Abstract
The platelet-derived growth factor (PDGF) signaling pathway is a critical regulator of animal development and homeostasis. Activation of the PDGF pathway leads to neointimal proliferative responses to artery injury; it promotes a switch of vascular smooth muscle cells (vSMC) to a less contractile phenotype by inhibiting the SMC-specific gene expression and increasing the rate of proliferation and migration. The molecular mechanism for these pleiotropic effects of PDGFs has not been fully described. Here, we identify the microRNA-221 (miR-221), a small noncoding RNA, as a modulator of the phenotypic change of vSMCs in response to PDGF signaling. We demonstrate that miR-221 is transcriptionally induced upon PDGF treatment in primary vSMCs, leading to down-regulation of the targets c-Kit and p27Kip1. Down-regulation of p27Kip1 by miR-221 is critical for PDGF-mediated induction of cell proliferation. Additionally, decreased c-Kit causes inhibition of SMC-specific contractile gene transcription by reducing the expression of Myocardin (Myocd), a potent SMC-specific nuclear coactivator. Our study demonstrates that PDGF signaling, by modulating the expression of miR-221, regulates two critical determinants of the vSMC phenotype; they are SMC gene expression and cell proliferation.
Unlike many terminally differentiated cells, smooth muscle cells (SMCs)3 can switch between differentiated and dedifferentiated phenotypes in response to changes in the local environment (2). In response to vascular injury, quiescent contractile vSMCs reduce the expression of SMC-specific genes such as α-smooth muscle actin (SMA), smooth muscle calponin (CNN), SM22α (SM22), and smooth muscle myosin heavy chain, increase proliferation, and synthesize collagens and matrix metalloproteinases (2, 9). Although this phenotype switch is believed to be essential for repair of vascular injury, deregulation of this process also plays a role in the pathogenesis of various human diseases, including atherosclerosis, hypertension, asthma, and cancer. Therefore, a complete understanding of the molecular mechanisms of vSMC phenotype regulation is essential for treatment or prevention of vascular disorders.
PDGFs potently mediate the vSMC phenotype switch from a differentiated, contractile state to a dedifferentiated, synthetic state by repressing SMC marker gene expression as well as promoting vSMC proliferation and migration (2, 10). Consistent with the effect of PDGF on vSMC, up-regulation of molecules in the PDGF signaling pathway is found in various cardiovascular disorders and vascular injuries, including atherosclerosis and restenosis (1). PDGF released from platelets and endothelial cells at sites of vascular injury is thought to be a contributing factor to atherosclerosis (11). Inhibition of PDGF signaling by the PDGF receptor (PDGFR) kinase inhibitor imatinib mesylate (Gleevec) demonstrated a major protective effect on atherosclerosis development (1). It is of note that hyperactivation of the PDGFR pathway has been implicated in the pathogenesis of idiopathic pulmonary artery hypertension (IPAH), and a clinical study indicates that imatinib mesylate improves both hemodynamics and exercise capacity of a patient with severe IPAH (12). A previous report suggested that PDGF-BB suppresses the expression of myocardin (Myocd), a potent transcription activator of all SMC-specific genes which contain the CArG box (CC(A/T)6GG) sequences in their promoter regions (13). As a result, PDGF-BB abolishes SMC gene transcription. It is unclear, however, whether PDGFs modulate cell growth and migration of vSMCs directly or indirectly as a result of modulation of SMC gene expression.
miRNAs are a class of small, noncoding RNAs that play a role in the negative post-transcriptional regulation of genes (3–7). miRNAs bind to the 3′-untranslated region (UTR) of target mRNAs and lead to mRNA degradation or translational inhibition. Recent studies indicate that a single miRNA affects the expression of a large number of protein coding genes, suggesting that modulation of one miRNA might be able to exhibit multiple cellular events simultaneously (14, 15). miRNA expression is spatially and temporally regulated both during development and in adult tissues (6, 16, 17). Growing evidence suggests that miRNAs play a key regulatory role in gene expression during embryogenesis and differentiation of adult tissues (6, 16, 18–22), and miRNA misexpression leads to developmental abnormalities or human diseases, including cancer and cardiovascular disorders (5, 19–24). Induction of specific miRNAs plays an essential role in a variety of processes such as osteoblast differentiation, transition from tumor stem cells to metastatic tumor cells, and differentiation of cardiac and skeletal muscle (21, 25, 26). However, no miRNA has been indicated in association with a dedifferentiation process under either pathologic or physiologic conditions. Furthermore, little is known about the signaling pathways that regulate miRNA expression or the mechanisms of this regulation (27).
miR-221 and its family member miR-222 are highly expressed in various cancer-derived cells, including prostate carcinoma PC3, HeLa, and thyroid carcinoma cells (28–30). High levels of miR-221 and miR-222 have been shown to promote proliferation of these cells through binding to the 3′-UTR of the cell cycle inhibitor and tumor suppressor p27Kip1 and inhibiting its expression (28–31). Additionally, the level of miR-221 and miR-222 is markedly decreased during the maturation of CD34-positive hematopoietic progenitor cells into erythroid cells, suggesting that miR-221 and miR-222 inhibit normal erythropoiesis (32). In this context, miR-221 and miR-222 down-regulate c-Kit, a tyrosine kinase receptor for the peptide growth factor SCF (stem cell factor), and thereby inhibit stem cell activity in hematopoietic progenitor cells to promote erythropoiesis. miR-221 and miR-222 have also been found to be highly expressed in endothelial cells, where they modulate the angiogenic activity of SCF by targeting c-Kit (33). There is no prior study of miR-221 or miR-222 function in vSMCs.
In this study we demonstrate that PDGF induces the expression of miR-221, which results in down-regulation of c-kit and p27Kip1 in human primary pulmonary artery smooth muscle cells (PASMC). Down-regulation of c-Kit inhibits the expression of SMC specific genes via inhibition of Myocd. Unlike c-Kit, down-regulation of p27Kip1 by miR-221 has no effect on SMC gene expression but promotes cell proliferation. These results demonstrate that PDGF exhibits its effects on both SMC gene expression and cell proliferation by inducing miR-221 expression, which results in down-regulation of multiple target genes with distinct functions.
EXPERIMENTAL PROCEDURES
Cell Culture—Human PASMC were purchased from Lonza (CC-2581) and were maintained in Sm-GM2 media (Lonza) containing 5% fetal calf serum. Early passage (passage 4–7) PASMC were used for this study. Recombinant human BMP4, PDGF-BB, and SCF were purchased from R&D Systems. All growth factor treatments were performed under starvation conditions (0.2% fetal calf serum) as described (10).
Real-time Reverse Transcription-PCR—Total RNA was extracted by TRIzol (Invitrogen), and 1 μg of RNA was subjected to reverse transcription using first-strand cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. The quantitative analysis of the change in expression levels was calculated by real-time PCR machine (iQ5, Bio-Rad) (34). PCR cycling conditions were 94 °C for 3 min and 40 cycles of (94 °C for 15 s, 60 °C for 20 s and 72 °C for 40 s). For detection of mature miRNAs, TaqMan MicroRNA assay kit (Applied Biosystems) was used according to the manufacturer's instructions. Data analysis was performed using comparative CT method in Bio-Rad software. Average of three experiments, each performed in triplicate with standard errors, are presented.
Reverse Transcription-PCR Primers—Primers used were: human c-Kit, 5′-CACCGAAGGAGGCACTTACAC-3′ and 5′-GGAATCCTGCTGCCACACA-3′; human p27Kip1, 5′-TTGCAGGAACCTCTTCGGCC-3′ and 5′-GGTCGCTTCCTTATTCCTGC-3′; human SMA, 5′-GCGTGGCTATTCCTTCGTTA-3′ and 5′-ATGAAGGATGGCTGGAACAG-3′; human CNN, 5′-AGCTAAGAGAAGGGCGGAAC-3′ and 5′-CATCTGCAGGCTGACATTGA-3′; human SM22, 5′-AACAGCCTGTACCCTGATGG-3′ and 5′-CGGTAGTGCCCATCATTCTT-3′; human myocardin, 5′-TGCATGCTGCTGTAAAGTCC-3′ and 5′-TAGCTGAATCGGTGTTGCTG-3′; human MRTFA, 5′-TGTGTCTCAACTTCCGATGG-3′ and 5′-TTCACCTTTGGCTTCAGCTC-3′; human MRTFB, 5′-GCAACTGCTGCACAAATACC-3′ and 5′-TTGATAAAGGGCTGCTGGAC-3′; human Pri-miR-221, 5′-CCAGTTTATCTATCCGACCTTC-3′ and 5′-CTTTCTTGCGGTCCTTTC-3′; human Pre-miR-221, 5′-TGAACATCCAGGTCTGGGGCA-3′ and 5′-GAGAACATGTTTCCAGGTAGC-3′.
miRNA Mimic—Chemically modified double-stranded RNAs designed to mimic the endogenous mature miR-21, miR-221, and negative control miRNA were purchased from Ambion. miRNA mimics were transfected using RNAi Max (Invitrogen) according to the manufacturer's directions at 0.3 or 3 nm as indicated.
