Abstract
Platelet microparticles are a normal constituent of circulating blood. Several studies have demonstrated positive correlations between thrombotic states and platelet microparticle levels. Yet little is known about the processes by which platelet microparticles are generated in vivo. We now characterize microparticles derived directly from megakaryocytes. Video microscopy of live mouse megakaryocytes demonstrated that microparticles form as submicron beads along the lengths of slender, unbranched micropodia. These microparticles are CD41+, CD42b+, and express surface phosphatidylserine. Megakaryocyte microparticle generation is resistant to inhibition of microtubule assembly, which is critical to platelet formation, and augmented by inhibition of actin polymerization. To determine whether circulating microparticles are derived primarily from activated platelets or megakaryocytes, we identified markers that distinguish between these 2 populations. CD62P and LAMP-1 were found only on mouse microparticles from activated platelets. In contrast, full-length filamin A was found in megakaryocyte-derived microparticles, but not microparticles from activated platelets. Circulating microparticles isolated from mice were CD62P−, LAMP-1− and expressed full-length filamin A, indicating a megakaryocytic origin. Similarly, circulating microparticles isolated from healthy volunteers were CD62P− and expressed full-length filamin A. Cultured human megakaryocytes elaborated microparticles that were CD41+, CD42b+, and express surface phosphatidylserine. These results indicate that direct production by megakaryocytes represents a physiologic means to generate circulating platelet microparticles.
Introduction
Many cells, including platelets, endothelial cells, leukocytes, and erythrocytes, shed fragments of their plasma membranes into the circulation. There is increasing evidence that these submicron fragments, termed microparticles, have important physiological roles.1 Platelet microparticles are the most abundant microparticles in the bloodstream, constituting approximately 70% to 90% of circulating microparticles.2–4 Evidence that platelet microparticles participate in thrombus formation is derived from several sources. Castaman defect, an isolated deficiency in the ability to generate platelet microparticles, is associated with a bleeding tendency.5,6 Platelets from patients with Scott syndrome also demonstrate an impaired ability to generate platelet microparticles and display a bleeding diathesis. In contrast, elevated platelet microparticle levels are associated with several disease states including heparin-induced thrombocytopenia,7 arterial thrombosis,8,9 idiopathic thrombocytopenic purpura, thrombotic thrombocytopenia,10 sickle cell anemia disease,11 and uremia.12 Platelet microparticles have also been implicated in the pathogenesis of atherosclerosis as well as the regulation of angiogenesis.13,14 Despite their apparent participation in important pathological processes, fundamental aspects of platelet microparticle physiology remain unexplored
The origin of circulating platelet microparticles is among the most poorly understood aspects of microparticle physiology. Platelet microparticles are routinely formed in vitro following exposure to pharmacological concentrations of platelet agonists such as the combination of thrombin and collagen. This observation has contributed to the widely held assumption that circulating platelet microparticles are derived from activated platelets. These microparticles are typically characterized on the basis of several factors, including their small size (< 1 μm), αIIbβ3 or GPIb expression, and exposure of phosphatidylserine (PS) on their outer membrane as indicated by annexin V binding. Although the mechanisms are not clearly elucidated at the molecular level, platelet microparticle formation appears to involve elevation of cytosolic calcium15–17 and loss of membrane-cytoskeletal adhesion.18–20 Calpain-dependent cleavage of proteins of the membrane skeleton underlying the plasma membrane contributes to loss of membrane-cytoskeletal adhesion and correlates with vesiculation of platelet membranes.18,21–23 Membrane composition, particularly PIP2 levels, also influences membrane-cytoskeletal adhesion19,24 and platelet microparticle formation.20 Other processes such as activation-induced protein tyrosine dephosphorylation,25 protein phosphorylation,26 and calmodulin activation26 have been implicated in the generation of microparticles from activated platelets.
Yet platelet microparticles circulate in healthy individuals and have been proposed to function in normal hemostasis.4 Microparticles expressing platelet-specific markers are present in the plasma of healthy individuals at concentrations approximately 3% of that of circulating platelet concentrations.27 Although platelet activation in disease states can result in elevated microparticle levels in vivo, there is no proof that platelet microparticles that circulate in healthy individuals are derived from activated platelets. Thus, a mechanism other than activation of mature platelets may be responsible for the population of platelet microparticles that circulates in healthy individuals.
