Abstract
The asialoglycoprotein receptor (ASGP-R) is an abundant, carbohydrate-specific, endocytic receptor expressed by parenchymal cells of the liver. We recently demonstrated that the ASGP-R mediates the clearance of glycoproteins bearing Siaα2,6GalNAc as well as those bearing terminal Gal or GalNAc. We now report that glycoproteins such as haptoglobin, serum amyloid protein (SAP), and carboxylesterase that bear oligosaccharides with terminal Siaα2,6Gal are elevated in the plasma of ASGP-R-deficient mice. Because of their abundance in plasma, glycoproteins bearing terminal Siaα2,6Gal will saturate the ASGP-R and compete with each other on the basis of their relative affinities for the ASGP-R and their relative abundance. We propose that the ASGP-R mediates the clearance of glycoproteins that bear oligosaccharides terminating with Siaα2,6Gal and thereby helps maintain the relative concentrations of these glycoproteins in the blood.
The asialoglycoprotein receptor (ASGP-R)3 was initially identified and characterized by Ashwell and co-workers (1, 2) on the basis of its ability to rapidly remove glycoproteins bearing oligosaccharides terminating with β1,4-linked Gal from the circulation. The ASGP-R has been extensively characterized since its initial discovery; however, its biologic function in vivo has remained unclear. This endocytic receptor is highly abundant with 500,000 receptors expressed at the plasma membrane of hepatocytes (3–5) and is rapidly internalized (3, 6). The abundance of the ASGP-R and its rapid rate of internalization in combination with the large number of hepatocytes that are present in the liver, 1.35 × 108/g of liver (7, 8), results in an enormous potential capacity to remove glycoproteins from the circulation. Until recently, mice that have had either subunit of the ASGP-R ablated, subunit 1 ASGP-R1(-/-) or subunit 2 ASGP-R2(-/-), have not been reported to have altered levels of circulating glycoproteins in their blood or to have a physiologic phenotype (9, 10). However, Grewal et al. (11) have reported that the ASGP-R plays a role in von Willebrand factor homeostasis and promotes thrombocytopenia during Steptococcus pneumoniae sepsis by removing platelets that have had their surface sialic acid removed by the bacterial neuraminidase.
We recently established that glycoproteins bearing Asn-linked oligosaccharides terminating with the sequence Siaα2,6GalNAcβ1,4GlcNAc are recognized by the ASGP-R and rapidly removed from the blood (12, 13). Glycoproteins bearing terminal Siaα2,6GalNAcβ1,4GlcNAc are the first examples of endogenous glycoproteins that can be recognized by the ASGP-R without further modification; i.e. removal of terminal Sia. Glycoproteins bearing these structures, for example the prolactin-like proteins (14), glycodelin (15), urokinase (16), and glycoprotein hormones (17), are not highly abundant, suggesting that the ASGP-R recognizes and clears additional more abundant glycoproteins. The most likely candidates are glycoproteins bearing Asn-linked oligosaccharides that terminate with the sequence Siaα2,6Galβ1, 4GlcNAc. We have reported that the ASGP-R recognizes these structures with an avidity that is in the micromolar range (13). The avidity of the ASGP-R for structures terminating with Siaα2,6Galβ1,4GlcNAc is predicted to be sufficient to mediate binding and clearance of glycoproteins bearing structures terminating with Siaα2,6Galβ1,4GlcNAc from the blood. This concept is supported by indications that neo-glycoproteins bearing structures terminating with Siaα2,6Galβ1,4GlcNAc are removed from the blood at a faster rate than those bearing Siaα2,3Galβ1,4GlcNAc (18). Slow clearance of glycoproteins bearing Siaα2,6Galβ1,4GlcNAc, however, hampers accurate measurement of their half-lives by injection of radiolabeled ligands.
We now report that multiple glycoproteins bearing oligosaccharides that terminate with Siaα2,6Galβ1,4GlcNAc are elevated in the plasma of ASGP-R-deficient ASGP-R2(-/-) mice as compared with wild-type (Wt) mice. The elevation of multiple glycoproteins bearing terminal Siaα2,6Galβ1,4GlcNAc supports our proposal that the ASGP-R accounts for the clearance of these glycoproteins. This previously undiscerned role of the ASGP-R now allows us to develop a model of how this receptor may contribute to the regulation of the concentration of many different glycoproteins in the blood.
MATERIALS AND METHODS
Mice—ASGP-R2(-/-) mice obtained from the Jackson Laboratories were kindly provided by David Ginsburg, University of Michigan, Dept. of Internal Medicine and Human Genetics. The mice were backcrossed with C57Bl/J6 for five generations. C57Bl/J6 were used as Wt controls.
Plasma Preparation—Blood was collected from the inferior vena cava of Wt and ASGP-R2(-/-) mice using a 1-ml syringe and a 23-gauge needle treated with EDTA to prevent coagulation. The blood was diluted 1:1 with 20 mm PO4, pH 7.4, 150 mm NaCl (PBS) containing 20 mm EDTA. Leukocytes and erythrocytes were separated from the plasma by sedimentation for 10 min at 0.5 × g. The protein concentration of the plasma was determined using a modified Lowry method (PlusOne 2D-Quant Kit, GE Healthcare).
