Abstract
Mitogen-activated protein kinases, originally known as microtubule-associated protein (MAP) kinases, are activated in response to a variety of stimuli. Here we report that microtubule-depolymerizing agents such as colchicine or nocodazole induced strong activation of MAP kinases including JNK, ERK, and p38. This effect was markedly attenuated by parkin, whose mutations are linked to Parkinson disease (PD). Our previous study has shown that parkin stabilizes microtubules through strong interactions mediated by three independent domains. We found that each of the three microtubule-binding domains of parkin was sufficient to reduce MAP kinase activation induced by microtubule depolymerization. The ability to attenuate microtubule depolymerization and the ensuing MAP kinase activation was abrogated in B-lymphocytes and fibroblasts derived from PD patients with parkin mutations such as exon 4 deletion. Such mutations produced truncated parkin proteins lacking any microtubule binding domain and prevented parkin from protecting midbrain dopaminergic neurons against microtubule-depolymerizing toxins such as rotenone or colchicine. Consistent with these, blocking MAP kinase activation in midbrain dopaminergic neurons by knocking down MAP kinase kinases (MKK) significantly reduced the selective toxicity of rotenone or colchicine. Conversely, overexpression of MAP kinases caused marked toxicities that were significantly attenuated by parkin. Thus, the results suggest that parkin protects midbrain dopaminergic neurons against microtubule-depolymerizing PD toxins such as rotenone by stabilizing microtubules to attenuate MAP kinase activation.
Mitogen-activated protein kinases are a superfamily of kinases that include the extracellular signal-related kinases (ERK1/2),2 Jun N-terminal kinases (JNK1/2/3), and p38 proteins (p38α/β/γ/δ) in mammalian species (1). Initially, MAP kinase stood for microtubule-associated protein kinase because microtubule-associated proteins such as MAP2 are excellent substrates of MAP kinases (2, 3). Previous studies have shown that a significant portion of ERK is associated with microtubules (4). It has also been shown that JNK1 is required for the maintenance of microtubule integrity in neurons through controlling the phosphorylation states of MAP2 and MAP1B (5). Phosphorylation of tau, an axon-enriched MAP, by p38δ promotes microtubule assembly (6). All MAP kinases are proline-directed kinases, preferring serine or threonine residues followed by proline. The abundance of such sites on many microtubule-associated proteins suggests that MAP kinases play critical roles in regulating the phosphorylation states of MAPs; hence, the dynamic properties of microtubules.
The selective loss of dopaminergic (DA) neurons in substantia nigra is the pathological hallmark of Parkinson disease and directly contributes to its locomotor symptoms. Nigral DA neurons project to striatal target areas with very long axons, which rely on microtubules to transport dopamine vesicles over long distances. Our previous studies have shown that these dopaminergic neurons are particularly vulnerable to microtubule-depolymerizing agents including rotenone (7), an environmental toxin that causes PD-like symptoms and pathologies in animal models (8). Microtubule depolymerization disrupts vesicular transport, which significantly elevates oxidative stress due to increased oxidation of cytosolic dopamine leaked from vesicles (7). On the other hand our previous studies have shown that parkin, a protein-ubiquitin E3 ligase linked to Parkinson disease, strongly binds to microtubules (9) through redundant, high affinity interactions mediated by three independent domains (10). In addition, parkin increases the ubiquitination and degradation of both α- and β-tubulin (9), whose complex folding reactions are prone to produce misfolded intermediates (11). These results suggest that parkin plays an important role in maintaining the stability and normal functions of microtubules, which are critically involved in the survival of nigral DA neurons (12).
In the present study we examined the impact of parkin on MAP kinase activation induced by microtubule depolymerization. Our results showed that MAP kinases, including JNK, ERK, and p38, were activated by microtubule-depolymerizing agents such as colchicine and nocodazole. This effect was greatly attenuated by overexpression of wild-type parkin or any one of its three microtubule binding domains. The ability of parkin to suppress microtubule depolymerization and the ensuing MAP kinase activation was abrogated in PD patients with parkin mutations such as exon 4 deletion, which produced a truncated protein lacking any microtubule-binding domain. This mutation also prevented parkin from protecting dopaminergic neurons against the selective toxicity of microtubule-depolymerizing toxins such as rotenone or colchicine. Blocking MAP kinase activation by small interfering RNA (siRNA) of MAP kinase kinases significantly reduced the selective toxicity of rotenone or colchicine, whereas overexpression of MAP kinases produced toxicities that were significantly reduced by parkin. Together, the results suggest that parkin protects midbrain dopaminergic neurons against microtubule-depolymerizing PD toxins by stabilizing microtubules to rein in MAP kinase activation.
EXPERIMENTAL PROCEDURES
Antibodies and Expression Constructs—Rabbit polyclonal antibodies against total or phosphorylated JNK1/2, c-Jun, ERK1/2, or p38 were purchased from Cell Signaling (Beverly, MA). Monoclonal antibody against FLAG (M2) and cy5-conjugated anti-rabbit IgG were purchased from Sigma. Polyclonal antibody against actin was from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-TH was from Affinity Bio-Reagents (Golden, CO). Various human parkin expression constructs and FLAG-tagged luciferase were generated previously (10). FLAG-tagged expression constructs containing exon 4-deleted parkin cDNA were generated by reverse transcription-PCR using total RNA isolated from the human B lymphocyte cell line ND01037, which was derived from a PD patient with homozygous deletion of exon 4 of parkin (13). The primers for the Δ4 construct were ATGATAGTGTTTGTCAGGTTCAAC and GGAATTAAAACATCATCCCAGCAA. The primers for the Δ4′ construct were ATGATAGTGTTTGTCAGGTTCAAC and CTACACGTCGAACCAGTGGTC. The PCR products were verified by sequencing and subcloned into the mammalian expression construct pCMV-tag 2B (Stratagene, La Jolla, CA), which added an N-terminal FLAG tag. Mouse cDNA for ERK1, JNK1, or p38α was purchased from Openbiosystems (Huntsville, AL) and was subcloned into pEGFP-C1 (Clontech, Mountain View, CA), which adds GFP at the N terminus of the MAP kinase. FLAG-parkin was subcloned into the pSinRep5 vector (Invitrogen) for production of recombinant sindbis virus according to the manufacturer's protocol.