Platelets form from progenitor cells termed megakaryocytes. Megakaryocytes assemble platelets along proplatelets, which are essential intermediate pseudopodial extensions. Proplatelets are generated by the outflow and evagination of the extensive internal membrane system of the mature megakaryocyte.28,29 Proplatelet extension is driven by microtubules30 as proplatelets fail to form in megakaryocytes treated with agents that inhibit microtubule assembly such as 1 to 10 μM nocodazole.30–32 Megakaryocytes from transgenic mice lacking β1-tubulin, the most abundant platelet β-tubulin isoform, assemble microtubules poorly and develop thrombocytopenia.33 In contrast, actin polymerization is not required for proplatelet extension. Rather, actin mediates the bending and branching of proplatelet extensions required for proplatelet end amplification.29,31
Although the mechanisms driving platelet formation have been studied extensively, the possibility that megakaryocytes actively produce microparticles as well as platelets has not been assessed in detail. In evaluating platelet production from live mouse megakaryocytes, we observed a dynamic process that resulted in the formation of submicron particles from slender, unbranched projections. Examination of these particles demonstrated that they were CD41+ and expressed PS on their outer surface. Unlike microparticles derived from activated platelets, however, megakaryocyte-derived microparticles did not express CD62P or lysosome-associated membrane protein-1 (LAMP-1) on their surface and contained full-length filamin A. These observations led us to evaluate the possibility that circulating CD41+ microparticles are constitutively produced directly from megakaryocytes. We found that like megakaryocyte-derived microparticles, circulating CD41+ microparticles are CD62−, LAMP−, and contain full-length filamin A. These results indicate that megakaryocytes represent a physiologic source of microparticles.
Methods
Materials
All chemicals and reagents were of molecular biology grade. Latrunculin A was obtained from Molecular Probes (Eugene, OR). Nocodazole was purchased from Sigma-Aldrich (St Louis, MO). The final concentration of DMSO in cell culture medium never exceeded 0.001% (vol/vol), and this concentration of DMSO had no effect on proplatelet formation, actin polymer levels, or megakaryocyte-derived microparticle formation. Thrombin was from Sigma-Aldrich and collagen was from Chrono-log (Havertown, PA). Annexin V–FITC, anti–human CD62P-PE, anti–mouse CD62P-FITC, anti–mouse LAMP-1-FITC, anti–human CD41-PE, anti–mouse CD41-PE, anti–human CD42b-FITC, and anti–mouse CD42b-PE antibodies were from BD Pharmingen (San Jose, CA). Anti–mouse GPVI-FITC and anti-mouse integrin α2-FITC were purchased from Emfret (Wurzburg, Germany). Rabbit polyclonal anti–mouse filamin A was generated using the ABD domain of filamin A.
Mouse megakaryocyte cultures
Livers were recovered from mouse fetuses and single-cell suspensions were generated using methods described previously.34 Cells were cultured in Dulbecco modified Eagle medium (DMEM; GIBCO, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM Glutamax, 50 U/mL penicillin, 50 mg/mL streptomycin, and 100 ng/mL recombinant thrombopoietin (DMEM complete). Cells were placed on a 1.5% to 3% albumin (fatty-acid free) step gradient and sedimented to obtain enriched populations of megakaryocytes. Day-3 megakaryocytes were evaluated. IACUC approval was obtained for the isolation and purification of mouse megakaryocytes from fetal mouse liver and for the drawing of blood for in vitro studies.
Megakaryocyte–bone marrow endothelial cell coculture
Megakaryocytes were cultured using methods adapted from those previously described.35 Mouse megakaryocytes (104) were plated in direct contact with mouse bone marrow endothelial cells (106; Dominion Pharmakine, Derio, Spain) that were isolated from bone marrow aspirates from femur and tibia of 6- to 8-week-old ICR/CD-1 mice. Megakaryocytes were plated in Transwell chambers alone or with bone marrow endothelial cells and cultured in medium supplemented with VEGF165, insulin-like growth factor, SDF-1, and FGF-4 (R&D Systems, Minneapolis, MN).35 After 24 hours, the media were collected and processed for microparticle isolation.