Anti-miRNA Oligonucleotides—2′-O-methyl-modified RNA oligonucleotides complementary to miRNA or green fluorescent protein (GFP; control) sequence were purchased from IDT and transfected at a concentration of 106 nm using Oligofectamine (Invitrogen) according to the manufacturer's directions. They are: anti-miR-221, 5′-GAAACCCAGCAGACAAUGUAGCU-3′, anti-miR-21, 5′-GUCAACAUCAGUCUGAUAAGCUA-3′; anti-GFP, 5′-AAGGCAAGCUGACCCUGAAGU-3′.
RNA Interference—Synthetic small interference RNA (siRNA) targeting human c-Kit or p27Kip1 were Stealth Select RNAi (Invitrogen) and HP Validated siRNA (Qiagen) respectively: C-kit, 5′-CAUGGACAUGAAACCUGGAGUUUCU-3′; p27Kip1, 5′-UUGAGUAGAAGAAUCGUCG-3′. An siRNA with a non-targeting sequence (scramble siRNA, Dharmacon) was used as a negative control. The siRNAs were transfected at 4 nm using RNAi Max (Invitrogen) according to manufacturer's directions. Fluorescein isothiocyanate (FITC)-conjugated fluorescent oligonucleotides (Block-it, Invitrogen) was used to evaluate transfection efficiency.
Plasmid DNA Transfection and cDNA Expression Constructs—PASMC were transfected using Effectene transfection reagent (Qiagen) according to the manufacturer's directions. Human c-kit expression plasmid was purchased from OriGene (catalog #SC120061). In brief, full-length human c-kit including 2 kilobases of 3′-UTR, which contains miR-221, target sequence was cloned into pCMV6-XL4 vector.
Immunoblot Assay—Cells were lysed in TNE buffer (1% Non-idet P-40, 10 mm Tris-HCl (pH 7.5), 1 mm EDTA, 150 mm NaCl). Total cell lysates were separated on a SDS-PAGE, transferred to PVDF membranes (Millipore), immunoblotted with antibodies, and visualized using an enhanced chemiluminescence detection system (Amersham Biosciences). Proteins bands were quantitated by densitometry using gel analysis software ImageJ (rsbweb.nih.gov/ij). All values are normalized to GAPDH. Antibodies used for immunoblotting are: anti-SMA antibody (clone 1A4, Sigma), anti-GAPDH antibody (2E3–2E10, Abnova), anti-p27Kip1 monoclonal antibody (clone 225501, R&D) anti-myocardin (Clone 355521, R&D Systems).
Immunofluorescence Staining—PASMC were fixed and permeabilized in a 50% acetone:50% methanol solution and subjected to staining using anti-SMA antibody (clone 1A4, Sigma) conjugated with FITC and nuclear staining with 4′-6-diamidino-2-phenylindole (DAPI, Invitrogen).
Luciferase Assay—Luciferase reporter construct containing CNN gene promoter (-549/+1700) (35) was transfected into PASMC with β-galactosidase plasmid as an internal transfection control. Luciferase assays were carried out as described (10), and luciferase activities were presented after normalization to β-galactosidase activities.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Cell Proliferation Assay—PASMC were transfected with anti-GFP, anti-miR-221, or miR-221, and 6 × 103 cells were split into 96-well plates. Cells were treated with or without 20 ng/ml PDGF-BB for 24 h followed by a cell proliferation assay using CellTiter96 nonradioactive cell proliferation assay (Promega) according to the manufacturer's directions. The absorbance at 490 nm was read by an enzyme-linked immunosorbent assay plate reader. Absorbencies were normalized to initial readings to verify equal cell numbers at the start of the assay. Data are presented as the mean of four measurements per condition.
Proliferating Cell Nuclear Antigen (PCNA) Staining—PASMC were stained with FITC-conjugated anti-PCNA antibody (clone PC10) from Biolegend as well as DAPI. At least 150 cells were counted per condition, and the percentages of PCNA-positive cells are presented. Results are the mean ± S.E. for triplicate assays.
Counting Cell Number—5 × 105 PASMC were plated in 12-well plates after siRNA transfection. At the times indicated, cells were trypsinized, and the total number of cells were counted using hemocytometer. The average of triplicates is presented.
In Vitro Scratch Wound Assay—PASMC transfected with anti-miR-221 or miR-221 mimic were plated in 12-well plates (12,000 cell/well), and a single scratch wound was generated with a 200-μl disposable pipette tip. Scratch wounds were photographed over 7 h with a Nikon inverted microscope with attached digital camera, and their widths were quantitated with the ImageJ software. Data were plotted as the percentage of wound closure setting the initial scratch width as 100% and are presented as the mean of triplicate measurements per condition in three independent experiments.
Statistical Analysis—The results presented are the average of at least three experiments, each performed in triplicate with standard errors. Statistical analyses were performed by analysis of variance followed by Tukey's multiple comparison test or by Student's t test as appropriate using Prism 4 (GraphPAD Software Inc.). p values of <0.05 were considered significant and are indicated with asterisks.
RESULTS
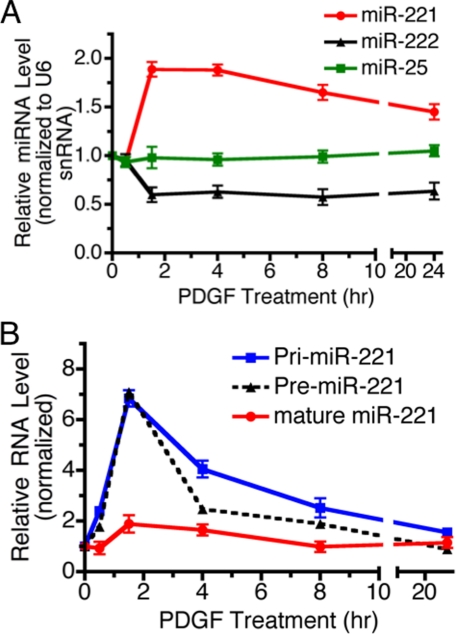
PDGF-BB Induces miR-221—Recent studies have revealed that miRNAs are involved in both physiological and pathological processes in various tissues, including vascular cells (3–7). Although the expression of miRNAs is spatially and temporally regulated, the mechanism of regulation is poorly understood. We recently discovered that the biosynthesis of miR-21, whose expression is frequently elevated in tumors, is regulated by bone morphogenetic protein (BMP) and transforming growth factor-β signaling (27). We hypothesized that PDGF signaling might also modulate vSMC phenotype via regulation of miRNA biogenesis. To this aim we cloned and sequenced miRNAs expressed in PASMC under vehicle- or PDGF-BB-treated conditions and then compared the relative abundance of specific miRNAs in the two populations as described (27). miR-221 was one of the few miRNAs enriched in PASMC treated with PDGF-BB (see supplemental Fig. S1), suggesting that miR-221 expression might be activated by PDGF signaling and mediate its action on the vSMC phenotype switch. A time-course expression of miR-221, miR-222, and miR-25 (control) was examined after PDGF-BB treatment in PASMC. miR-221 was induced 2-fold after 1.5 h of treatment with PDGF and gradually decreased by 8 h (Fig. 1A, red line). The miR-221 gene is encoded as a gene cluster with miR-222 on the X chromosome, and the expression of these miRNAs appears to be co-regulated in some tissues (28–30). Interestingly, our result indicated that PDGF treatment weakly inhibits the expression of miR-222 contrary to miR-221, suggesting that miR-221 and miR-222 are differentially regulated by PDGF in PASMC (Fig. 1A, black line). PDGF did not alter the level of miRNAs globally as miR-25 (Fig. 1A, green line) and miR-100 (data not shown) expression were not affected by PDGF treatment.
FIGURE 1.
miR-221 is regulated by the PDGF-BB signaling pathway. A, time-course expression of miR-221 (red), miR-222 (black), and miR-25 (green) normalized to U6 small nuclear RNA was examined by qRT-PCR in PASMC stimulated with 20 ng/ml PDGF-BB for 0.5–24 h as indicated. Changes of miR-25 upon PDGF-BB treatment was not statistically significant. B, time-course analysis of the relative expression of miR-221 transcripts (Pri-miR-221), Pre-miR-221 or mature miR-221 normalized to GAPDH (for Pri-miR-221 or Pre-miR-221), or U6 small nuclear RNA (for mature miR-221) in PASMC treated with PDGF-BB (20 ng/ml) for 0.5–24 h as indicated. The average of three experiments, each performed in triplicate with S.E., are presented.