Cell Lines, Neuronal Cultures, B-lymphocytes, and Skin Fibroblasts from PD Patients—Human embryonic kidney (HEK) 293 and SH-SY5Y cells were purchased from American Type Culture Collection (Manassas, VA). HEK293 and SH-SY5Y cells stably expressing FLAG-parkin were generated previously (14). All cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% bovine fetal calf serum and antibiotics. Primary midbrain neuronal cultures were prepared from rat embryos at E18 as previously described (7). Cultures were maintained in 24-well plates in neurobasal media supplemented with 2% B27 (Invitrogen) and AraC (5 μm, Sigma) for 14 days. Before neuronal cultures were treated with colchicine or nocodazole, they were incubated in neurobasal media without B27 for 2 h to lower the basal level of MAP kinase activation induced by growth factors in B27. Epstein-Barr virus-transformed human B lymphocyte cell lines were purchased from Coriell Cell Repositories (Camden, NJ). ND01037 was derived from a PD patient (male Caucasian, 33 years of age at sampling) with homozygous deletion of exon 4 of parkin. ND01038 (male Caucasian, 41 years) and ND01040 (female Caucasian, 51 years) were derived from unaffected siblings of ND01037 and carried heterozygous deletion of exon 4 (13). Two unrelated and unaffected controls were used, ND00092 (male Caucasian, 37 years) and ND05706 (male Caucasian, 39 years). These cells were maintained in RPMI 1640 medium containing 2 mm l-glutamine and 15% heat-inactivated fetal bovine serum (Invitrogen). Primary skin fibroblasts from two PD patients with parkin mutations and two normal subjects are described previously. One patient carried a homozygous deletion of exon 2, whereas the other carries compound heterozygous deletions of exons 3-4 and exons 3-5 (15). These primary fibroblasts were maintained in Dulbecco's modified Eagle's medium supplemented with 10% bovine fetal calf serum and antibiotics. The protocols covering the use of PD cell lines have been determined to meet exempt criteria for human subject research by the Health Sciences Institutional Review Board of the State University of New York at Buffalo.
Transfection and Western Blotting—Transient transfection of various constructs in HEK293 cells was performed using the calcium phosphate method for 48 h. Cells cultured in 6-cm dishes were washed 3 times in cold phosphate-buffered saline and lysed on ice in cold lysis buffer (1% Triton X-100, 10 mm Tris, pH 7.6, 50 mm NaCl, 30 mm sodium pyrophosphate, 50 mm NaF, 5 mm EDTA, and 0.1 mm Na3VO4) for 20 min. Lysates were centrifuged at 16,000 × g at 4 °C, and the supernatant fractions containing equal amounts of total proteins were boiled in 2× SDS loading buffer for 5 min followed by separation on SDS-polyacrylamide gel and analysis by Western blots with various antibodies. Western blotting was carried out using the ECL method according to the manufacturer's protocol (GE Healthcare).
Extraction of Free or Polymerized Tubulin—Free or polymerized tubulin from HEK293 cells, B-lymphocytes, or primary fibroblasts was extracted as described previously (7). After the cells were treated with 10 μm colchicine or nocodazole for 2 h, they were washed twice at 37 °C with 1 ml of buffer A containing 0.1 m MES, pH 6.75, 1 mm MgSO4, 2 mm EGTA, 0.1 mm EDTA, and 4 m glycerol. The cultures were then incubated at 37 °C for 5 min in 600 μl of free tubulin extraction buffer (buffer A plus 0.1% v/v Triton X-100 and protease inhibitors). The extracts were centrifuged at 37 °C for 2 min at 16,000 × g. The supernatant fractions contained free tubulin extracted from the cytosol. The pellet fraction and lysed cells in the culture dish were dissolved in 600 μl of 25 mm Tris, pH 6.8, plus 0.5% SDS and contained tubulin originally in a polymerized state (i.e. as microtubules). Equal amounts of total proteins from free or polymerized tubulin fractions were analyzed by Western blotting with anti-α-tubulin to assess the amount of tubulin or antiactin to show equal loading.
Transfection of Midbrain Neuronal Culture, Immunocytochemistry, Image Acquisition, and Analysis—Primary embryonic rat midbrain neuronal cultures maintained for 10 days were transfected with 3 μg of various constructs using Lipofectamine 2000 (Invitrogen). Four days later (i.e. at 14 days in vitro) the cultures were treated with rotenone (100 nm) or colchicine (10 μm) for 12 h. Cell death was detected by TdT-mediated dUTP-X nick end labeling (TUNEL) staining using the In Situ Cell Death Detection kit from Roche Applied Science. Fluorescence images were acquired on a Nikon fluorescence microscope with a CCD camera (Diagnostic Instrument, Sterling Heights, MI) and merged using the SPOT software (Diagnostic Instrument). The cultures contained many dead glial cells (TUNEL+), which were killed by AraC in the media. Double-stranded siRNA targeting rat MKK1 (NM_031643, CGUGUACAUCAUGGUAUUUtt), MKK2 (NM_ 133283, GGACAUCUUAGGGAAGGUCtt), MKK3 (NM_008928, GCCAUAAGCUACUGCCAUUtt), MKK4 (NM_001030023, GGACGAGUCCAAAAGGCCAtt), MKK6 (NM_053703, GCCUUCAAAUGUGCUCAUUtt), and MKK7 (NM_ 001025425, GCGUUAUCAGGCAGAAAUCtt) were purchased from Ambion (Austin, TX). At 11 days in vitro, rat midbrain neuronal cultures were co-transfected with siRNA and an enhanced green fluorescence protein plasmid (Clontech, Cambridge, UK) using Lipofectamine 2000 (Invitrogen) (16). To test the effectiveness of MKK siRNAs, 2-3 days after transfection cultures were treated with 10 μm colchicine for 2 h and stained for the corresponding activated MAP kinases: anti-phospho- (p)-ERK for siRNA of MKK1 or MKK2; anti-p-p38 for siRNA of MKK3 or MKK6; anti-p-JNK for siRNA of MKK4 or MKK7. The effects of these siRNAs on the selective toxicity of rotenone or colchicine were examined in rat midbrain neuronal cultures as described above. In experiments requiring the coexpression of a MAP kinase and parkin, we first transfected the cultures with a GFP-MAPK plasmid and 24 h later infected the cultures with the FLAG-parkin sindbis virus for another 24 h before the cultures were treated with 30 nm rotenone or 10 μm colchicine for 12 h. The lower concentration of rotenone was used because the combined toxicity of MAP kinase and 100 nm rotenone was too high for reliable quantification. For each condition we counted 6 coverslips with at least 50 transfected TH+ neurons on each coverslip and in some experiments with at least 25 TH+ neurons co-expressed a GFP-MAPK and FLAG-parkin. The sindbis virus infected at least 50% neurons. The total number of TH+ neuron per coverslip was about 400-500. All data were expressed as the mean ± S.E. Statistical analyses were performed with unpaired t test using the software Origin (Origin Lab, Northampton, MA).