Human megakaryocyte cultures
Human cord blood CD34+ cells were purchased from StemCell Technologies (Vancouver, BC) and plated in 24-well plates at 4 × 104 cells/mL in IMDM with 1% BSA, 740 μg/mL partially saturated human transferrin, 10 μg/mL insulin, 40 μg/mL low-density lipoproteins, and 50 ng/mL recombinant thrombopoietin.36,37 Cells were maintained at less than 105/mL with biweekly media changes. On day 14 of culture, cells were harvested, counted, and prepared for electron microscopy. Greater than 90% of these cells were CD41+ by flow cytometry. The supernatant was collected for microparticle isolation and frozen at −80°C. Supernatants were subsequently thawed and microparticles analyzed by flow cytometry.
DIC microscopy of microparticle formation
Megakaryocytes were cultured in DMEM within video chambers and were kept at 37°C and a 5% CO2 atmosphere using methods described previously.31 Preparations were maintained at 37°C with constant 5% carbon dioxide gas infusion using an Incubator XL-3 incubation chamber (Carl Zeiss, Heidelberg, Germany), and examined on a Zeiss Axiovert 200 microscope equipped with a 100× objective (NA 1.4) and 1.6× optivar. Images were captured with an Orca-II ER cooled CCD camera (Hamamatsu, Hamamatsu City, Japan). Illumination was shuttered between exposures. Electronic shutters and image acquisition were under the control of Metamorph software (Universal Imaging Corporation of Molecular Devices, Westchester, PA). Images were captured every 5 seconds for video microscopy.
Examination of microparticle formation by high-contrast microscopy
Megakaryocytes were pipetted into chambers formed by mounting a 24 × 60-mm glass coverslip onto a glass slide with 2 strips of hematoseal tube sealant. All glassware was washed with ethanol and blocked with 3% bovine serum albumin in PBS for 2 hours before use. The Richardson Technology Microscope-3 (RTM-3; Richardson Technologies, Bolton, ON) was used to image white-light in the RTM image contrast mode. An extreme-dark field condenser was used in combination with an infinity-corrected 100× objective HCX PL APO, 1.4 NA, corrected for 0.17-mm coverslip thickness. The images were captured every 3 seconds using a high-resolution, color, analog output with a Sony 3CCD ExwaveHAD video camera (San Diego, CA), and recorded by Openlab Version 3.0 software system (Improvision, Waltham, MA). The light source on the RTM-3 consisted of a 30-W halogen bulb for transmitted light imaging. All live cell microscopy was performed at 37°C.
Thin-section electron microscopy
Megakaryocytes were fixed with 1.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, for 8 hours. Cells were dehydrated through a series of alcohols, infiltrated with propylene oxide, and embedded in epoxy resin in an inverted beam capsule. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined with a Tecnai G2 Spirit BioTWIN transmission electron microscope (Hillsboro, OR) at an accelerating voltage of 80 kV, and images were recorded with an AMT 2k CCD camera (Danvers, MA).
Microparticle preparations
For analysis of murine platelet microparticles, blood was drawn from wild-type C57BL/6J mice by vena caval puncture and anticoagulated with acid citrate dextrose (ACD). Platelet-rich plasma (PRP) was subsequently isolated from blood by centrifugation at 400g for 5 minutes. Platelets were removed from PRP following addition of 1 μM PGE1 (Calbiochem, San Diego, CA) by serial centrifugation at 1000g for 10 minutes. Microparticles within platelet-poor plasma were used for subsequent studies. To prepare microparticles from activated platelets, washed mouse platelets were incubated with 1 U/mL thrombin and 50 μg/mL collagen at 37°C for 45 minutes. Following activation, platelets were removed by serial centrifugation at 1000g for 10 minutes. Microparticles were isolated from megakaryocyte cultures by centrifugation at 200g for 20 minutes to remove megakaryocytes followed by centrifugation at 1000g for 10 minutes to remove any platelets or proplatelets. Freshly prepared microparticles were analyzed by flow cytometry.
Evaluation of resting platelets
Platelet-rich plasma was prepared by centrifugation and incubated with 25 μg/mL lepirudin, a direct thrombin inhibitor. Samples were incubated end-over-end in an orbital shaker. Samples were analyzed at the indicated times for P-selectin surface expression by flow cytometry, cleavage of filamin A by immunoblot analysis, and platelet microparticle production by flow cytometry.