To understand at which biosynthetic step PDGF-BB regulates miR-221 expression, we examined by qRT-PCR the level of primary transcripts (Pri-miR-221) and the intermediate product (Pre-miR-221) as well as mature miR-221 after PDGF treatment. Robust induction of both Pri-miR-221 and Pre-miR-221 was observed as early as 1.5 h after PDGF treatment, suggesting that miR-221 is likely to be transcriptionally induced by PDGF signaling (Fig. 1B, blue and black dotted line).
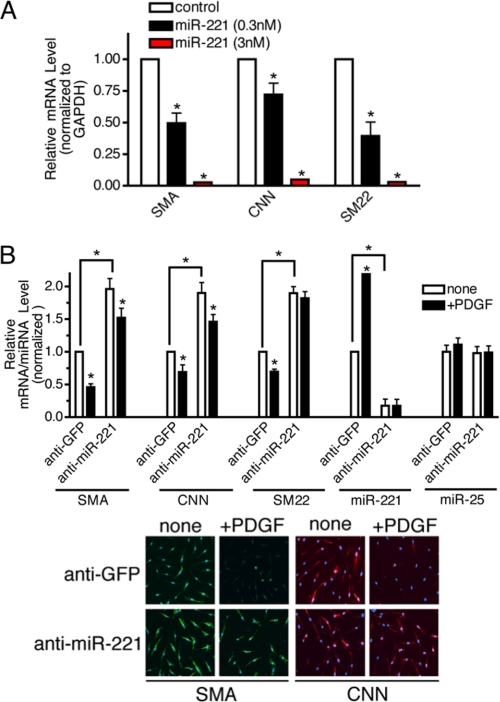
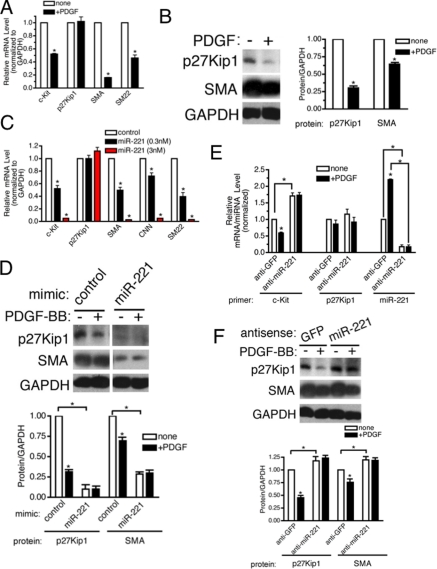
miR-221 Is Essential for the PDGF-mediated Repression of SMC Genes—To examine the effect of increased expression of miR-221 on the vSMC phenotype, we transfected PASMC with increasing amounts (0.3 and 3 nm) of chemically modified, synthetic miR-221 (miR-221 mimic) or control mimic (containing a sequence from GFP) and examined the mRNA level of SMC-specific genes (SMA, CNN, or SM22) by qRT-PCR. According to a qRT-PCR analysis, ectopic expression of 0.3 and 3 nm miR-221 mimic led to ∼180- and 450-fold higher expression levels than the endogenous miR-221 level, respectively (data not shown). Exogenous miR-221 reduced the expression of all three SMC marker mRNAs in a dose-dependent manner, similar to the effect of PDGF (Fig. 2A). It is of note that high magnitude of expression of miR-221 mimic was required to reduce the level of SMC markers similar to the level mediated by PDGF treatment. This observation suggests that the miR-221 level is not the sole determinant of SMC gene expression. It is plausible that miRNA mimic does not down-regulate its targets as efficiently as endogenous miRNA due to chemical modifications on miRNA mimic.
FIGURE 2.
miR-221 is critical for the PDGF-dependent inhibition of SMC gene markers. A, PASMC were transfected with negative control mimic or two different doses of miR-221 mimic (0.3 or 3 nm). Total RNA was harvested 48 h after transfection. The level of expression of SMC genes (SMA, CNN, and SM22) was examined by qRT-PCR analysis. B, PASMC were transfected with antisense oligonucleotides (106 nm) to miR-221 (anti-mIR-221) or GFP (anti-GFP) as control. Cells were then treated with PDGF-BB (20 ng/ml) for 48 h and subjected to qRT-PCR analysis or immunofluorescence staining of SMC markers (SMA, CNN, and SM22). The effect and specificity of anti-miR-221 on miR-221 expression was evaluated by qRT-PCR analysis of miR-221 and miR-25 (control) presented as a ratio to the U6 small nuclear RNA level. SMA and CNN protein expression was examined by FITC-conjugated anti-SMA antibody (green) and Cy3-conjugated anti-CNN antibody (red), respectively. Nuclei were stained with DAPI (blue).
Next, 2′-O-methyl-modified RNA oligonucleotides complementary to the miR-221 sequence (anti-miR-221) were transfected into PASMC to inhibit miR-221 function, and the levels of SMC marker genes were examined. As a control, we used RNA oligonucleotides against GFP (anti-GFP). Anti-miR-221 transfection reduced both the basal and the PDGF-induced expression of miR-221 by more than 80% (Fig. 2B). Anti-miR-221 did not affect the level of miR-25 (Fig. 2B) or other miRNAs tested (data not shown), confirming its specificity. Transfection of anti-miR-221 in PASMC elevated 2-fold the basal mRNA expression of three SMC markers, SMA, CNN, and SM22. Both qRT-PCR (Fig. 2B) and immunofluorescence staining (Fig. 2B) indicated that the PDGF-BB-mediated repression of SMC-specific genes was significantly impaired when miR-221 was inhibited. These results confirm that miR-221 plays an essential role in PDGF-mediated down-regulation of SMC-specific genes.
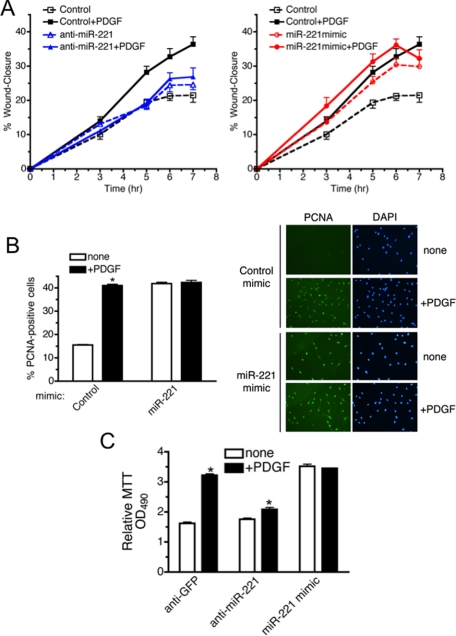
miR-221 Is Essential for the PDGF-mediated Stimulation of Cell Migration and Growth—Not only can PDGF mediate down-regulation of SMC genes, it can also promote cell migration and proliferation; both effects are fundamental characteristics of the dedifferentiated state of vSMCs. We first examined whether induction of miR-221 by PDGF plays a role in cell migration regulation by an in vitro scratch wound assay. One day after PASMC were transfected with anti-miR-221, miR-221 mimic, or anti-GFP (control), a single scratch wound was created in the well, and the time-course of wound closure was monitored (Fig. 3A). In control cells PDGF treatment strongly promoted cell migration (Fig. 3A, left panel, black dotted line versus solid line). When miR-221 function was blocked by anti-miR-221, PDGF did not alter the rate of cell migration, suggesting that induction of miR-221 is essential for PDGF-dependent cell migration (Fig. 3A, left panel, blue dotted line versus solid line). Conversely, transfection of miR-221 mimic alone significantly elevated cell migration to a level similar to untransfected cells treated with PDGF (Fig. 3A, right panel, red dotted line versus black solid line). These results indicate that PDGF-dependent induction of miR-221 is essential for promoting cell migration.
FIGURE 3.
miR-221 is critical for the PDGF-dependent promotion of cell migration and growth. A, PASMC transfected with anti-GFP (Control), anti-miR-221 (left panel), or 0.3 nm miR-221 mimic (right panel) were subjected to the scratch wound assay in the presence or absence of 20 ng/ml PDGF-BB. Results are the mean ± S.E. of triplicate measurements of three independent experiments. B, PASMC transfected with miR-221 mimic (0.3 nm) or control mimic followed by PDGF-BB treatment for 24 h were stained with FITC-conjugated antibody against proliferation marker PCNA and DAPI. >150 cells were counted per condition, and the percentage of PCNA-positive cells is presented. Results are the mean ± S.E. for triplicate assays of three independent experiments. C, PASMC transfected with anti-miR-221, anti-GFP (control), or miR-221 mimic (0.3 nm) followed by PDGF-BB treatment for 24 h were subjected to MTT cell proliferation assay. Results are indicated as the absorbance readings at 490 nm. Results are the mean ± S.E. for triplicate assays of three independent experiments.