RESULTS
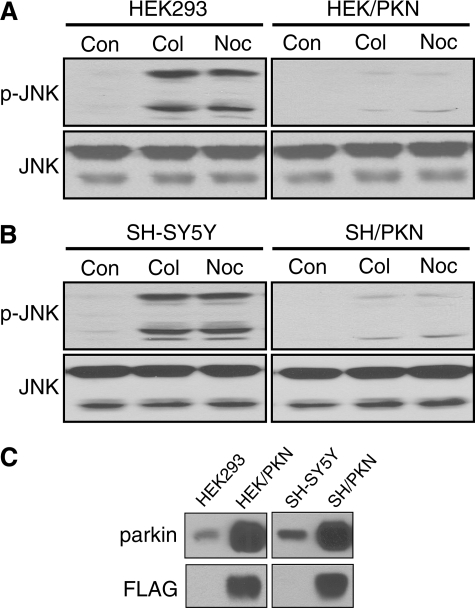
Parkin Attenuates JNK Activation Induced by Microtubule Depolymerization—To examine the impact of microtubule depolymerization on MAP kinases, we treated the human embryonic kidney cell line HEK293 or the human dopaminergic neuroblastoma cell line SH-SY5Y with the microtubule-depolymerizing agent colchicine or nocodazole (both at 10 μm for 2 h). As shown in Fig. 1, colchicine or nocodazole treatment activated all three JNK isoforms (JNK1 at 46 kDa, JNK2 and JNK3 at 54 kDa), which were recognized by the antibodies against phospho-JNK and total JNK. To identify the optimal treatment regimen, we treated both cell lines with 10 μm colchicine or nocodazole for various durations. JNK activation in the two cell lines had a similar time course, which peaked at 1-2 h (data not shown). We also treated both cell lines for 2 h with different concentrations of colchicine or nocodazole. JNK activation reached a plateau at 10 μm concentrations of either drug (data not shown). Thus, we used 10 μm and 2 h for all subsequent experiments for consistency and maximal activation of MAP kinases. Stable expression of parkin in HEK293 or SH-SY5Y cells greatly blocked JNK activation without affecting the expression level of JNK (Fig. 1).
FIGURE 1.
Parkin attenuates JNK activation induced by microtubule depolymerization. A, HEK293 cells or HEK293 cells stably expressing parkin (HEK/PKN) were treated with vehicle control (Con) or the microtubule-depolymerizing agent colchicine (Col) or nocodazole (Noc) for 2 h at 10 μm. Total cell lysates were Western-blotted with anti-phospho-JNK (p-JNK) or anti-JNK. JNK activation was markedly reduced by stable expression of parkin. B, the same results were obtained when the experiments were repeated in SH-SY5Y cells and SH-SY5Y cells stably expressing parkin (SH/PKN). C, expression levels of FLAG-parkin and endogenous parkin in HEK293, HEK/PKN, SH-SY5Y, and SH/PKN cells. The experiments were repeated at least four times with similar results.
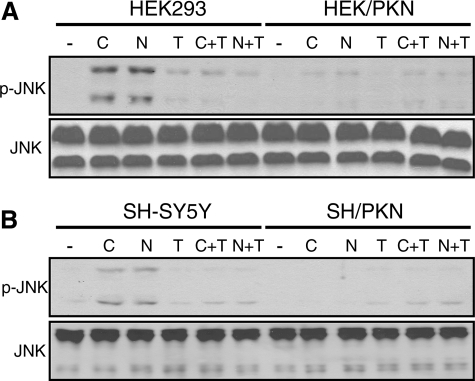
The Effect of Parkin on JNK Activation Is Occluded by Taxol—Because our previous study has shown that parkin stabilizes microtubules against depolymerizing agents (10), we tested whether the effect of parkin was occluded by the microtubule-stabilizing agent taxol. As shown in Fig. 2, taxol (10 μm for 2 h) blocked JNK activation induced by colchicine or nocodazole in both HEK293 and SH-SY5Y cells. By itself, taxol did not have any significant effect on JNK activation. Overexpression of parkin attenuated JNK activation to similar extents in the absence or presence of taxol. The occlusive effects of parkin and taxol suggest that both act on the same molecular target—microtubules.
FIGURE 2.
The effect of parkin on JNK activation is occluded by taxol. HEK293 cells or HEK293 stably expressing parkin (A), SH-SY5Y cells or SH-SY5Y stably expressing parkin (B) were treated with 10 μm colchicine (C) or nocodazole (N) with or without taxol (T) for 2 h. Cell lysates were Western-blotted with phospho-JNK (p-JNK) or JNK antibodies. -, vehicle (DMSO) control. The experiments were repeated at least three times with similar results.
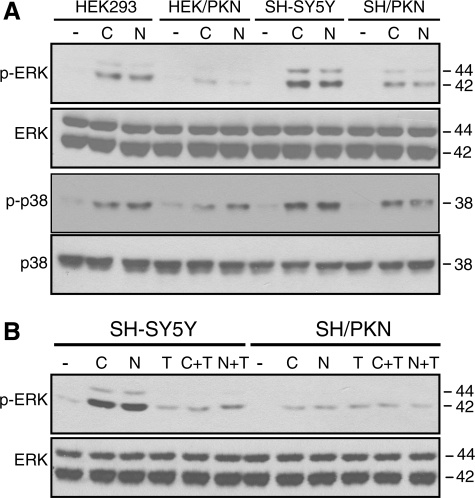
Microtubule Depolymerization-induced ERK and p38 Activation Are Also Attenuated by Parkin—Colchicine or nocodazole (both at 10 μm for 2 h) also induced the activation of ERK and p38 MAP kinases in HEK293 and SH-SY5Y cells (Fig. 3A). Stable expression of parkin in both cells significantly attenuated the activation of ERK and p38 (Fig. 3A). The effect of parkin on ERK activation was occluded by taxol in SH-SY5Y cells (Fig. 3B) and HEK293 cells (data not shown). Similarly, parkin and taxol occluded each other on p38 activation induced by colchicine or nocodazole (data not shown). These results suggest that microtubule depolymerization-induced activation of all three major MAP kinases is significantly reduced by parkin through a mechanism occluded by taxol.
FIGURE 3.
Parkin attenuates colchicine- or nocodazole-induced ERK and p38 activation in a manner occluded by taxol. A and B, HEK293 or HEK293 cells stably expressing parkin, SH-SY5Y, or SH-SY5Y cells stably expressing parkin were treated with 10 μm colchicine (C) or nocodazole (N) without or with taxol (T) for 2 h. Cell lysates were blotted with antibodies against phospho (p)-ERK, ERK, p-p38, or p38, respectively. -, vehicle (DMSO) control. The experiments were repeated at least three times with similar results.
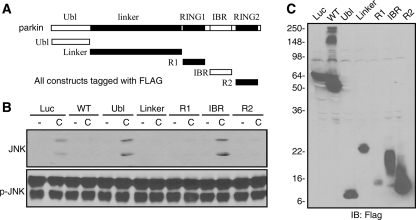
Parkin Attenuates MAP Kinase Activation through Any One of Its Three Microtubule Binding Domains—Our previous study has shown that parkin stabilizes microtubules against depolymerizing agents through three independent domains: linker, RING1, and RING2. Any one of these domains stabilizes microtubules to a similar extent as the full-length parkin protein (10). To dissect the mechanism by which parkin suppressed MAP kinase activation, we transfected HEK293 cells with each of the five different domains of parkin (Fig. 4A) and treated the cells without or with colchicine (10 μm for 2 h). Expression of the Linker, RING1, or RING2 domain blocked colchicine-induced JNK activation, just as wild-type parkin. In contrast, the Ubl or IBR domain, like the luciferase control, did not significantly affect JNK activation (Fig. 4B). Expression levels of these constructs were shown in Fig. 4C. We found that each of the three microtubule-stabilizing domains of parkin (Linker, RING1, and RING2) also significantly attenuated colchicine-induced activation of ERK and p38 (data not shown). Thus, each of the microtubule binding domains of parkin is sufficient not only to stabilize microtubule but also to suppress MAP kinase activation induced by microtubule depolymerization.
FIGURE 4.