Cyanine Dye Labeling—Plasma samples from Wt and ASGP-R2(-/-) mice containing 25 μg of protein were separately diluted 1:10 in 30 mm Tris-HCl, pH 8.5, 7 m urea, 2 m thiourea, and 4% CHAPS (w/v). The diluted samples from Wt and ASGP-R2(-/-) mice were labeled with 200 pmol of either 3-(4-carboxymethyl)phenylmethyl-3′-ethyloxacarbocyanine halide N-hydroxysuccinimidyl ester (Cy2), or 1-(5-scarboxypentyl)-1′-methylindodicarbocyanine halide N-hydroxysuccinimidyl ester (Cy5), respectively, in the dark for 30 min at 4 °C in a total volume of 20 μl. Excess N-hydroxysuccinimidyl esters were consumed by adding 1 μl of 10 mm lysine and incubating for 10 min at 4 °C in the dark. The labeled Wt and ASGP-R2(-/-) samples were combined and brought to a final volume of 450 μl with 400 μl of 7 m urea, 2 m thiourea, 4% CHAPS, 10% isopropanol, 5% glycerol (w/v), 5.4 μl Destreak Rehydration Solution (GE Healthcare), and 2.25 μl of ampholyte pH 3–11 (GE Healthcare).
Two-dimensional Differential In-gel Electrophoresis (2D-DIGE)—Isoelectric focusing of the combined, labeled samples was conducted using 24-cm 3–10 NL-immobilized pH gradient (IPG) strips for 6500 Volt-h using an IPGPhor (GE Healthcare). After focusing, the IPG strips were incubated in 10 ml of 50 mm Tris, pH 8.8, 6 m urea, 30% glycerol, and 2% SDS containing 50 mg dithiothreitol. The reduced proteins were alkylated by incubation in the same buffer containing 600 mg of iodoacetamide. Electrophoretic separation by SDS-PAGE was performed by layering the IPG strips onto 10% polyacrylamide gels. The Cy2- and Cy5-labeled images were acquired using a Typhoon Imager (GE Healthcare, Cy2: Blue 488 nm laser, 520 BP40 filter; Cy5: Red 633 nm laser, 670BP30 filter). Relative quantification of specific gel spots was determined using the DIA and BVA modules of the DeCyder software.
Protein Identification of Gel Spots by Mass Spectrometry—Specific gel spots were selected using the Decyder Software and excised from the gel using an x,y robot (ProPic, Genomic Solutions, Ann Arbor, MI). The gel pieces were trypsin-digested. Mass spectrometric analysis was performed on the tryptic peptides as previously described (19) using either a MALDI-TOF/TOF instrument (ABI4700) or a nano LC-MS using a QSTAR-L mass spectrometer.
Lectin Blots—Plasma proteins, 75 μg, from Wt and ASGP-R2(-/-) mice were labeled with Cy5 and individually separated by two-dimensional gel electrophoresis as described above on 12–16% polyacrylamide gels and electrophoretically transferred to Immobilon-FL PVDF membranes. The membranes were each incubated in 125 ml of horseradish peroxidase-conjugated Sambucus nigra I (SNA-1) lectin (EY Labs) diluted 1:1000 in 20 mm Tris, 500 mm NaCl, 0.05% Tween 20, pH 7.5 containing 1% (w/v) bovine serum albumin (Sigma A-7030) for 45 m. The membranes were washed and developed by incubation in 30 ml of ECL plus (GE Healthcare) working solution at 25 °C for 5 m. Digital images were generated by scanning the blots with Typhoon Imager (HRP: 457 nm laser 520BP40 filter; Cy5: 633 nm laser, 670BP40 filter).
Neuraminidase Treatment, Wisteria Floribunda (WFA)-Agarose Affinity Chromatography, and Western Blotting—Plasma proteins, 400 μg, from Wt and ASGP-R2(-/-) mice was digested with neuraminidase from Arthrobacter ureafaciens (Roche Applied Science) at 37 °C overnight. WFA-agarose (EY Laboratories) was prepared for incubation with each sample by washing 50 μl, with 2.5 ml of PBS. Each sample of neuraminidase-treated plasma was applied to WFA-agarose in a Handee mini-spin column (Pierce) and incubated overnight at 4 °C. Unbound proteins were collected by sedimenting the WFA-agarose (200 × g for 1 min) in the spin column. The columns were washed three times with 200 μl of PBS, and each wash fraction was collected by centrifugation. Bound proteins were eluted with 50 mm GalNAc and analyzed by Western blot following separation by SDS-PAGE using 4–12% Bis-Tris gels. Glycoproteins bearing terminal β1,4-linked GalNAc were identified using the monoclonal antibody SMLDN1:1 that is specific for this structure (20) at a dilution of 1:1,000,000 followed by horseradish peroxidase-conjugated goat anti-mouse IgM at a dilution of 1:500,000. Glycoproteins recognized by SMLDN1:1 were excised from a gel, trypsin-digested, and analyzed by mass spectrometry.