The role of miR-221 in PDGF-induced cell proliferation was also examined. PASMC transfected with miR-221 mimic or control mimic were stained with PCNA to measure the number of proliferating cells under each condition (Fig. 3B, right panel). Quantitative analysis of PCNA staining (Fig. 3B, left panel) indicates that PDGF increased the number of proliferating cells ∼2.6-fold in cells transfected with control mimic (Fig. 3B, left panel). Transfection of miR-221 mimic dramatically increased the number of proliferating cells to a level similar to PDGF-treated control cells (Fig. 3B, left panel). PDGF is unable to further stimulate PCNA expression in cells transfected with miR-221 mimic (Fig. 3B), suggesting that induction of miR-221 is a major mechanism of regulation of cell growth by PDGF.
We then confirmed these results using a MTT cell proliferation assay. PASMC transfected with anti-miR-221, anti-GFP (control), or miR-221 mimic were stimulated with PDGF-BB for 24 h, and the number of viable cells was determined by MTT assay. Transfection of miR-221 mimic increased the MTT activity to a level similar to PDGF treatment, consistent with the result of PCNA staining in Fig. 3B (Fig. 3C). The PDGF-mediated increase in cell proliferation (1.8-fold) was significantly reduced (to 1.1-fold) in anti-miR-221-transfected cells (Fig. 3C). A similar effect of anti-miR-221 was observed by quantitative analysis of PCNA staining (see Fig. 5B). These results demonstrate that the PDGF-dependent increase in cell proliferation requires induction of miR-221. Thus, miR-221 mediates the effects of PDGF on migration, proliferation, and differentiation of PASMC. This raises the question of whether a single miR-221 target may be responsible for all these phenomena or whether miR-221 might dispatch the signal through multiple functionally and physically distinct targets.
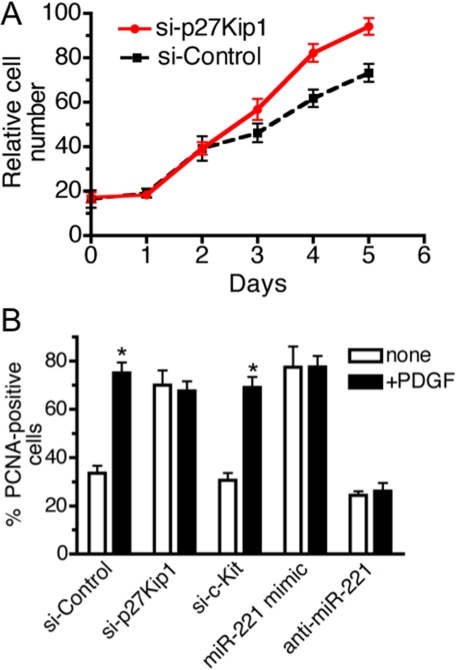
FIGURE 5.
p27Kip1 is responsible for PDGF-mediated promotion of cell growth. A, after transfection of si-Control or si-p27Kip1 in PASMC, 5 × 105 cells were seeded, and proliferation was determined by counting cell number over 5 days. Transfection efficiency of siRNA was at least 95% as determined by FITC-labeled siRNA (data not shown). B, PASMC transfected with si-p27Kip1, si-c-Kit, si-Control, miR-221 mimic (0.3 nm), or anti-miR-221 followed by PDGF-BB treatment for 24 h. Cells were stained with FITC-conjugated anti-PCNA antibody and DAPI. A total of >150 cells were counted per condition, and the percentages of PCNA-positive cells are presented.
miR-221 Targets c-Kit and p27Kip1 in PASMC—miR-221 has previously been shown to target the 3′-UTR of c-kit and p27Kip1 mRNAs and to lead to reduced expression of the gene products through mRNA degradation and/or translational inhibition (28–33). A previous study has shown that human vSMCs express both the ligand of c-Kit, SCF, and c-Kit, which would allow the SCF/c-Kit signaling pathway to affect vSMC function via an autocrine pathway (36). We first investigated whether the c-kit or p27Kip1 mRNA or protein levels are modulated by PDGF-BB in PASMC. c-kit mRNA level was decreased about 50% upon PDGF treatment of PASMC under conditions that significantly inhibited SMC markers expression (Fig. 4A). P27Kip1 mRNA was not affected by PDGF-BB treatment (Fig. 4A). Unlike c-Kit mRNA, the 27Kip1 mRNA level was constant throughout the 24-h PDGF-BB treatment (see supplemental Fig. S2). However, immunoblot analysis indicated that the p27Kip1 protein level was significantly reduced upon PDGF treatment (Fig. 4B). Therefore, both c-Kit and p27Kip1 are regulated by PDGF signaling in vSMCs.
FIGURE 4.
miR-221 mediates reduced expression of c-kit and p27Kip1. A, PASMC were treated with 20 ng/ml PDGF-BB for 24 h followed by qRT-PCR analysis of two known targets of miR-221, c-Kit, and p27Kip1 and two SMC marker genes, SMA and SM22. B, immunoblotting with anti-p27Kip1, anti-SMA, and anti-GAPDH antibody (loading control) was performed in parallel with qRT-PCR analysis (right panel). Proteins bands were quantitated by densitometry, and relative amounts of proteins normalized to GAPDH are presented. C, PASMC were transfected with negative control miRNA or two different doses of miR-221 mimic (0.3 or 3 nm). Total RNA was harvested 48 h after transfection, and the level of expression of c-kit, p27Kip1, and SMC genes (SMA, CNN, and SM22) was examined by qRT-PCR analysis. D, immunoblot analysis of p27Kip1, SMA, and GAPDH using cell lysates of PASMC treated equally to B. Proteins bands were quantitated by densitometry, and relative amounts of proteins normalized to GAPDH are presented. E, PASMC were transfected with antisense oligonucleotides (106 nm) to miR-221 (anti-miR-221) or GFP (anti-GFP) as control. Cells were then treated with PDGF-BB (20 ng/ml) for 48 h and subjected to qRT-PCR analysis of c-kit and p27Kip1. The effect of anti-miR-221 was evaluated by qRT-PCR analysis of miR-221 normalized to U6 small nuclear RNA. F, immunoblot analysis of p27Kip1, SMA, or GAPDH using cell lysates of PASMC treated as described in E. Proteins bands were quantitated by densitometry, and relative amounts of proteins normalized to GAPDH are presented.
To assess if miR-221 is responsible for the PDGF-dependent down-regulation of c-Kit and p27Kip1, miR-221 mimic was transfected into PASMC, and the mRNA level of c-kit, p27Kip1, and SMC markers (SMA, CNN, and SM22) was examined by qRT-PCR. Exogenous miR-221 significantly reduced the expression of c-kit mRNA as well as SMC marker mRNAs (Fig. 4C), confirming that the miR-221 directly targets c-kit mRNA, as previously observed (32, 33). The level of p27Kip1 mRNA was not affected by exogenous miR-221, which is consistent with the result obtained with PDGF treatment in Fig. 4A (Fig. 4C). Immunoblot analysis indicated, however, that miR-221 mimic dramatically reduces p27Kip1 protein as well as SMA protein (Fig. 4D). Therefore, these results confirm that both c-kit and p27Kip1 are repressed by miR-221 in PASMC, with p27Kip1 inhibited at the translation step and c-Kit at the mRNA level.
Next, we tested whether PDGF could down-regulate c-Kit and p27Kip1 when endogenous miR-221 function was blocked by anti-miR-221. When more than 80% of endogenous miR-221 was depleted by anti-miR-221, the c-kit mRNA level was elevated 1.7-fold (Fig. 4E). More importantly, PDGF-BB treatment did not repress c-kit in the presence of anti-miR-221 (Fig. 4E). Similarly, the basal level of p27Kip1 protein was elevated by anti-miR-221, and PDGF was unable to repress it (Fig. 4F). In conclusion, these results confirm that PDGF leads to down-regulation of c-Kit and p27Kip1 through induction of miR-221 in PASMC.