Microtubule binding domains of parkin are sufficient to attenuate JNK activation induced by microtubule depolymerization. A, microtubule binding domains of parkin. B, expression of microtubule-binding domains of parkin in 293 cells was sufficient to attenuate JNK activation induced by colchicine. WT, wild type; C, colchicine. C, expression levels of constructs used in B. -, DMSO; Luc, FLAG-luciferase control vector; R1, RING1 domain; R2, RING2 domain; IB, immunoblot. The experiments were repeated at least three times with similar results.
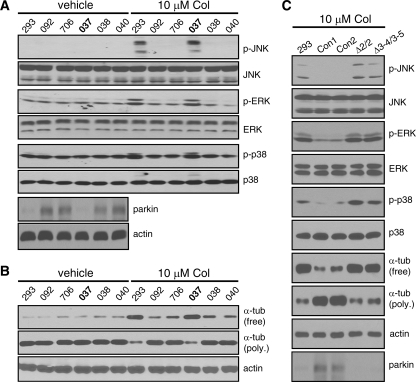
The Ability of Parkin to Suppress Microtubule Depolymerization and the Ensuing MAP Kinase Activation Is Lost in PD Patients with Parkin Mutations—To test whether the ability of parkin to suppress microtubule depolymerization-induced MAP kinase activation is involved in Parkinson disease, we treated B-lymphocytes cell lines derived from a family carrying exon 4 deletion of parkin (ND01037, ND01038, and ND01040) as well as two unrelated normal subjects (ND00092 and ND05706). The human embryonic kidney cell line HEK293, which expresses very little endogenous parkin, was used as a negative control. ND01037 was derived from a PD patient with homozygous deletion of exon 4, whereas ND01038 and ND01040, which were derived from unaffected siblings of the patient, carried the same mutation in the heterozygous state (13). As shown in Fig. 5A, this panel of cells was treated with 10 μm colchicine for 2 h. Significant activation of JNK, ERK, and p38 was only seen in HEK293 cells and ND01037, which had homozygous deletion of parkin exon 4. In contrast, none of the three MAP kinases was significantly activated in the heterozygous carriers or the unaffected normal controls. Anti-parkin immunoblot confirmed that parkin expression was lost in ND01037 and was very low in HEK293 cells (Fig. 5A). The same results were obtained when we used nocodazole (10 μm for 2 h) instead of colchicine (data not shown). The data suggest that homozygous exon 4 deletion of parkin abrogates the ability of parkin to attenuate MAP kinase activation induced by microtubule-depolymerizing agents.
FIGURE 5.
The ability of parkin to suppress MAP kinase activation and microtubule depolymerization is lost in PD patients with parkin mutations. A and B, HEK293 cells (293) and B-lymphocytes derived from two normal controls (092 and 706) and a family with exon 4 deletion of parkin (037, homozygote with PD; 038 and 040, unaffected heterozygous siblings) were treated without or with colchicine (Col, 10 μm) for 2 h. Colchicine-induced activation of MAP kinases was greatly attenuated in normal subjects and unaffected heterozygotes but not in PD patient with homozygous exon 4 deletion or 293 cells. Parkin immunoblot confirmed the loss of parkin protein in the homozygote and the very low level of endogenous parkin in 293 cells (A). The amounts of free tubulin (tub) and polymerized (poly.) tubulin in microtubules showed that colchicine-induced microtubule depolymerization was significantly attenuated in normal controls or unaffected heterozygotes but not in PD patient with homozygous exon 4 deletion or 293 cells (B). Anti-actin blot showed equal loading. Cell lines: 037, ND01037; 038, ND01038; 040, ND01040; 092, ND00092; 706, ND05706. C, the same experiments were repeated in primary skin fibroblasts derived from two normal controls (Con1 and Con2) and two PD patients with parkin homozygous exon 2 deletion (Δ2/2) or compound heterozygous deletions of exons 3-4 and 3-5 (Δ3-4/3-5). Fibroblasts from PD patients with parkin mutations lost the ability to attenuate colchicine-induced MAP kinase activation and microtubule depolymerization. Parkin expression was lost in cells from the two patients. All experiments were performed at least three times with similar results.
Next, we examined the impact of exon 4 deletion on the ability of parkin to suppress microtubule depolymerization. Free tubulin or polymerized tubulin was extracted from the same panel of cells treated with colchicine at 10 μm for 2 h. The amounts of free tubulin after treatment, which indicate the degree of microtubule depolymerization, were much higher in HEK293 cells and ND01037 than in the heterozygous carriers or the normal controls (Fig. 5B). Reciprocal changes were observed in the amount of polymerized tubulin (Fig. 5B). Thus, cells that contain homozygous exon 4 deletion of parkin behaved like HEK293 cells, which expressed very little parkin. In contrast, cells that carry the heterozygous mutation responded like cells with wild-type parkin. Same results were obtained when we used nocodazole (10 μm for 2 h) instead of colchicine (data not shown).
To substantiate these results, we used primary skin fibroblasts derived from two normal subjects (Con1 and Con2) and two PD patients with parkin mutations (Δ2/2, homozygous exon 2 deletion; Δ3-4/3-5, compound heterozygous deletions of exons 3-4 and 3-5). These fibroblasts and HEK293 cells (as a negative control) were treated with 10 μm colchicine for 2 h. Activation of all three MAP kinases (JNK, ERK, and p38) was markedly attenuated in the two normal fibroblasts compared with the two fibroblasts with parkin mutations or HEK293 cells (Fig. 5C). A colchicine-induced increase in free tubulin and decrease in polymerized tubulin was greatly attenuated in normal but not parkin-deficient fibroblasts (Fig. 5C). Western blotting with parkin antibody showed that parkin expression was indeed lost in cells from the two PD patients (Fig. 5C). Thus, results from three PD patients with different parkin mutations in two different cellular environment consistently showed that these mutations abrogate the ability of parkin to attenuate microtubule depolymerization and the ensuing activation of MAP kinases.