Quantitative Western Blotting—The relative quantities of multiple plasma proteins, haptoglobin, SAP, hemopexin, transferrin, and C-reactive protein (CRP) were determined. Plasma proteins from individual Wt and ASGP-R2(-/-) mice were separated by SDS-PAGE and electrophoretically transferred to Immobilon-FL PVDF membranes (Millipore). For hemopexin detection, 1 μg of plasma proteins was loaded onto a 4–12% Bis-Tris gel. For haptoglobin, transferrin, and CRP detection, 10 μg of plasma proteins was loaded onto 4–12% Bis-Tris gels. For SAP detection, 100 μg of plasma proteins was loaded onto 10% Bis-Tris gels (Invitrogen). The plasma was separated using MOPS Running Buffer for all gels (Invitrogen). Following electrophoretic transfer membranes were incubated in 0.1% casein (Hammersten grade, BDH Chemicals) for 1 h to block nonspecific binding during incubation with antibodies. The relative quantity of each protein in each sample was determined by incubating the membranes overnight at 4 °C with the appropriate antibody in 25 ml of PBS. The dilutions were: rabbit anti-mouse haptoglobin (Life Diagnostics) 1:20,000, rabbit anti-rat SAP 1:20,000 rabbit anti-rat hemopexin (Life Diagnostics) 1:2,500, rabbit anti-rat transferrin 1:10,000, and rabbit anti-rat CRP 1:10,000. The membranes were washed three times for 10 min at 25 °C with 0.01% Tween 20 in PBS and incubated for 1 h at room temperature with IReDye800 labeled goat anti-rabbit IgG (Rockland). After washing three times for 10 min at 25 °C with 0.01% Tween 20 in PBS, a LICOR Odyssey was used to scan and quantitate the relative amount of protein in each fraction.
Sambucus Nigra I (SNA-I)-Agarose and Ricinus Communis I (RCA-I)-Agarose Affinity Chromatography—SNA-I-agarose and RCA-I-agarose (EY Laboratories) were prepared for incubation with each sample by washing 50 μl with 2.5 ml of PBS. Plasma proteins, 25 μg in 50 μl of PBS, from Wt or ASGP-R2(-/-) mice were incubated overnight at 4 °C with 50 μl SNA-I or RCA-I-agarose in Handee mini-spin columns (Pierce). Unbound proteins were collected by centrifugation (200 × g for 1 min). The columns were washed three times with 200 μl PBS and each wash fraction was collected by centrifugation. The bound proteins were eluted from the column using 45 μl of NuPAGE LDS sample buffer and 5 μl of NuPAGE sample reducing agent (Invitrogen) and boiling for 5 min. The amount of haptoglobin in each fraction was determined by Western blot analysis as described above.
RESULTS
Multiple Plasma Proteins Are Elevated in the Plasma of ASGP-R2(-/-) Mice as Compared with Wt Mice—The levels of ASGP-R1 are markedly reduced in ASGP-R2(-/-) mice (21), and clearance of glycoproteins bearing terminal β1,4-linked Gal is abolished (13, 21). In addition, membranes prepared from ASGP-R2(-/-) mice do not exhibit detectable binding of glycoproteins such as orosomucoid that have been treated with neuraminidase to expose terminal Gal (not shown). Thus, even though the minor subunit of the ASGP-R has been ablated in ASGP-R2(-/-) mice, the mice are functionally deficient in ASGP-R activity in the liver.
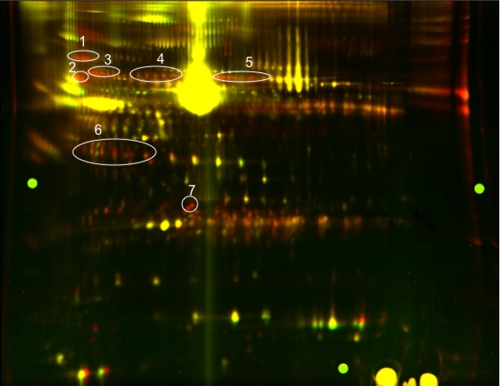
The vast majority of proteins found in plasma are glycosylated. Many of these plasma glycoproteins bear one or more N-linked oligosaccharides that have branches that terminate with either Siaα2,6Gal or Siaα2,3Gal. If, as we have proposed (13), oligosaccharides terminating with Siaα2,6Gal are recognized by the ASGP-R and cleared from the blood at a more rapid rate than those terminating with Siaα2,3Gal, glycoproteins bearing oligosaccharides terminating with Siaα2,6Gal should be elevated in the plasma of ASGP-R2(-/-) mice. We have used fluorescence 2D-DIGE to address this issue. Plasma was isolated from Wt and ASGP-R2(-/-) mice, and equal amounts of plasma protein were fluorescently labeled with Cy2 and Cy5, respectively. The Cy2- and Cy5-labeled samples were combined and then separated into components by two-dimensional electrophoresis. The gel was scanned using the excitation and emission wavelengths specific for Cy2 or Cy5, and the false colored images were digitally combined to produce the composite image illustrated in Fig. 1.
FIGURE 1.
