. Chem. 262 14683–14688 [PubMed] [Google Scholar]
- 55.Motley, A., Bright, N. A., Seaman, M. N., and Robinson, M. S. (2003) J. Cell. Biol. 162 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.