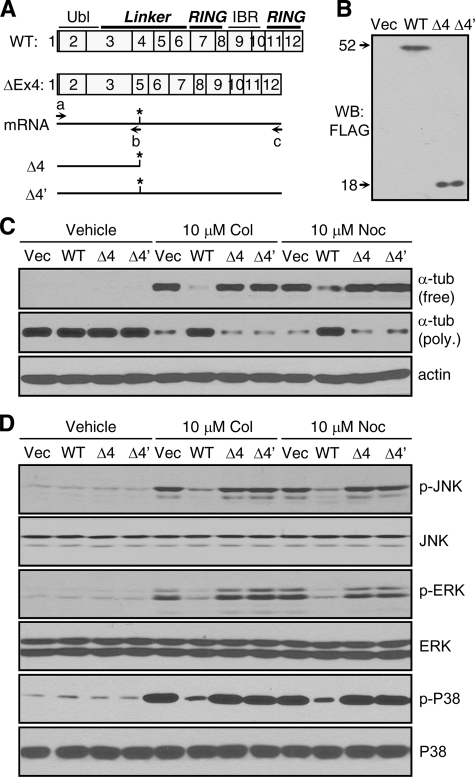
Exon 4 Deletion Disrupts the Ability of Parkin to Attenuate Microtubule Depolymerization and MAP Kinase Activation—To understand the molecular mechanism underlying the above changes in PD patients with parkin mutations, we cloned the cDNA of exon 4-deleted parkin by reverse transcription-PCR using total RNA isolated from ND01037. As shown in Fig. 6A, exon 4 deletion produces a stop codon in exon 5. The resulting polypeptide is much longer than the protein products of exon 2 deletion or compound deletions of exons 3-4 and 3-5. Thus, we studied exon 4 deletion as a representative mutation. Two exon 4 deletion cDNA constructs (Δ4 and Δ4′) were generated by using a common primer spanning the starting codon (a) and reverse primers covering the new stop codon (b) or the original stop codon (c). These cDNAs were subcloned into the mammalian expression vector pCMV-Tag2B, which added a FLAG tag at the N terminus. Transfection of the two mutant parkin constructs in HEK293 cells produced a protein of the same predicted size (18 kDa), suggesting that Δ4′ and Δ4 are essentially the same, as the new stop codon truncates the parkin protein (Fig. 6B). Because the truncated protein contained only the Ubl domain and a small part of the Linker domain, we tested whether it lost the ability to stabilize microtubules against depolymerizing agents. HEK293 cells transfected with Δ4, Δ4′, wild-type parkin, or empty vector were treated with 10 μm colchicine or nocodazole for 2 h. Neither Δ4 nor Δ4′ had any significant effect on microtubule depolymerization compared with the empty vector. In contrast, wild-type parkin greatly attenuated microtubule depolymerization induced by colchicine or nocodazole (Fig. 6C). Consistent with these results, Δ4 or Δ4′ did not suppress MAP kinase activation induced by these microtubule-depolymerizing agents (Fig. 6D). Together, the above results suggest that exon 4 deletion as well as similar mutations such as Δ2/2 or Δ3-4/3-5 abrogates the ability of parkin to attenuate microtubule depolymerization and the ensuing MAP kinase activation.
FIGURE 6.
Exon 4 deletion abrogates the ability of parkin to attenuate microtubule depolymerization and the ensuing MAP kinase activation. A and B, exon 4 deletion in the human parkin gene produced a truncated protein. Exon 4-deleted parkin mRNA had a stop codon (*) in exon 5. Two cDNA constructs, Δ4 and Δ4′, were cloned by reverse transcription-PCR from lymphocytes with homozygote exon 4 deletion of parkin (ND01037) using primers a and b or primers a and c, respectively (A). Both constructs produced truncated parkin of 18 kDa in HEK293 cells (B). WB, Western blot; WT, wild type. C and D, microtubule depolymerization and the ensuing activation of MAP kinases were significantly blocked by wild-type but not exon 4-deleted parkin. HEK293 cells transfected with empty vector (Vec), wild-type parkin, or the exon 4-deleted parkin constructs (Δ4 or Δ4′) were treated without or with colchicine or nocodazole (both at 10 μm for 2 h). The amounts of free tubulin (tub) in the supernatant fractions or polymerized (poly.) tubulin in the pellet fractions were analyzed by Western blotting with anti-α-tubulin. Anti-actin blots showed equal loading of total proteins (C). Total cell lysates were blotted with antibodies against the indicated MAP kinases and their activated forms (D). The experiments were repeated at least three times with similar results. p-, phospho-.
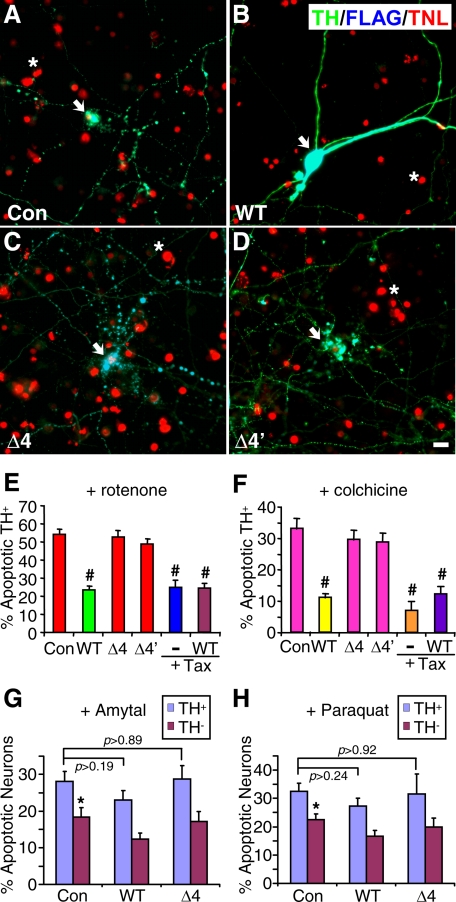
Exon 4 Deletion Abrogates the Ability of Parkin to Protect Dopaminergic Neurons against Microtubule-depolymerizing Toxins—Because our previous studies have shown that dopaminergic neurons are particularly vulnerable to microtubule-depolymerizing agents including the PD toxin rotenone, we examined whether parkin, which stabilizes microtubules, could attenuate the selective toxicity of these agents on DA neurons. The impact of parkin exon 4 deletion was examined as a representative of parkin mutations studied above, as the mutation abrogated the microtubule-stabilizing effect of parkin. Rat embryonic midbrain neuronal cultures were transfected with FLAG-tagged wild-type parkin, the Δ4 or Δ4′ mutant, or the luciferase control. As shown in our previous studies (7), rotenone treatment (100 nm for 12 h) exhibited much greater toxicity on TH+ neurons than on TH- neurons. Ectopic expression of wild-type parkin markedly attenuated the selective toxicity of rotenone on TH+ neurons (Fig. 7B), compared with the situation with the luciferase control (Fig. 7A). Rotenone killed 54.4 ± 2.7% TH+ neurons transfected with FLAG-luciferase, but only 23.5 ± 2.2% of TH+ neurons transfected with FLAG-parkin (p < 0.001, n = 6 coverslips, each with at least 50 transfected TH+ neurons). In contrast, transfection of the Δ4 (Fig. 7C) or Δ4′ (Fig. 7D) parkin construct did not significantly change the selective toxicity of rotenone on TH+ neurons (Fig. 7E, toxicity at 52.7 ± 3.8 or 49.1 ± 2.6%, respectively). Our previous study showed that taxol, a microtubule-stabilizing agent, significantly reduced the selective toxicity of rotenone (7). We found that the protective effect of wild-type parkin (23.5 ± 2.2%) was similar to that of taxol (25.2 ± 3.9%) or taxol plus parkin (24.7 ± 2.2%) (p > 0.70, Fig. 7E).
FIGURE 7.