Multiple proteins are elevated in the plasma of ASGP-R2(-/-) mice. Equal amounts of plasma protein, 25 μg, from Wt and ASGP-R2(-/-) mice were labeled with Cy2 and Cy5, respectively, as described under “Materials and Methods.” The cyanine dye-labeled samples were subsequently combined and separated by 2D-DIGE. The images were acquired with a Typhoon Imager and combined to generate the false colored image shown. Wt Cy2-labeled proteins are colored green and ASGP-R2(-/-) Cy5-labeled proteins are colored red. Spots, designated 1–7, that are red represent plasma proteins that are elevated in ASGP-R2(-/-) mice and were subsequently identified by MALDI-TOF/TOF following trypsin digestion. The majority of proteins are colored yellow indicating they are present in equal amounts in plasma from Wt and ASGP-R2(-/-) mice. DeCyder Software was used to quantitate the relative levels of plasma proteins in these images as summarized in Table 1.
As many as 160 individual spots were elevated >3-fold in the plasma of ASGP-R2(-/-) mice as compared with Wt mice. Seven of the most prominent groups of spots that were elevated are circled and designated 1–7 in Fig. 1. The presence of multiple spots differing in charge but not size is characteristic of glycoproteins. Tryptic peptides from individual spots within the regions marked 1–7 were identified by tandem mass spectrometry as summarized in Table 1. All of the spots identified are glycoproteins. Several are associated with the acute phase response including SAP, haptoglobin, serine protease inhibitor clade A, inter-α-trypsin inhibitor, and α1-B glycoprotein. Carboxylesterase and the IgM heavy chain are also glycoproteins but are not considered acute phase proteins. The majority of spots are not elevated in ASGP-R2(-/-) mice as indicated by the features colored yellow in Fig. 1. Thus, only specific plasma proteins are elevated in ASGP-R2(-/-) mice. DeCyder Software was used to determine the relative fold-change for each of the spots that were identified as summarized in Table 1. Individual spots were elevated between 2.8 (inter-α trypsin inhibitor) and 33-fold (SAP) in ASGP-R2(-/-) mice as compared with Wt mice (Table 1).
TABLE 1.
Identification of proteins increased in plasma from ASGP-R2(–/–) mice Spots identified as being elevated in the plasma of ASGP-R2(–/–) mice, indicated by the numbers 1–7 in Fig. 1, were identified by tandem mass spectrometry of tryptic fragments. The identification and fold increase in level of plasma proteins from ASGP-R2(–/–) as compared to Wt mice are indicated for individual spots in each group. APP indicates acute phase proteins; i.e. protein that increase during the acute phase response.
| Protein ID | Location | GI number | Fold change (–/–)/Wt | Function |
|---|---|---|---|---|
| Serine protease Inhibitor Clade A Member 1A | 1 | 6678079 | 6.934.48 | Endopeptidase inhibitor, APP |
| Carboxylesterase | 2 | 2921308 | 3.739.987.35 | Hydrolyze and inactivate toxins |
| α1-B glycoprotein | 3 | 63680085 | 8.92 | Not known, APP |
| Inter-α trypsin inhibitor Heavy chain 4 | 4 | 12836422 | 3.972.78 | Inhibits trypsin and plasmin, APP |
| IgM | 5 | 52382 | 3.163.67 | Antibody |
| Haptoglobin | 6 | 2144486 | 8.954.14 | Bind hemoglobin to prevent oxidative damage, APP |
| SAP | 7 | 54045 | 33.3820.11 | Controls chromatin degradation, APP |
Plasma Glycoproteins Bearing Terminal Siaα2,6Gal Are Elevated in the Plasma of ASGP-R2(-/-) Mice—The results obtained by 2D-DIGE suggest that the ASGP-R regulates the concentration of a select group of plasma proteins. Based on the ability of the ASGP-R to bind glycoproteins bearing structures that terminate in Siaα2,6Gal (13), we predicted that the glycoproteins that are elevated in the plasma of ASGP-R2(-/-) mice will be enriched for structures that terminate with Siaα2,6Gal.
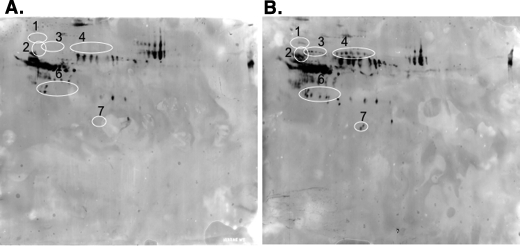
The lectin SNA-I binds saccharides terminating with Siaα2,6Gal/GalNAc but not those terminating with Siaα2,3Gal (22, 23). Cyanine dye-labeled plasma proteins from Wt and ASGP-R2(-/-) mice were separately subjected to two-dimensional gel electrophoresis and electrophoretically transferred to PVDF membranes. The relative locations and levels of spots containing terminal Siaα2,6Gal/GalNAc were determined by Western blot using SNA-I-HRP as shown in Fig. 2. Both the level and number of spots that react with SNA-I are increased in the plasma from ASGP-R2(-/-) mice. Furthermore, at least 6 of the 7 groups of spots identified in Fig. 1 (labeled 1–4, 6–7) and Table 1 as representing elevated plasma glycoproteins correspond to the spots that react with SNA-I. Because the proteins were cyanine dye-labeled, we could confirm the correspondence between the SNA-I reactive spots and the labeled spots.
FIGURE 2.