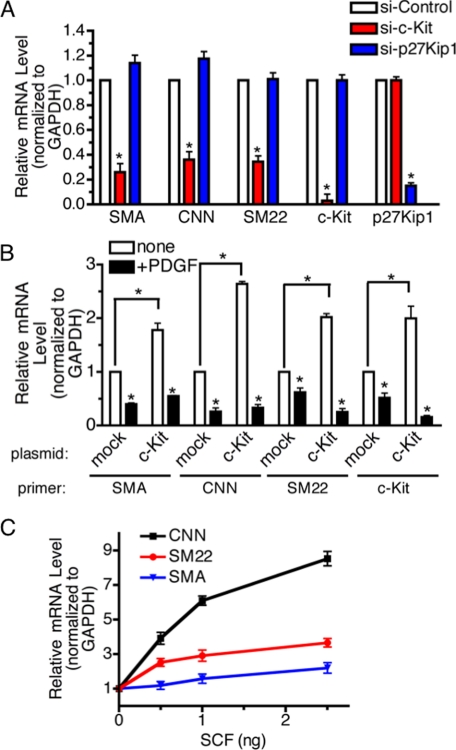
Down-regulation of p27Kip1 Promotes Cell Growth—P27Kip1 is a member of the Cip/Kip family of cyclin-dependent kinase (CDK) inhibitors that function to negatively control cell cycle progression from G1 to S phase through binding to CDK2 and cyclin E complexes (37). In vivo studies suggest that p27Kip1 is critical for vSMC proliferation upon vascular injury (38). Therefore, we hypothesized that PDGF-dependent promotion of vSMC proliferation might be mediated through an increase of miR-221 and down-regulation of p27Kip1. To test the role of p27Kip1 in PASMC proliferation, we reduced endogenous p27Kip1 by transfecting PASMC with siRNA against p27Kip1 (si-p27Kip1) and measured cell growth over 5 days. Based on co-transfection of FITC-conjugated control siRNA, we confirmed that nearly 100% cells were transfected with siRNA (data not shown). As a control, we transfected a siRNA with a non-targeting sequence (si-Control). qRT-PCR analysis confirmed that cells transfected with si-p27Kip1 exhibit more than 80% reduction of p27Kip1 compared with si-Control cells (see Fig. 6A). Under these conditions, the proliferation of si-p27Kip1-transfected cells was significantly increased in comparison to si-Control cells, suggesting that decreased expression of p27Kip1 can promote cell growth in PASMC (Fig. 5A).
FIGURE 6.
Targeted inhibition of c-kit mediates PDGF-dependent suppression of SMC genes. A, PASMC were transfected with non-targeting control siRNA (si-Control) or siRNA for c-kit (si-c-Kit), or p27Kip1 (si-p27Kip1). Forty-eight hours after transfection, total RNAs were extracted and subjected to qRT-PCR analysis to examine mRNA levels of SMA, CNN, SM22, c-kit, or p27Kip1 normalized to GAPDH. The average of three experiments, each performed in triplicate with S.E., are presented. B, PASMC were transfected with empty vector (mock) or human c-Kit expression plasmid followed by PDGF-BB treatment for 24 h and qRT-PCR analysis of SMC genes (SMA, CNN, and SM22) and human c-kit. C, PASMC were treated with 0.5, 1.0, or 2.5 ng of SCF for 24 h followed by qRT-PCR analysis to examine mRNA levels of SMA, CNN, or SM22.
Quantitative analysis of PCNA staining confirmed that transfection of si-p27Kip1 increased the number of proliferating cells as much as PDGF treatment and that PDGF could not further stimulate cell growth in the presence of si-p27Kip1, similar to cells transfected with miR-221 mimic (Fig. 5B). Interestingly, down-regulation of c-Kit did not affect the number of proliferating cells, suggesting that miR-221-mediated inhibition of c-Kit does not play a role in cell growth in PASMC (Fig. 5B). Thus, the PDGF-dependent increase in cell proliferation appears to be mediated by inhibition of a specific target of miR-221, p27Kip1, but not by c-kit.
Inhibition of c-Kit Reduces SMC Gene Expression—We next examined whether either c-Kit or p27Kip1 plays a role in the regulation of SMC gene expression by PDGF. Endogenous c-kit or p27Kip1 was knocked down by siRNA, and SMC gene expression was examined by qRT-PCR. When the c-Kit level was reduced to 3% that of the level in control cells, the basal expression of all three SMC markers was significantly decreased in comparison to si-Control-transfected cells (60–70% inhibition of SMC markers; Fig. 6A, red bars). Despite significant down-regulation of p27Kip1 (15% of si-Control cells), si-p27Kip1 did not inhibit any of the three SMC markers (Fig. 6A, blue bars). These results suggest that c-Kit, but not p27Kip1, is a critical mediator of the PDGF-dependent SMC gene repression.
To examine whether overexpression of c-Kit alone is sufficient to modulate SMC gene expression, we transiently transfected into PASMC a c-Kit expression plasmid containing the coding region and the 3′-UTR which includes the miR-221 seed sequence. A 2-fold increase of c-kit expression resulted in a ∼2-fold increase of all three SMC markers tested (Fig. 6B). PDGF-BB treatment, which causes miR-221 induction, decreased total c-kit expression, presumably through the miR-221 target site in the 3-UTR of the transfected c-kit cDNA plasmid (Fig. 6B, c-Kit). Consistent with the level of c-kit, SMC gene expression was decreased upon PDGF-BB treatment (Fig. 6B). To examine whether the effect of exogenous c-Kit on the expression of SMC gene markers is a result of increased c-Kit signaling, PASMC were treated with increasing amounts of the c-Kit ligand SCF followed by reverse transcription-PCR analysis of SMC gene markers. The level of SMC markers expression was increased in a dose-dependent manner (Fig. 6C). Altogether, these results demonstrate that c-Kit signaling promotes SMC differentiation, although down-regulation of c-kit by miR-221 leads to repression of SMC marker genes.
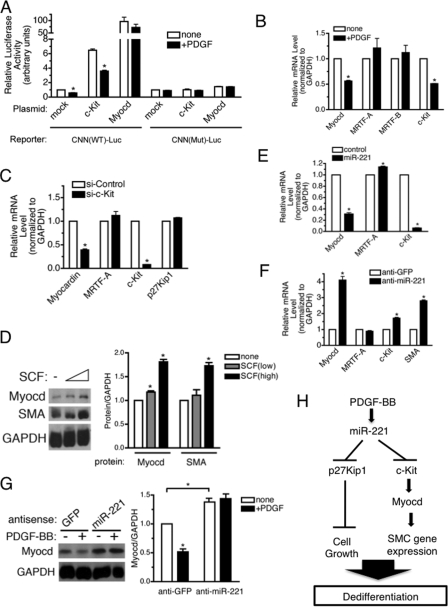
c-Kit Regulates Myocardin Expression—To examine whether the induction of SMC genes by c-Kit is due to transcriptional activation, PASMC were transfected with a luciferase reporter construct driven by the CNN promoter containing the CArG box (CNN(WT))-Luc). About 50% reduction of the reporter activity was observed upon PDGF-BB treatment, confirming that PDGF signaling modulates CNN expression through transcriptional repression (Fig. 7A, mock). Overexpression of Myocd, a potent transcriptional activator of SMC-specific genes which acts through the CArG sequence, induced the reporter activity ∼100-fold (Fig. 7A, Myocd). Exogenous c-Kit, expressed 2-fold over endogenous levels, elevated the reporter activity 6.5-fold, and PDGF-BB inhibited the reporter activity to ∼50%, as PDGF treatment decreases not only endogenous c-kit but exogenous c-kit expression through the miR-221 target site in the 3-UTR of the transfected c-kit cDNA plasmid (Fig. 7A, c-Kit). Furthermore, c-Kit was unable to stimulate a CNN reporter containing a mutation in the CArG sequence (CNN(Mut)-Luc) (Fig. 7A). Similar results were obtained using reporter constructs containing the SMA or SM22 promoters (data not shown). Based on these results, we speculate that c-Kit modulates SMC marker gene expression through a direct transcriptional activation through the CArG box.