Wild-type, but not Exon 4-deleted, parkin protects dopaminergic neurons against the selective toxicity of microtubule-depolymerizing agents. A-D, rat midbrain neuronal cultures were transfected with FLAG-tagged luciferase control (Con, A), wild-type (B), Δ4 (C), or Δ4′ (D) mutant parkin. After rotenone treatment (100 nm for 12 h), cultures were co-stained for TH (green), FLAG (blue) and TUNEL (TNL, red). Ectopic expression of wild-type, but not mutant, parkin markedly attenuated the selective toxicity of rotenone on TH+ neurons. Arrows, TH+ neurons; *, dead glial cells (killed by AraC in the media); bar, 10 μm. E, a statistical summary of data represented in A-D as well as the protective effects of taxol (1 μm, Tax) alone or with wild type parkin. #, p < 0.001, versus control, n = 6 coverslips, each with at least 50 TH+ neurons transfected by various constructs. The protective effects of parkin and taxol against rotenone toxicity occluded each other (p > 0.70, WT versus taxol or wild type + taxol). F, a statistical summary of similar experiments with colchicine treatment (10 μm for 12 h) instead. Wild-type, but not exon 4-deleted, parkin significantly reduced the selective toxicity of colchicine on TH+ neurons (#, p < 0.001 versus control, n = 6 coverslips, each with at least 50 transfected TH+ neurons). The protective effects of parkin and taxol against colchicine toxicity occluded each other (p > 0.20, WT versus taxol or wild type + taxol). G and H, parkin did not significantly protect against the toxicity of amytal (G) or paraquat (H) on TH+ or TH- neurons. Midbrain neuronal cultures were transfected with FLAG-tagged luciferase control, wild-type, or Δ4 mutant parkin and treated with amytal (G) or paraquat (H) at 10 μm for 12 h. The toxicity of either agent was moderately selective on TH+ versus TH- neurons (p < 0.05 versus the preceding bar) and was not significantly changed by wild-type or mutant parkin (p values as indicated, n = 6 coverslips, each with at least 50 transfected TH+ neurons).
To substantiate that parkin acts against the microtubule-depolymerizing activity of rotenone, we repeated the experiments by replacing rotenone with colchicine (10 μm for 12 h), which selectively killed TH+ neurons (7). As shown in Fig. 7F, wild-type parkin significantly reduced the selective toxicity of colchicine (11.2 ± 1.3%, p < 0.001, n = 6 coverslips) compared with the situation with the luciferase control (33.2 ± 3.3%, n = 6). Neither the Δ4 nor the Δ4′ parkin mutant exhibited any significant protection against colchicine toxicity (29.6 ± 3.1 or 29.0 ± 2.8%, respectively). There was no significant difference between the protective effects of wild-type parkin (11.2 ± 1.3%), taxol (7.1 ± 2.8%), or taxol plus parkin (12.2 ± 2.4%) (p > 0.20, Fig. 7F).
To examine whether the protective effect of parkin is selective against microtubule depolymerization, we tested whether expression of wild-type or the Δ4 mutant parkin had any significant effects on the toxicity of amytal or paraquat (both at 10 μm for 12 h), two toxins that inhibit mitochondrial complex I but have no known interaction with microtubules. Wild-type parkin caused a very modest and statistically insignificant reduction in the toxicity of amytal (p > 0.19, Fig. 7G) or paraquat (p > 0.24, Fig. 7H) on TH+ neurons. Similarly, the Δ4 mutant parkin had no significant effect on the toxicity of amytal (p > 0.89, Fig. 7G) or paraquat (p > 0.92, Fig. 7H). In contrast to rotenone or colchicine, which exhibits much higher toxicity on TH+ versus TH- neurons (7), amytal or paraquat only showed moderate but significant selectivity in their toxicity on TH+ versus TH- neurons (p < 0.05, Fig. 7, G and H). The toxicity of amytal or paraquat on TH- neurons was also not significantly changed by wild-type or mutant parkin (p > 0.07, Fig. 7, G and H). Thus, parkin does not appear to protect against the toxicity of complex I inhibitors such as amytal or paraquat.
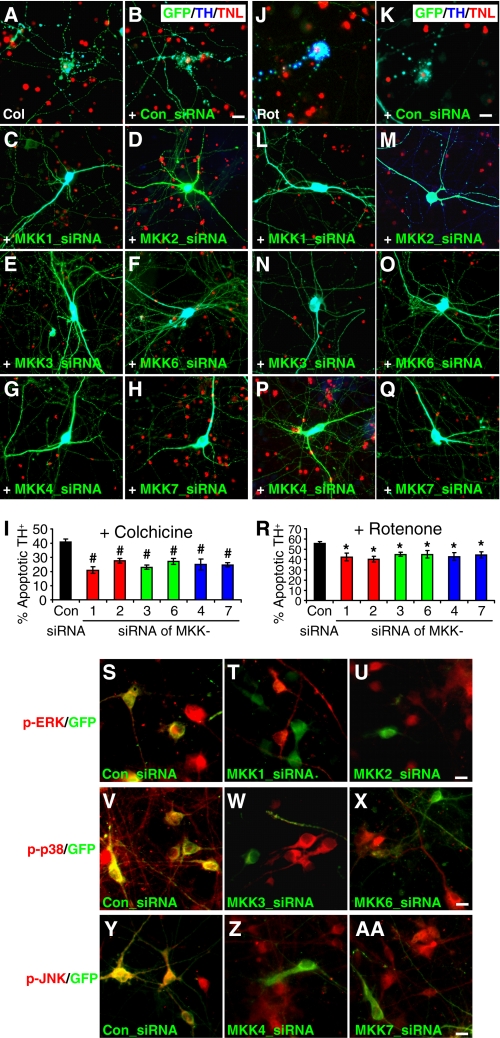
Blocking MAP Kinase Activation Attenuates the Selective Toxicity of Microtubule-depolymerizing Agents on Dopaminergic Neurons—Because parkin attenuated the selective toxicity of microtubule-depolymerizing toxins and suppressed MAP kinase activation induced by these depolymerizing agents, it is possible that blocking MAP kinase activation may protect midbrain DA neurons from these toxins. To test this, we transfected rat midbrain neuronal cultures with siRNA against each of the MAP kinase kinases (MKK or MAP2K) to block the activation of its corresponding MAP kinase. MKKs have quite selective actions on MAP kinases (1). In general, ERK is activated by MKK1 and MKK2, p38 is activated by MKK3 and MKK6, and JNK is activated by MKK4 and MKK7 (17). After midbrain neuronal cultures were co-transfected with GFP and siRNA against each MKKs, the cells were treated with 10 μm colchicine (Fig. 8, A-I) or 100 nm rotenone (Fig. 8, J-R) for 12 h. TH+ neurons transfected with each MKK siRNA showed significantly less death than TH+ neurons transfected with a control siRNA of scrambled sequence (p < 0.01 for colchicine, p < 0.05 for rotenone, n = 6 coverslips). We confirmed the efficacy of siRNA against each MKK by treating transfected midbrain neuronal cultures with 10 μm colchicine for 2 h and staining the cells for the activated MAP kinases that correspond to each MKK. In midbrain neurons transfected with siRNA against MKK1 (Fig. 8T) or MMK2 (Fig. 8U), colchicine-induced ERK activation (as indicated by the level of phospho-ERK) was markedly decreased compared with untransfected neurons in the same field or neurons transfected with control siRNA (Fig. 8S). Similarly, p38 activation was greatly reduced by siRNA against MKK3 (Fig. 8W) or MKK6 (Fig. 8X) compared with control siRNA (Fig. 8V). JNK activation was significantly diminished by siRNA against MKK4 (Fig. 8Z) or MKK7 (Fig. 8AA) compared with control siRNA (Fig. 8Y).