Glycoproteins bearing terminal Siaα2,6Galβ1,4GlcNAc are elevated in the plasma of ASGP-R2(-/-) mice. Equal amounts of Cy5-labeled plasma proteins, 75 μg from Wt (panel A) and ASGP-R2(-/-) (panel B) mice, respectively, were separately subjected to 2D-GE and electrophoretically transferred to Immobilon-FL PVDF membranes. The membranes were then probed with HRP-SNA-I, which recognizes structures terminating with Siaα2,6Galβ1,4GlcNAc. All spots except one that were identified with an increase in ASGP-R2(-/-) mice corresponded to glycoproteins that are recognized by SNA-I and show increased reactivity in plasma from ASGP-R2(-/-) mice. Compare circled regions in panels A and B.
SNA-I will bind to structures terminating with Siaα2,6GalNAc as well those terminating with Siaα2,6Gal; however, the only glycoproteins in plasma known to bear Siaα2,6GalNAc in rodents are prolactin-like proteins (PLP) in the rat (14) and glycoprotein hormones such as luteinizing hormone (LH) in the mouse (24). The former are only present in plasma during pregnancy (14) and both are present in small quantities. We determined that the predominant, if not exclusive, structure detected by SNA-I in the plasma of Wt and ASGP-R2(-/-) mice is Siaα2,6Gal. Plasma from Wt and ASGP-R2(-/-) mice was treated with neuraminidase to remove sialic acid and expose underlying Gal or GalNAc. The neuraminidase-digested plasma was then incubated with WFA-agarose, which binds oligosaccharides bearing terminal β1,4-linked GalNAc but not those bearing terminal β1,4-linked Gal (25). Glycoproteins bound to WFA-agarose were eluted analyzed by Western blot using the SMLDN1:1 mAb, a monoclonal antibody specific for terminal β1,4-linked GalNAc (20). There was no evidence of any glycoprotein in either the bound or unbound fraction containing terminal GalNAc (not shown). Carbonic anhydrase-VI, which bears oligosaccharides terminating with Siaα2,6GalNAc (26), was used as a positive control. Thus, the glycoproteins in plasma that react with SNA-I bear oligosaccharides that terminate with Siaα2,6Gal.
Taken together, the results indicate that glycoproteins bearing oligosaccharides terminating with Siaα2,6Gal represent the major fraction of glycoproteins that are increased in the plasma of ASGP-R2(-/-) mice.
Haptoglobin and SAP Are Elevated in Plasma from ASGP-R2(-/-) Mice—Spots identified on two-dimensional gels represent individual isoforms of glycoproteins. It is possible that individual isoforms are elevated at the expense of other isoforms, but that the overall level of a given plasma glycoprotein is not increased in ASGP-R2(-/-) mice. Western blot analysis using antibodies specific for haptoglobin and SAP was used to determine if the total level of these two glycoproteins was elevated in the plasma of ASGP-R2(-/-) mice.
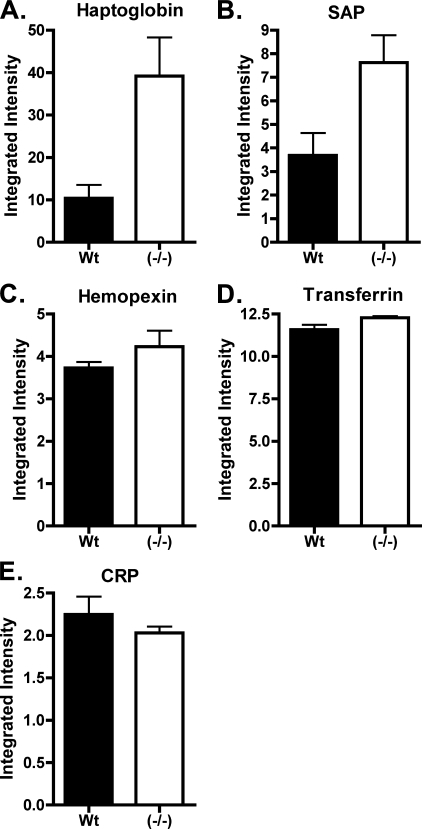
Plasma obtained from 7 Wt and 11 ASGP-R2(-/-) mice was examined as summarized in Fig. 3. Haptoglobin increased 3–5-fold in ASGP-R2(-/-) plasma (p = 0.0112) when compared with Wt (Fig. 3A). SAP increased 2–3-fold (p = 0.0146) when compared with Wt (Fig. 3B). These represent highly significant increases for these two major plasma glycoproteins.
FIGURE 3.
Levels of haptoglobin and SAP but not hemopexin, transferrin, or CRP are elevated in the plasma of ASGP-R2(-/-) mice. Equal amounts of plasma protein per sample, 1 μg for hemopexin, 10 μg for haptoglobin, transferrin, and CRP or 100 μg for SAP detection, from 7 Wt and 11 ASGP-R2(-/-) mice were separated by SDS-PAGE and electrophoretically transferred to Immobilon-FL PVDF membranes. Western blots were performed to quantitate the relative amount of: (panel A) haptoglobin, (panel B) SAP, (panel C) hemopexin, (panel D) transferrin, and (panel E) CRP in each sample. Haptoglobin is elevated 4-fold, p = 0.01, and SAP 2-fold, p = 0.01 in plasma from ASGP-R2(-/-) mice. Hemopexin, transferrin, and CRP did not differ significantly in levels in Wt and ASGP-R2(-/-) mice.