FIGURE 7.
c-Kit pathway induces SMC gene expression through induction of a critical transcription factor Myocd. A, wild type (CNN(WT)-Luc) or mutant (CNN(Mut)-Luc) CNN gene promoter-luciferase reporter construct was transiently transfected into PASMC. Cells were treated with or without PDGF-BB for 20 h followed by luciferase assay. Luciferase activities were normalized to the β-galactosidase activities. B, PASMC were treated with 20 ng/ml PDGF-BB for 24 h followed by RNA extraction and qRT-PCR analysis of mRNA encoding Myocd family proteins (Myocd, MRTF-A, and MRTF-B) or c-kit. C, PASMC were transfected with 4 nm non-targeting control siRNA (si-Control) or siRNA for c-kit (si-c-Kit). Forty-eight hours after transfection, total RNAs were extracted and subjected to qRT-PCR analysis to examine mRNA levels of Myocd, MRTF-A, c-kit, or p27Kip1 normalized to GAPDH. D, PASMC were treated with 2.5 or 5 ng of SCF for 8 h followed by immunoblot analysis to examine protein levels of Myocd, SMA, and GAPDH (loading control). Proteins bands were quantitated by densitometry, and relative amounts of proteins normalized to GAPDH are presented. E, PASMC were transfected with control mimic, or miR-221 mimic RNA was harvested 48 h after transfection and subjected to qRT-PCR analysis of mRNA encoding Myocd family proteins (Myocd and MRTF-A) or c-kit normalized to GAPDH. F, PASMC were transfected with anti-GFP (control) or anti-miR-221 mimic followed by qRT-PCR analysis of mRNA encoding Myocd family proteins (Myocd and MRTF-A), c-kit or SMA normalized to GAPDH. G, PASMC transfected with anti-GFP (control) or anti-miR-221 mimic were treated with 20 ng/ml PDGF-BB for 48 h. Total cell lysates were subjected to immunoblotting with anti-Myocd and anti-GAPDH antibody (loading control). Proteins bands were quantitated by densitometry, and relative amounts of proteins normalized to GAPDH are presented. H, schematic diagram of pleiotropic PDGF action mediated by induction of miR-221.
It has been demonstrated that PDGF represses the expression of Myocd in SMCs but not of the related proteins MRTF-A and MRTF-B, although the mechanism of this effect is unknown (13). We speculated that PDGF might modulate Myocd expression indirectly through miR-221 and c-Kit and subsequently affect SMC gene expression. qRT-PCR analysis and immunoblotting confirmed that Myocd mRNA and protein were both reduced to ∼50% upon PDGF-BB treatment, whereas no significant change in MRTF-A or MRTF-B was observed (Fig. 7B). When c-Kit was down-regulated by siRNA, Myocd (but not MRTF-A or p27Kip1) was reduced ∼60% (Fig. 7C). Activation of c-Kit by increasing doses of SCF resulted in an elevated protein level of Myocd as well as SMA, suggesting that the c-Kit signaling pathway is a critical regulator of Myocd, which subsequently modulates SMC gene transcription (Fig. 7D). We then examined whether modulation of miR-221 levels affects Myocd expression. Overexpression of miR-221 reduced Myocd to 30% that of the endogenous level (Fig. 7E). Conversely, inhibition of endogenous miR-221 by anti-miR-221 transfection resulted in a 4-fold increase in Myocd as well as a 1.7-fold increase in c-Kit and a 2.8-fold induction of SMA (Fig. 7F). Furthermore, immunoblot analysis indicates that PDGF-mediated down-regulation of Myocd protein was blocked by anti-miR-221 (Fig. 7G). Therefore, PDGF represses SMC gene expression through down-regulation of c-Kit by miR-221, which subsequently inhibits Myocd. In conclusion, induction of miR-221 by PDGF represses target genes c-kit and p27Kip1, which in turn modulate SMC gene expression and cell growth, respectively (Fig. 7H).
DISCUSSION
Intimal hyperplasia, which is characterized by dedifferentiation of medial SMCs from a quiescent, contractile phenotype to a synthetic, proliferative phenotype, is an essential process of repair after vascular injury that can lead to various vascular disorders (2). Increased expression of PDGF ligands and receptors after vascular injury amplifies the process by promoting a synthetic proliferative phenotype (1). Increased PDGF signaling in PASMC is also implicated in the pathogenesis of IPAH (1), whereas the BMP signaling pathway is generally repressed in IPAH through a variety of mechanisms (39–41). In this study we demonstrate that PDGF signaling reduces the expression of c-kit through up-regulation of miR-221 in vSMCs. We show that c-Kit is critical for the induction of Myocd and activation of SMC genes in PASMC. We observe only an ∼50% reduction of c-kit and Myocd mRNAs upon PDGF treatment, but we speculate that even a moderate change in c-Kit expression in vSMCs can have a significant impact on c-Kit signaling and its biological outcomes. Similarly, overexpression of c-Kit by as little as 2-fold above its endogenous level induces Myocd expression (4-fold) as well as SMC gene expression (3-fold). Furthermore, PDGF-mediated induction of miR-221 is critical for SMC dedifferentiation, as knock-down of miR-221 dramatically increases the levels of Myocd through its upstream modulator, c-kit.
Studies of the maturation of bone marrow stem cells and intestinal development indicate that c-Kit expression is a hall-mark of an undifferentiated, “stem cell-like” state, and that loss of c-kit expression coincides with cell differentiation (42, 43). Therefore, the finding that c-Kit promotes the expression of Myocd, a critical transcriptional regulator in smooth and cardiac muscle differentiation (8, 44, 45), was unexpected. It has recently been shown that Myocd is repressed in transformed cell lines; restoration of Myocd expression results in differentiation to myofibroblasts, expression of SMA, and inhibition of malignant cell growth (46). We speculate that the high level of miR-221 found in various cancers might contribute to the malignant behavior of cancer cells via down-regulation of Myocd in addition to the cell proliferative effect due to down-regulation of p27Kip1 (28–30). In this respect, repression of Myocd by PDGF-mediated induction of miR-221 dedifferentiates PASMC to a state reminiscent of “transformed” cells and characterized by decreased expression of differentiation markers and increased cell growth and motility. This is the first example in which induction of a miRNA is associated with a dedifferentiation process.
Imatinib mesylate has been developed as an effective treatment for cancer and results in decreased proliferation and enhanced apoptosis of malignant cells. Interestingly, imatinib mesylate inhibits the tyrosine kinase activity of both PDGFR and c-Kit. Because PDGFRs and c-Kit synergistically promote tumor growth and invasion, it is thought that imatinib mesylate may be particularly effective against cancer due to its dual inhibitory activity (47, 48). Imatinib mesylate has recently been shown to reverse experimental hypertension in two pulmonary artery hypertension (PAH) animal models as well as in one case of a human idiopathic PAH patient (12, 49), suggesting that this inhibitor may be an effective new therapy for PAH. Our study, however, indicates that a specific antagonist of PDGFR, which does not inhibit c-Kit activity, may be more effective for treating pulmonary artery hypertension, as it would prevent the direct effect of PDGF without promoting vSMC dedifferentiation through inhibition of c-Kit and Myocd.
A critical role of miR-221 has been demonstrated in several human cell types including various carcinomas, hematopoietic cells, and endothelial cells. High expression of miR-221 has been associated with increased proliferation of carcinomas or resistance to chemotherapy agents (28–31, 50–53). It was suggested that silencing of the tumor suppressor gene p27Kip1, which leads to proliferation, is a critical role of miR-221 in cancer cells (28, 29). Our results confirm that miR-221-dependent down-regulation of p27Kip1 promotes cell proliferation in vSMCs. We speculate that the miR-221-p27Kip1 axis might play a role in neointima formation after vascular injury or other pathological conditions. In addition to a role as an inhibitor of cell proliferation in the nucleus, p27Kip1 is known to interact with RhoA in the cytoplasm to modulate cytoskeletal organization and cell migration (54). Therefore, it is intriguing to speculate that the miR-221-p27Kip1 axis might also affect the PDGF-dependent change in cell migration.
In hematopoietic cells miR-221 expression is markedly down-regulated during erythropoietic differentiation and maturation and is inversely related to c-Kit expression, a key regulator of erythropoiesis (32). In endothelial cells, high expression of miR-221 blocks migration, proliferation, and angiogenesis via down-regulation of c-Kit (33). The finding that miR-221 promotes proliferation in cancer cells but blocks endothelial cell proliferation suggests that the role of miR-221 and the regulation of its target genes are cell type-dependent. The inhibition of Myocd and SMC gene expression by miR-221 unveils a novel and specific function of miR-221 in vSMCs. Because modulation of c-Kit alone is able to affect Myocd expression (see Fig. 5, B and C) and miR-221 seed sequences are not present in the 3′-UTR of Myocd transcripts, we speculate that miR-221 down-regulates Myocd indirectly through silencing c-Kit, although we have not completely ruled out the possibility that miR-221 may also directly target Myocd.