FIGURE 8.
Blocking MAP kinase activation attenuates the selective toxicity of microtubule-depolymerizing agents on dopaminergic neurons. A-H, rat midbrain neuronal cultures were transfected with GFP only (A) or together with siRNA against a scrambled control sequence (Con, B) or the MAP kinase kinase MKK1 (C), MKK2 (D), MKK3 (E), MKK6 (F), MKK4 (G), MKK7 (H). After colchicine treatment (Col, 10 μm for 12 h), cultures were co-stained for GFP (green), TH (blue), and TUNEL (TNL)(red). Knocking down of each MKK markedly attenuated the selective toxicity of colchicine on DA neurons. I, statistical summary of data represented in A-H.#, p < 0.01, versus control siRNA, n = 6 coverslips, each with 40-60 transfected TH+ neurons. J-R, the same experiments were repeated with rotenone (100 nm for 12 h) instead of colchicine. Knocking down each MKK also significantly reduced the selective toxicity of rotenone on DA neurons. *, p < 0.05, versus control siRNA, n = 6 cover-slips, each with 40-60 transfected TH+ neurons. S-AA, midbrain neuronal cultures co-transfected with GFP and siRNA against each MKK were treated with colchicine (10 μm for 2 h) and stained with antibodies against their corresponding activated MAP kinases to confirm the effectiveness of each MKK siRNA. Anti-phospho-ERK staining (red) showed that colchicine-induced ERK activation was markedly reduced in neurons transfected with GFP (green) and siRNA against MKK1 (T) or MKK2 (U) compared with control (S). Anti-p-p38 staining (red) showed that colchicine-induced p38 activation was markedly reduced in neurons transfected with GFP (green) and siRNA against MKK3 (W) or MKK6 (X) compared with control (V). Anti-p-JNK staining (red) showed that colchicine-induced JNK activation was markedly reduced in neurons transfected with GFP (green) and siRNA against MKK4 (Z) or MKK7 (AA) compared with control (Y). Bars, 10 μm.
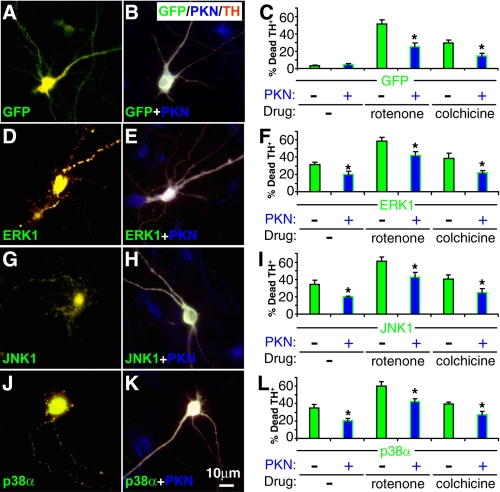
Parkin Attenuates the Toxicity Caused by Overexpression of MAP Kinases in DA Neurons—To investigate whether the protective effect of parkin against rotenone or colchicine is mediated by MAP kinases, we examined whether overexpression of MAP kinases caused any toxicity to midbrain TH+ neurons and whether the effects were changed by parkin. The cDNA for ERK1, JNK1, or p38α was cloned to represent the three major MAP kinase families in this study. The GFP-MAPK fusion constructs were transfected in midbrain neuronal cultures, which were also infected with sindbis virus expressing FLAG-parkin. Sindbis virus, which infected about 50% of the neurons, was used to ensure that there were sufficient numbers of TH+ neurons doubly expressing GFP-MAPK and FLAG-parkin. In contrast to GFP (Fig. 9A), which did not cause any significant toxicity to midbrain TH+ neurons, overexpression of ERK1 (Fig. 9D), JNK1 (Fig. 9G), or p38α (Fig. 9J) caused marked cell death in TH+ neurons. These toxicities were significantly suppressed by coexpression of FLAG-parkin (p < 0.05, Fig. 9). Overexpression of each MAP kinase also greatly enhanced the toxicity of rotenone (30 nm for 12 h) or colchicine (10 μm for 12 h). The combined toxicities of each MAP kinase and each drug were significantly reduced by coexpression of parkin (p < 0.05, Fig. 9).
FIGURE 9.
Parkin reduces the toxicity caused by MAP kinase overexpression in dopaminergic neurons. Midbrain neuronal cultures were transfected with GFP (A-C) or GFP-tagged ERK1 (D-F), JNK1 (G-I), or p38α (J-L) and infected without (A, D, G, and J) or with sindbis virus expressing FLAG-parkin (B, E, H, and K). These cultures were then treated without or with rotenone (30 nm for 12 h) or colchicine (10 μm for 12 h) and were co-stained for GFP (green), FLAG (blue), and TH (red) to examine the survival of TH+ neurons. GFP-tagged MAP kinases caused significant toxicity on TH+ neurons, which was significantly attenuated by coexpression of parkin. The toxicity of MAP kinases was additive to that of rotenone or colchicine. Parkin also significantly reduced the combined toxicity. Bar, 10 μm. *, p < 0.05 versus the preceding bar, n = 6 coverslips for each condition, with at least 50 TH+ neurons on each coverslip singly transfected with GFP or GFP-MAPK and at least 25 TH+ neurons also infected with FLAG-parkin virus.
DISCUSSION
Parkinson disease is characterized by the degeneration of dopaminergic neurons in substantia nigra. Both genetic mutations and environmental toxins play important roles in the selective demise of nigral DA neurons and the ensuing locomotor symptoms. Our previous studies suggest that the integrity of the microtubule network is vital to the survival of midbrain DA neurons (12). Microtubule depolymerization exerts much greater toxicity on DA neurons than on non-DA neurons, because DA neurons need to sequester and transport an easily oxidizable neurotransmitter over long distance on microtubules. Disruption of microtubule-based transport leads to increased accumulation of vesicles in the cell body and elevated cytosolic concentrations of dopamine leaked from the vesicles. This gives rise to increased oxidative stress and cell death from dopamine oxidation (7). Thus, agents that stabilize microtubules should protect dopaminergic neurons against environmental PD toxins such as rotenone, which strongly depolymerizes microtubules (7, 18, 19).
Our previous study has shown that parkin, whose mutations cause Parkinson disease, strongly binds to microtubules (9) and stabilizes microtubules against depolymerizing agents (10). The results predict that parkin should be able to protect midbrain DA neurons against microtubule-depolymerizing agents. In the present study we showed that ectopic expression of parkin, but not its PD-linked mutant, indeed protected against the selective toxicity of rotenone or colchicine on TH+ neurons in rat midbrain neuronal cultures (Fig. 7, A-F). In contrast, parkin did not significantly protect against the toxicity of amytal or paraquat, which inhibits mitochondrial complex I but has no known interaction with microtubules (Fig. 7, G and H).