Hemopexin, transferrin, and CRP provide examples of plasma proteins that were not elevated in ASGP-R2(-/-) mice when examined by 2D-DIGE. Quantitation was performed by Western blot using antibodies specific for hemopexin, transferrin, and CRP respectively. In agreement with the 2D-DIGE analysis, neither hemopexin (Fig. 3D) or transferrin (Fig. 3E), which are glycoproteins, nor CRP (Fig. 3C), which is not glycosylated, were elevated. The absence of an increase in CRP levels in ASGP-R2(-/-) mice indicates that the increased levels of Siaα2,6Gal-bearing glycoproteins in ASGP-R2(-/-) mice is not due to induction of the acute phase response.
Haptoglobin from Both Wt and ASGP-R2(-/-) Mice Is Modified with Siaα2,6Gal—Haptoglobin is a major plasma glycoprotein known to bear two N-linked oligosaccharides that are extensively modified with terminal Siaα2,6Gal (27). The SNA-I lectin blot in Fig. 2 shows that the isoforms of haptoglobin that are elevated in plasma from ASGP-R2(-/-) mice bear terminal Siaα2,6Gal; but do not establish that these are the major isoforms of haptoglobin.
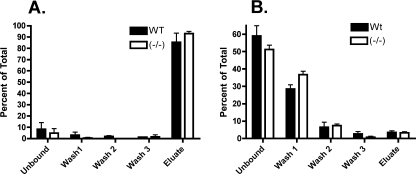
Plasma, 25 μg, from Wt and ASGP-R2(-/-) mice was incubated overnight at 4 °C with SNA-I immobilized on agarose (Fig. 4A). After washing to remove unbound proteins, bound glycoproteins were eluted from the column by boiling in SDS. Less than 10% of the haptoglobin remained in the unbound fraction following incubation with SNA-I agarose and >90% of the bound haptoglobin was present in the fraction eluted with SDS (Fig. 4A). In contrast, when Wt or ASGP-R2(-/-) plasma was incubated with immobilized RCA-I, which binds oligosaccharides with terminal Gal (28), the major fraction of haptoglobin was present in the unbound fraction and the first wash fraction. The differences in the amount of haptoglobin in the Unbound and Wash1 fractions from Wt and ASGP-R2(-/-) mice does not reach statistical significance. The amount of haptoglobin present in the bound fraction did not differ, 3% in plasma from Wt mice versus 4% in plasma of ASGP-R2(-/-) (Fig. 4B). Thus, the major, if not exclusive, form of haptoglobin in both Wt and ASGP-R2(-/-) mice bears oligosaccharides terminating with Siaα2,6Gal whereas haptoglobin bearing terminal Gal is a minor fraction in Wt and ASGP-R2(-/-) mice. Furthermore, the amount of haptoglobin bearing terminal Gal does not increase significantly in ASGP-R2(-/-) mice as compared with Wt mice. Thus, the increase in haptoglobin in ASGP-R2(-/-) mice represents a reduced clearance rate for glycoproteins bearing oligosaccharides that terminate with Siaα2,6Gal.
FIGURE 4.
Haptoglobin-bearing oligosaccharides that terminate with Siaα2,6Gal predominate in the plasma of Wt and ASGP-R2(-/-). Plasma, 25 μg, from Wt and ASGP-R2(-/-) mice was incubated with SNA-I-agarose (panel A) or RCA-I-agarose (panel B). Equal aliquots of the supernatant containing unbound haptoglobin, each of three wash fractions, and material eluted by warming the agarose in SDS-PAGE-loading buffer was subjected to SDS-PAGE and electrophoretic transfer to Immobilon-F PVDF membranes. The amount of haptoglobin in each fraction was determined by Western blotting using rabbit anti-mouse haptoglobin. The amount of haptoglobin in each fraction is shown as a percent of the total.
DISCUSSION
Our observation that multiple glycoproteins bearing terminal Siaα2,6Gal are elevated in the plasma of ASGP-R2(-/-) mice indicates that the ASGP-R serves to control the concentration of glycoproteins bearing structures terminating with Siaα2,6Gal by clearing them from the blood. The ASGP-R, an abundant carbohydrate-specific, endocytic receptor that is expressed by parenchymal cells in the liver (1), is well suited for this purpose. It is among the most abundant receptors known in mammals with as many as 500,000 asialoglycoprotein binding sites expressed at the plasma membrane of hepatocytes (3–5). The receptor is rapidly endocytosed and recycled back to the cell surface. The ASGP-R is capable of internalizing 0.1 pmol of ligand per minute per 106 parenchymal cells (3, 29). The 1.5 g of liver in the mouse is capable of removing 1.2 nmol of ligand per h from the blood if it is saturated with ligand! This is the equivalent of clearing 110 μg of haptoglobin dimer with a molecular weight of 90,000 per hour. The normal concentration of haptoglobin, one of the most abundant glycoproteins present in plasma, is 600–2700 μg/ml or 7.0–30 μm. If the ASGP-R binds haptoglobin with a Kd of 3.6 μm, the Kd that we obtained for binding bovine serum albumin chemically modified with oligosaccharides terminating with Siaα2,6Gal (13), the receptor would be saturated at the concentrations of haptoglobin seen in vivo. As a result, if haptoglobin were the only glycoprotein recognized the ASGP-R would remove 110 μg of haptoglobin from the blood per h or between 2 and 9% of the haptoglobin present in the entire circulation of the mouse every h.