The level of Myocd is augmented in pathological conditions such as dilated cardiomyopathy and cerebral angiopathy in Alzheimer disease. However, it is unclear how Myocd is elevated under these conditions (55, 56). Myocd expression is regulated by transcription factors, including Nkx2.5 and Mef2, during cardiovascular development (57, 58). It is of note that only c-Kit/Nkx2.5-double positive embryonic stem cells demonstrated the capacity to differentiate into both cardiomyocytes and SMCs, suggesting that c-Kit and Nkx2.5 may cooperatively regulate SMC fate via induction of Myocd in vivo (59). Recently, it has been reported that Myocd protein is stabilized by the interaction with the 4.5 LIM domain protein 2 (FHL2), which prevents Myocd from undergoing ubiquitin-proteasome-dependent degradation (60). It is plausible that the c-Kit signaling pathway may regulate the expression of FHL2 or the complex formation between FHL2 and Myocd, in turn promoting the stabilization and accumulation of Myocd.
We demonstrated that miR-221 and miR-222 are differentially regulated upon PDGF treatment in vSMCs. As miR-221 and miR-222 are encoded on the X chromosome in tandem as a gene cluster, and have an identical seed sequence, it has been speculated that these two miRNA genes might be regulated in a coordinated manner and control common targets. Indeed, many different cancer tissues, including solid tumors of the colon, pancreas, and stomach, non-solid tumors, and chronic lymphocytic leukemia overexpress both miR-221 and miR-222 (28–31). However, it has also been suggested that miR-221 and miR-222 are differentially expressed and may have a slightly different role in some tumors (61, 62). We speculate that expression of miR-221 and miR-222 could be differentially regulated by growth factors in a context-dependent manner. Spatial and temporal expression of miRNAs can be regulated by different mechanisms such as transcriptional regulation or the processing of pri-miRNA or pre-miRNA by Drosha or Dicer RNase III enzymes (27, 63, 64). We have recently demonstrated that miR-21 is regulated by the transforming growth factor-β and BMP pathway at the first processing step from pri-miR-21 to pre-miR-21 by the Drosha microprocessor complex (27). Here, we demonstrate that miR-221 gene transcription is regulated by PDGF signaling. The PDGF pathway is well known as a modulator of the expression of various protein coding genes, but miR-221 is the first miRNA gene found to be regulated by PDGF. The promoter structure of the miR-221 gene has recently been characterized (65). The microphthalmia-associated transcription factor, a protein known to bind to the E-box, has been shown to bind to the miR-221 promoter in melanocytes (65). As the PDGF pathway modulates transcription through different transcription factor binding sites, including the E-box (66), it is intriguing to speculate that PDGF may activate miR-221 transcription through recruitment of microphthalmia-associated transcription factor or other E-box binding proteins. Binding sites of other transcription factors acting downstream of the PDGF pathway, such as AP-1, Stat, and Elk1, were also found by in silico analysis within the evolutionarily conserved regions of the miR-221 promoter. Further studies, such as chromatin immunoprecipitation and reporter assays, will enable the identification of the transcription factor that is essential for PDGF-dependent regulation of miR-221 in vSMC.
Our study demonstrates that the PDGF pathway is likely to modulate vSMC phenotype through a combination of transcriptional regulation and the miRNA-dependent gene silencing effect. Recent genome-wide studies have revealed that a single miRNA is able to simultaneously modulate expression of a number of genes (14, 15). Our study demonstrates for the first time that the regulation of a single miRNA affects multiple cellular responses through distinct targets. Our finding raises the possibility that the inhibition of miR-221 function in vSMCs, either by treatment with anti-miR-221 or by overexpression of the miR-221 target sequence (miRNA sponge) (67), represents a potential new therapy for vascular proliferative disorders caused by activation of the PDGF pathway.
Supplementary Material
Acknowledgments
We thank all members of the Hata laboratory for critical discussions and comments.
This work was supported, in whole or in part, by National Institutes of Health Grants HL082854 (to A. Hata) and HL086572 (to G. L.) (NIHLB). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: SMC, smooth muscle cell(s); vSMC, vascular SMC; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; UTR, untranslated region; mi-, micro-; CNN, smooth muscle calponin; IPAH, idiopathic pulmonary artery hypertension; Myocd, myocardin; PASMC, pulmonary artery smooth muscle cells; siRNA, small interference RNA; DAPI, 4′-6-Diamidino-2-phenylindole; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SCF, stem cell factor; qRT, quantitative real-time; GFP, green fluorescent protein; FITC, fluorescein isothiocyanate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PCNA, proliferating cell nuclear antigen; BMP, bone morphogenetic protein; SMA, α-smooth muscle actin.
References
- 1.Andrae, J., Gallini, R., and Betsholtz, C. (2008) Genes Dev. 22 1276-1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens, G. K., Kumar, M. S., and Wamhoff, B. R. (2004) Physiol. Rev. 84 767-801 [DOI] [PubMed] [Google Scholar]
- 3.Kim, V. N. (2005) Nat. Rev. Mol. Cell Biol. 6 376-385 [DOI] [PubMed] [Google Scholar]
- 4.Niwa, R., and Slack, F. J. (2007) Curr. Opin. Genet. Dev. 17 145-150 [DOI] [PubMed] [Google Scholar]
- 5.Hammond, S. M. (2006) Cancer Chemother. Pharmacol. 58 63-68 [DOI] [PubMed] [Google Scholar]
- 6.Zhao, Y., and Srivastava, D. (2007) Trends Biochem. Sci. 32 189-197 [DOI] [PubMed] [Google Scholar]
- 7.Bartel, D. P. (2004) Cell 116 281-297 [DOI] [PubMed] [Google Scholar]
- 8.Miano, J. M. (2004) Circ. Res. 95 340-342 [DOI] [PubMed] [Google Scholar]
- 9.Owens, G. (1995) Physiol. Rev. 75 487-517 [DOI] [PubMed] [Google Scholar]
- 10.Lagna, G., Ku, M. M., Nguyen, P. H., Neuman, N. A., Davis, B. N., and Hata, A. (2007) J. Biol. Chem. 282 37244-37255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNamara, C. A., Sarembock, I. J., Bachhuber, B. G., Stouffer, G. A., Ragosta, M., Barry, W., Gimple, L. W., Powers, E. R., and Owens, G. K. (1996) Semin. Thromb. Hemostasis 22 139-144 [DOI] [PubMed] [Google Scholar]
- 12.Ghofrani, H. A., Seeger, W., and Grimminger, F. (2005) N. Engl. J. Med. 353 1412-1413 [DOI] [PubMed] [Google Scholar]
- 13.Yoshida, T., Gan, Q., Shang, Y., and Owens, G. K. (2007) Am. J. Physiol. Cell Physiol. 292 886-895 [DOI] [PubMed] [Google Scholar]
- 14.Selbach, M., Schwanhausser, B., Thierfelder, N., Fang, Z., Khanin, R., and Rajewsky, N. (2008) Nature 455 58-63 [DOI] [PubMed] [Google Scholar]
- 15.Baek, D., Villen, J., Shin, C., Camargo, F. D., Gygi, S. P., and Bartel, D. P. (2008) Nature 455 64-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harfe, B. D. (2005) Curr. Opin. Genet. Dev. 15 410-415 [DOI] [PubMed] [Google Scholar]
- 17.Wienholds, E., and Plasterk, R. H. (2005) FEBS Lett. 579 5911-5922 [DOI] [PubMed] [Google Scholar]
- 18.Croce, C. M., and Calin, G. A. (2005) Cell 122 6-7 [DOI] [PubMed] [Google Scholar]
- 19.Calin, G. A., and Croce, C. M. (2006) Nat. Rev. Cancer 6 857-866 [DOI] [PubMed] [Google Scholar]
- 20.Hammond, S. M. (2006) Curr. Opin. Genet. Dev. 16 4-9 [DOI] [PubMed] [Google Scholar]
- 21.Callis, T. E., Chen, J. F., and Wang, D. Z. (2007) DNA Cell Biol. 26 219-225 [DOI] [PubMed] [Google Scholar]