These results suggest that the protective effect of parkin is correlated to its ability to stabilize microtubules. Indeed, PD-linked mutations such as exon 4 deletion abrogated the ability of parkin to stabilize microtubules against depolymerizing agents (Fig. 6) and also disrupted the protective effect (Fig. 7). Consistent with this, MAP kinase activation induced by microtubule depolymerizing agents was greatly suppressed in B-lymphocytes and skin fibroblasts derived from normal subjects but not PD patients with parkin mutations (Fig. 5). Our results showed that any one of the three microtubule-stabilizing domains of parkin (Linker, RING1, or RING2) attenuated MAP kinase activation, whereas the other two domains that do not bind to microtubules (Ubl or IBR) failed to reduce MAP kinase activation (Fig. 4). Thus, it seems plausible that parkin protects midbrain DA neurons against microtubule-depolymerizing toxins by stabilizing microtubules and reducing MAP kinase activation. When we blocked MAP kinase activation by using siRNAs against MAP kinase kinases (MKKs or MAP2Ks), the selective toxicity of rotenone or colchicine was indeed reduced significantly (Fig. 8). Conversely, overexpression of ERK1, JNK1, or p38α exhibited marked toxicity on TH+ neurons and exacerbated the toxicity of rotenone or colchicine. Co-expression of parkin significantly attenuated the toxicity of MAP kinase overexpression and the combined toxicity with rotenone or colchicine. Together, these results suggest that the protective effect of parkin against rotenone or colchicine is mediated by MAP kinase.
The ability of parkin to attenuate MAP kinase activation appears to be directly related to its capacity to bind and stabilize microtubules rather than to its E3 ligase activity. Any one of the three tubulin binding domains (Linker, RING1, or RING2) stabilizes microtubules (10) and reduced MAP kinase activation (Fig. 4). Because each of these domains alone does not have any E3 ligase activity, the ability of parkin to reduce microtubule depolymerization and the ensuing MAP kinase activation is independent of its catalytic activity as an E3 ligase. Consistent with this, the total levels of JNK (Fig. 1), p38, or ERK (Fig. 3) were not appreciably changed by parkin even though parkin greatly reduced their activation in response to microtubule depolymerization. Thus, the effect of parkin on MAP kinase activation is not mediated by E3-dependent degradation of MAP kinases. This conclusion is further corroborated by the results that taxol, a microtubule-stabilizing compound, occluded the effects of parkin in reducing MAP kinase activation (Figs. 2 and 3) and the selective toxicity of rotenone (Fig. 7E) or colchicine (Fig. 7F). Essentially, parkin acted like taxol and directly stabilized microtubules against depolymerizing agents. The definitive molecular details of parkin-tubulin/microtubule interaction await further structural studies, which should offer interesting insights into the extremely high affinity of the complex (10).
In conclusion, this study provides evidence that the ability of parkin to stabilize microtubules plays a critical role in attenuating MAP kinase activation induced by microtubule depolymerization. Because midbrain dopaminergic neurons are particularly vulnerable to microtubule depolymerization (7), the present study showed that parkin protected midbrain DA neurons by stabilizing microtubules and attenuating the ensuing activation of MAP kinases. This protective effect was abrogated by PD-linked parkin mutations, suggesting its important involvement in Parkinson disease.
This work was supported, in whole or in part, by National Institutes of Health Grant NS41722 (to J. F.). This work was also supported by the New York State Center of Excellence in Bioinformatics and Life Sciences (to J. F.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ERK, extracellular signal-regulated kinase; PD, Parkinson disease; DA, dopamine; TH, tyrosine hydroxylase; JNK, Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MAP, microtubule-associated protein; MKK (MAP2K), MAP kinase kinase; siRNA, small interfering RNA; TUNEL, TdT-mediated dUTP-X nick end labeling; siRNA, small interfering RNA; E3, ubiquitin-protein isopeptide ligase; PKN, parkin; Ubl, ubiquitin-like domain; IBR, in-between RING finger; GFP, green fluorescent protein; HEK, human embryonic kidney; MES, 4-morpholineethanesulfonic acid.
References
- 1.Chang, L., and Karin, M. (2001) Nature 410 37-40 [DOI] [PubMed] [Google Scholar]
- 2.Sturgill, T. W., and Ray, L. B. (1986) Biochem. Biophys. Res. Commun. 134 565-571 [DOI] [PubMed] [Google Scholar]
- 3.Ray, L. B., and Sturgill, T. W. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 3753-3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reszka, A. A., Seger, R., Diltz, C. D., Krebs, E. G., and Fischer, E. H. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 8881-8885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, L., Jones, Y., Ellisman, M. H., Goldstein, L. S., and Karin, M. (2003) Dev. Cell 4 521-533 [DOI] [PubMed] [Google Scholar]
- 6.Feijoo, C., Campbell, D. G., Jakes, R., Goedert, M., and Cuenda, A. (2005) J. Cell Sci. 118 397-408 [DOI] [PubMed] [Google Scholar]
- 7.Ren, Y., Liu, W., Jiang, H., Jiang, Q., and Feng, J. (2005) J. Biol. Chem. 280 34105-34112 [DOI] [PubMed] [Google Scholar]
- 8.Betarbet, R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V., and Greenamyre, J. T. (2000) Nat. Neurosci. 3 1301-1306 [DOI] [PubMed] [Google Scholar]
- 9.Ren, Y., Zhao, J. H., and Feng, J. (2003) J. Neurosci. 23 3316-3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang, F., Jiang, Q., Zhao, J., Ren, Y., Sutton, M. D., and Feng, J. (2005) J. Biol. Chem. 280 17154-17162 [DOI] [PubMed] [Google Scholar]
- 11.Lewis, S. A., Tian, G. L., and Cowan, N. J. (1997) Trends Cell Biol. 7 479-484 [DOI] [PubMed] [Google Scholar]
- 12.Feng, J. (2006) Neuroscientist 12 469-476 [DOI] [PubMed] [Google Scholar]
- 13.Dogu, O., Johnson, J., Hernandez, D., Hanson, M., Hardy, J., Apaydin, H., Ozekmekci, S., Sevim, S., Gwinn-Hardy, K., and Singleton, A. (2004) Movement Disorders 19 812-816 [DOI] [PubMed] [Google Scholar]
- 14.Jiang, H., Ren, Y., Zhao, J., and Feng, J. (2004) Hum. Mol. Genet. 13 1745-1754 [DOI] [PubMed] [Google Scholar]
- 15.Nakaso, K., Adachi, Y., Yasui, K., Sakuma, K., and Nakashima, K. (2006) Neurosci. Lett. 400 44-47 [DOI] [PubMed] [Google Scholar]
- 16.Yuen, E. Y., Jiang, Q., Chen, P., Gu, Z., Feng, J., and Yan, Z. (2005) J. Neurosci. 25 5488-5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo, K. A., and Johnson, G. L. (2002) Nat. Rev. Mol. Cell Biol. 3 663-672 [DOI] [PubMed] [Google Scholar]
- 18.Brinkley, B. R., Barham, S. S., Barranco, S. C., and Fuller, G. M. (1974) Exp. Cell Res. 85 41-46 [DOI] [PubMed] [Google Scholar]
- 19.Marshall, L. E., and Himes, R. H. (1978) Biochim. Biophys. Acta 543 590-594 [DOI] [PubMed] [Google Scholar]