However, haptoglobin is not the only glycoprotein that bears oligosaccharides that terminate with Siaα2,6Gal, nor is it the only glycoprotein that is elevated in the plasma of ASGP-R2(-/-) mice. The glycoproteins listed in Table 1 as well as a number of other glycoproteins also bear oligosaccharides terminating with Siaα2,6Gal and will compete with haptoglobin as well as each other for binding to the ASGP-R. The ASGP-R will, therefore, be saturated with respect to binding ligands that bear terminal Siaα2,6Gal. As a result the fraction of ASGP-R binding sites that are occupied by individual glycoproteins will reflect their relative affinity and concentration relative to the other glycoproteins in plasma that are recognized. For example, if haptoglobin is bound with a Kd of 3.0 μm and SAP with a Kd of 15 μm and both are present in plasma at the same concentration, 80% of the ASGP-R will be occupied with haptoglobin and 20% with SAP. Under this condition haptoglobin will be cleared at a 4-fold greater rate than SAP.
The affinity of individual plasma glycoproteins that bear oligosaccharides that terminate with Siaα2,6Gal for the ASGP-R will reflect the number and location of these structures. The concentrations of plasma glycoproteins will reflect their rates of synthesis and their rates of removal by mechanisms related to their function such as the binding of free hemoglobin by haptoglobin as well as clearance by the ASGP-R. We propose that the ASGP-R acts much like a “buffer” in vivo. If the concentration of a plasma glycoprotein increases due to increased synthesis, it will occupy a greater fraction of the ASGP-R and be cleared at a more rapid rate. If the concentration of a plasma glycoprotein decreases due to consumption, for example in the case of haptoglobin by increased hemolysis, a smaller fraction of the ASGP-R will be occupied by that glycoprotein and it will be cleared at a slower rate.
We postulate that the ASGP-R serves to help regulate the relative concentrations of a wide range of plasma glycoproteins that all bear terminal Siaα2,6Gal. Because the total amount of these glycoproteins is more than sufficient to saturate the binding sites on the hepatocytes, each glycoprotein will occupy a fraction of the receptor proportionate to the concentration and affinity of that glycoprotein relative to all the other glycoproteins in the plasma. Should the concentration of an individual glycoprotein increase or decrease relative to all the others due to some perturbation, the ASGP-R will serve to return the glycoprotein to its same relative concentration after the perturbation has ceased by increasing or decreasing its rate of clearance. Such a function may well explain why so many different glycoproteins in plasma bear oligosaccharides that terminate with Siaα2,6Gal. An attractive feature of such a mechanism is that it would also serve to return glycoproteins to hepatocytes where their amino acids and sugars can be recycled following degradation in lysosomes. The rapid clearance of glycoproteins bearing terminal Siaα2, 6GalNAc, Gal, or GalNAc will continue because glycoproteins bearing these structures will be bound with affinities that are 100-fold stronger than those for glycoproteins bearing structures terminating with Siaα2,6Gal. The rapid clearance of glycoproteins bearing terminal Siaα2,6GalNAc, Gal, or GalNAc may reflect additional functions that require rapid clearance by the ASGP-R. For example, the reported removal of platelets from the blood by the ASGP-R during S. pneumoniae sepsis due to the action of the bacterial neuraminidase may comprise such a distinct function (11).
The results we have presented indicate that the ASGP-R does indeed mediate the clearance of a wide range of glycoproteins that bear terminal Siaα2,6Gal. Ablation of the ASGP-R results in significant increases in the plasma concentrations of these glycoproteins. We propose that an important function of the ASGP-R is to contribute to the regulation of the relative concentrations of plasma glycoproteins bearing terminal Siaα2,6Gal.
This work was supported, in whole or in part, by National Institutes of Health Grant R37-CA21923 (to J. U. B.). This work was also supported by the National Centers of Research Resources P41-RR00954, the Siteman Cancer Center, and institutional resources to the Proteomics Center. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ASGP-R, asialoglycoprotein receptor; ASGP-R1, subunit 1 of the ASGP-R; ASGP-R2, subunit 2 of the ASGP-R; Gal, galactose; GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine; Sia, sialic acid; SAP, serum amyloid protein; Wt, wild-type; PBS, phosphate-buffered saline; 2D-DIGE, two-dimensional difference gel electrophoresis; IPG, immobilized pH gradient; SNA-I, S. nigra I; RCA-I, R. communis I; APP, acute phase protein; PVDF, polyvinylidene difluoride; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; CRP, C-reactive protein; MOPS, 4-morpholinepropanesulfonic acid; MALDI-TOF, matrix-assisted laser desorption/ionization-time of flight.