- 22.Mann, D. L. (2007) N. Engl. J. Med. 356 2644-2645 [DOI] [PubMed] [Google Scholar]
- 23.Yang, Z., and Wu, J. (2007) DNA Cell Biol. 26 257-264 [DOI] [PubMed] [Google Scholar]
- 24.Wiemer, E. A. (2007) Eur. J. Cancer 43 1529-1544 [DOI] [PubMed] [Google Scholar]
- 25.Yu, F., Yao, H., Zhu, P., Zhang, X., Pan, Q., Gong, C., Huang, Y., Hu, X., Su, F., Lieberman, J., and Song, E. (2007) Cell 131 1109-1123 [DOI] [PubMed] [Google Scholar]
- 26.Li, Z., Hassan, M. Q., Volinia, S., van Wijnen, A. J., Stein, J. L., Croce, C. M., Lian, J. B., and Stein, G. S. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 13906-13911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis, B. N., Hilyard, A. C., Lagna, G., and Hata, A. (2008) Nature 454 56-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.le Sage, C., Nagel, R., Egan, D. A., Schrier, M., Mesman, E., Mangiola, A., Anile, C., Maira, G., Mercatelli, N., Ciafre, S. A., Farace, M. G., and Agami, R. (2007) EMBO J. 26 3699-3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galardi, S., Mercatelli, N., Giorda, E., Massalini, S., Frajese, G. V., Ciafre, S. A., and Farace, M. G. (2007) J. Biol. Chem. 282 23716-23724 [DOI] [PubMed] [Google Scholar]
- 30.Visone, R., Pallante, P., Vecchione, A., Cirombella, R., Ferracin, M., Ferraro, A., Volinia, S., Coluzzi, S., Leone, V., Borbone, E., Liu, C. G., Petrocca, F., Troncone, G., Calin, G. A., Scarpa, A., Colato, C., Tallini, G., Santoro, M., Croce, C. M., and Fusco, A. (2007) Oncogene 26 7590-7595 [DOI] [PubMed] [Google Scholar]
- 31.Fornari, F., Gramantieri, L., Ferracin, M., Veronese, A., Sabbioni, S., Calin, G. A., Grazi, G. L., Giovannini, C., Croce, C. M., Bolondi, L., and Negrini, M. (2008) Oncogene 27 5651-5661 [DOI] [PubMed] [Google Scholar]
- 32.Felli, N., Fontana, L., Pelosi, E., Botta, R., Bonci, D., Facchiano, F., Liuzzi, F., Lulli, V., Morsilli, O., Santoro, S., Valtieri, M., Calin, G. A., Liu, C. G., Sorrentino, A., Croce, C. M., and Peschle, C. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 18081-18086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urbich, C., Kuehbacher, A., and Dimmeler, S. (2008) Cardiovasc. Res. 79 581-588 [DOI] [PubMed] [Google Scholar]
- 34.Schmittgen, T. D., Lee, E. J., Jiang, J., Sarkar, A., Yang, L., Elton, T. S., and Chen, C. (2008) Methods 44 31-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miano, J. M., Carlson, M. J., Spencer, J. A., and Misra, R. P. (2000) J. Biol. Chem. 275 9814-9822 [DOI] [PubMed] [Google Scholar]
- 36.Hollenbeck, S. T., Sakakibara, K., Faries, P. L., Workhu, B., Liu, B., and Kent, K. C. (2004) J. Surg. Res. 120 288-294 [DOI] [PubMed] [Google Scholar]
- 37.Koff, A. (2006) Cancer Cell 9 75-76 [DOI] [PubMed] [Google Scholar]
- 38.Chen, D., Krasinski, K., Sylvester, A., Chen, J., Nisen, P. D., and Andres, V. (1997) J. Clin. Investig. 99 2334-2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naeije, R., and Rondelet, B. (2004) Bull. Mem. Acad. R. Med. Belg. 159 219-226 [PubMed] [Google Scholar]
- 40.Adnot, S. (2005) J. Clin. Investig. 115 1461-1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrell, N. W. (2006) Proc. Am. Thorac. Soc. 3 680-686 [DOI] [PubMed] [Google Scholar]
- 42.Kent, D., Copley, M., Benz, C., Dykstra, B., Bowie, M., and Eaves, C. (2008) Clin. Cancer Res. 14 1926-1930 [DOI] [PubMed] [Google Scholar]
- 43.Sanders, K. M., and Ward, S. M. (2007) J. Physiol. (Lond.) 578 33-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida, T., Sinha, S., Dandre, F., Wamhoff, B. R., Hoofnagle, M. H., Kremer, B. E., Wang, D. Z., Olson, E. N., and Owens, G. K. (2003) Circ. Res. 92 856-864 [DOI] [PubMed] [Google Scholar]
- 45.Du, K. L., Ip, H. S., Li, J., Chen, M., Dandre, F., Yu, W., Lu, M. M., Owens, G. K., and Parmacek, M. S. (2003) Mol. Cell. Biol. 23 2425-2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milyavsky, M., Shats, I., Cholostoy, A., Brosh, R., Buganim, Y., Weisz, L., Kogan, I., Cohen, M., Shatz, M., Madar, S., Kalo, E., Goldfinger, N., Yuan, J., Ron, S., MacKenzie, K., Eden, A., and Rotter, V. (2007) Cancer Cell 11 133-146 [DOI] [PubMed] [Google Scholar]
- 47.Ali, S., and Ali, S. (2007) Gene (Amst.) 401 38-4517659849 [Google Scholar]
- 48.Gross, D. J., Munter, G., Bitan, M., Siegal, T., Gabizon, A., Weitzen, R., Merimsky, O., Ackerstein, A., Salmon, A., Sella, A., and Slavin, S. (2006) Endocr. Relat. Cancer 13 535-540 [DOI] [PubMed] [Google Scholar]
- 49.Schermuly, R. T., Dony, E., Ghofrani, H. A., Pullamsetti, S., Savai, R., Roth, M., Sydykov, A., Lai, Y. J., Weissmann, N., Seeger, W., and Grimminger, F. (2005) J. Clin. Investig. 115 2811-2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim, H. J., Kim, Y. H., Lee, D. S., Chung, J. K., and Kim, S. (2008) J. Nucl. Med. 49 1686-1693 [DOI] [PubMed] [Google Scholar]
- 51.Nikiforova, M. N., Tseng, G. C., Steward, D., Diorio, D., and Nikiforov, Y. E. (2008) J. Clin. Endocrinol. Metab. 93 1600-1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciafre, S. A., Galardi, S., Mangiola, A., Ferracin, M., Liu, C. G., Sabatino, G., Negrini, M., Maira, G., Croce, C. M., and Farace, M. G. (2005) Biochem. Biophys. Res. Commun. 334 1351-1358 [DOI] [PubMed] [Google Scholar]
- 53.Miller, T. E., Ghoshal, K., Ramaswamy, B., Roy, S., Datta, J., Shapiro, C. L., Jacob, S., and Majumder, S. (2008) J. Biol. Chem. 283 29897-29903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Besson, A., Dowdy, S. F., and Roberts, J. M. (2008) Dev. Cell 14 159-169 [DOI] [PubMed] [Google Scholar]
- 55.Chow, N., Bell, R. D., Deane, R., Streb, J. W., Chen, J., Brooks, A., Van Nostrand, W., Miano, J. M., and Zlokovic, B. V. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 823-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torrado, M., Lopez, E., Centeno, A., Medrano, C., Castro-Beiras, A., and Mikhailov, A. T. (2003) J. Mol. Med. 81 566-577 [DOI] [PubMed] [Google Scholar]
- 57.Creemers, E. E., Sutherland, L. B., McAnally, J., Richardson, J. A., and Olson, E. N. (2006) Development 133 4245-4256 [DOI] [PubMed] [Google Scholar]
- 58.Ueyama, T., Kasahara, H., Ishiwata, T., Nie, Q., and Izumo, S. (2003) Mol. Cell. Biol. 23 9222-9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christoforou, N., Miller, R. A., Hill, C. M., Jie, C. C., McCallion, A. S., and Gearhart, J. D. (2008) J. Clin. Investig. 118 894-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinson, J. S., Medlin, M. D., Taylor, J. M., and Mack, C. P. (2008) Am. J. Physiol. Heart Circ. Physiol. 295 1067-1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ladeiro, Y., Couchy, G., Balabaud, C., Bioulac-Sage, P., Pelletier, L., Rebouissou, S., and Zucman-Rossi, J. (2008) Hepatology 47 1955-1963 [DOI] [PubMed] [Google Scholar]
- 62.Marsit, C. J., Eddy, K., and Kelsey, K. T. (2006) Cancer Res. 66 10843-10848 [DOI] [PubMed] [Google Scholar]
- 63.Obernosterer, G., Leuschner, P. J., Alenius, M., and Martinez, J. (2006) RNA 12 1161-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wulczyn, F. G., Smirnova, L., Rybak, A., Brandt, C., Kwidzinski, E., Ninnemann, O., Strehle, M., Seiler, A., Schumacher, S., and Nitsch, R. (2007) FASEB J. 21 415-426 [DOI] [PubMed] [Google Scholar]
- 65.Ozsolak, F., Poling, L. L., Wang, Z., Liu, H., Liu, X. S., Roeder, R. G., Zhang, X., Song, J. S., and Fisher, D. E. (2008) Genes Dev. 22 3172-3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiner, J. A., Chen, A., and Davis, B. H. (2000) Biochem. J. 345 225-231 [PMC free article] [PubMed] [Google Scholar]
- 67.Ebert, M. S., Neilson, J. R., and Sharp, P. A. (2007) Nat. Methods 4 721-726 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.