References
- 1.Ashwell, G., and Harford, J. (1982) Annu. Rev. Biochem. 51 531-554 [DOI] [PubMed] [Google Scholar]
- 2.Morell, A. G., Gregoriadis, G., Scheinberg, I. H., Hickman, J., and Ashwell, G. (1971) J. Biol. Chem. 246 1461-1467 [PubMed] [Google Scholar]
- 3.Schwartz, A. L., Rup, D., and Lodish, H. F. (1980) J. Biol. Chem. 255 9033-9036 [PubMed] [Google Scholar]
- 4.Weigel, P. H. (1980) J. Biol. Chem. 255 6111-6120 [PubMed] [Google Scholar]
- 5.Baenziger, J. U., and Fiete, D. (1980) Cell 22 611-620 [DOI] [PubMed] [Google Scholar]
- 6.Tolleshaug, H., Berg, T., Nilsson, M., and Norum, K. R. (1977) Biochim. Biophys. Acta 499 73-84 [DOI] [PubMed] [Google Scholar]
- 7.Kedderis, G. L., and Held, S. D. (1996) Toxicol. Appl. Pharmacol. 140 124-130 [DOI] [PubMed] [Google Scholar]
- 8.Kawada, N. (1997) Histol. Histopathol. 12 1069-1080 [PubMed] [Google Scholar]
- 9.Braun, J. R., Willnow, T. E., Ishibashi, S., Ashwell, G., and Herz, J. (1996) J. Biol. Chem. 271 21160-21166 [DOI] [PubMed] [Google Scholar]
- 10.Tozawa, R., Ishibashi, S., Osuga, J., Yamamoto, K., Yagyu, H., Ohashi, K., Tamura, Y., Yahagi, N., Iizuka, Y., Okazaki, H., Harada, K., Gotoda, T., Shimano, H., Kimura, S., Nagai, R., and Yamada, N. (2001) J. Biol. Chem. 276 12624-12628 [DOI] [PubMed] [Google Scholar]
- 11.Grewal, P. K., Uchiyama, S., Ditto, D., Varki, N., Le, D. T., Nizet, V., and Marth, J. D. (2008) Nat. Med. 14 648-655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park, E. I., Manzella, S. M., and Baenziger, J. U. (2003) J. Biol. Chem. 278 4597-4602 [DOI] [PubMed] [Google Scholar]
- 13.Park, E. I., Mi, Y., Unverzagt, C., Gabius, H. J., and Baenziger, J. U. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 17125-17129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzella, S. M., Dharmesh, S. M., Cohick, C. B., Soares, M. J., and Baenziger, J. U. (1997) J. Biol. Chem. 272 4775-4782 [DOI] [PubMed] [Google Scholar]
- 15.Dell, A., Morris, H. R., Easton, R. L., Panico, M., Patankar, M., Oehniger, S., Koistinen, R., Koistinen, H., Seppala, M., and Clark, G. F. (1995) J. Biol. Chem. 270 24116-24126 [DOI] [PubMed] [Google Scholar]
- 16.Bergwerff, A. A., Van Oostrum, J., Kamerling, J. P., and Vliegenthart, J. F. G. (1995) Eur. J. Biochem. 228 1009-1019 [DOI] [PubMed] [Google Scholar]
- 17.Stockell Hartree, A., and Renwick, A. G. C. (1992) Biochem. J. 287 665-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unverzagt, C., Andre, S., Seifert, J., Kojima, S., Fink, C., Srikrishna, G., Freeze, H., Kayser, K., and Gabius, H. J. (2002) J. Med. Chem. 45 478-491 [DOI] [PubMed] [Google Scholar]
- 19.Bredemeyer, A. J., Lewis, R. M., Malone, J. P., Davis, A. E., Gross, J., Townsend, R. R., and Ley, T. J. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 11785-11790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyame, A. K., Leppanen, A. M., DeBose-Boyd, R., and Cummings, R. D. (1999) Glycobiology 9 1029-1035 [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi, S., Hammer, R. E., and Herz, J. (1994) J. Biol. Chem. 269 27803-27806 [PubMed] [Google Scholar]
- 22.Shibuya, N., Goldstein, I. J., Broekaert, W. F., Nsimba-Lubaki, M., Peeters, B., and Peumans, W. J. (1987) J. Biol. Chem. 262 1596-1601 [PubMed] [Google Scholar]
- 23.Van Damme, E. J., Barre, A., Rouge, P., Van Leuven, F., and Peumans, W. J. (1996) Eur. J. Biochem. 235 128-137 [DOI] [PubMed] [Google Scholar]
- 24.Mi, Y., Fiete, D., and Baenziger, J. U. (2008) J. Clin. Investig. 118 1815-1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mengeling, B. J., Smith, P. L., Stults, N. L., Smith, D. F., and Baenziger, J. U. (1991) Anal. Biochem. 199 286-292 [DOI] [PubMed] [Google Scholar]
- 26.Hooper, L. V., Beranek, M. C., Manzella, S. M., and Baenziger, J. U. (1995) J. Biol. Chem. 270 5985-5993 [DOI] [PubMed] [Google Scholar]
- 27.Dalziel, M., Lemaire, S., Ewing, J., Kobayashi, L., and Lau, J. T. (1999) Glycobiology 9 1003-1008 [DOI] [PubMed] [Google Scholar]
- 28.Baenziger, J. U., and Fiete, D. (1979) J. Biol. Chem. 254 9795-9799 [PubMed] [Google Scholar]
- 29.Schwartz, A. L., Fridovich, S. E., and Lodish, H. F. (1982) J. Biol. Chem. 257 4230-4237 [PubMed] [Google Scholar]