Flow cytometry
Flow cytometry was performed using a Becton Dickinson FACSCalibur flow cytometer (Lincoln Park, NJ). Samples were incubated with FITC-labeled or PE-labeled antibodies as indicated in the figure legends. PBS (500 μL) was added to the sample after a 20-minute incubation and the microparticles were analyzed immediately by flow cytometry. Fluorescent channels were set at logarithmic gain. Ten thousand particles were acquired for each sample. A 530/30 band pass filter was used for FL1 fluorescence to measure binding of FITC-labeled antibodies and annexin V. A 585/42 band pass filter was used for FL2 fluorescence to measure binding of PE-labeled antibodies. Data were analyzed using CellQuest software (Becton Dickinson) on a MacIntosh PowerPC (Apple, Cupertino, CA).
Analysis of filamin A
Microparticle lysates were diluted in sample buffer at 95°C for 5 minutes. Equal amounts of proteins were then separated by SDS–polyacrylamide gel electrophoresis (PAGE). Immunoblotting was performed using an anti–filamin A primary antibody and FITC-labeled secondary antibodies. Bands were visualized using fluorescence detection on a Typhoon 9400 Molecular Imager (GE Healthcare, Piscataway, NJ).
Statistical analysis
For measurement of microparticle formation by flow cytometry, mean plus or minus standard deviations were calculated from 3 to 6 independent studies, as indicated in the figure legends. Statistical significance of differences between groups was determined using a 2-tailed Student t test. Significance was set at P value less than or equal to .05.
Results
Visualization of microparticle formation from mouse megakaryocytes
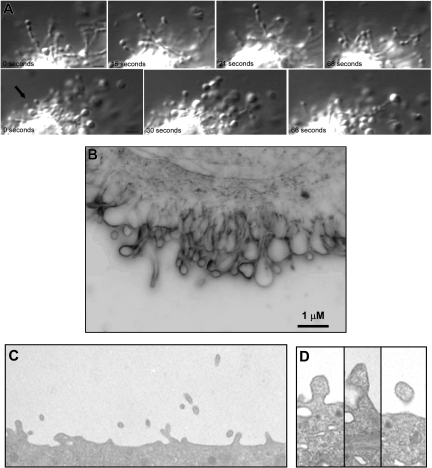
Preliminary observations made while studying platelet formation in mouse megakaryocytes indicated that, in addition to generating proplatelets, megakaryocytes form markedly smaller particles that are distinct from platelets. To evaluate the hypothesis that megakaryocytes produce microparticles, megakaryocytes isolated from mouse fetal liver were cultured in suspension as previously described.31,34 High-resolution differential-interference contrast (DIC) microscopy of day-3 megakaryocytes revealed striking features that have not been previously recognized, and emphasizes the dynamic nature of microparticle generation. These studies showed that microparticles formed as submicron beads along the length of slender micropodia (Figure 1A; Videos S1-S3, available on the Blood website; see the Supplemental Materials link at the top of the online article). These beads were substantially smaller than platelet-sized bulges that form proplatelets. The beads formed submicron particles that were released from the megakaryocytes into the culture medium. The degree of shedding of microparticles was indicated by the numerous vesicles observed surrounding the cell.
Figure 1.
Megakaryocytes produce microparticles via an active, constitutive process. (A) Video-enhanced differential-interference contrast microscopy showing 2 examples of a mouse megakaryocyte forming microparticles in vitro. Small vesicles in linear arrays were observed extending from the surface of megakaryocytes. The microparticle at the tip of this process is 0.5 μm (). Scale bar represents 1 μm. (B) Surface blebs were visualized in unstained, living mouse megakaryocytes imaged by the RTM-3 in light modality using a 100× objective lens. This representative frame shows a membrane region that exhibited active surface blebbing. Scale bar represents 1 μm. (C) Thin-section electron micrograph showing the surface of a mouse megakaryocyte. Numerous small projections were observed emanating from the surface. The width of this field is 4 μm. (D) Thin-section electron micrographs showing blebs at various stages along the surface of a mouse megakaryocyte. The surface projections are approximately 300 nm in diameter.
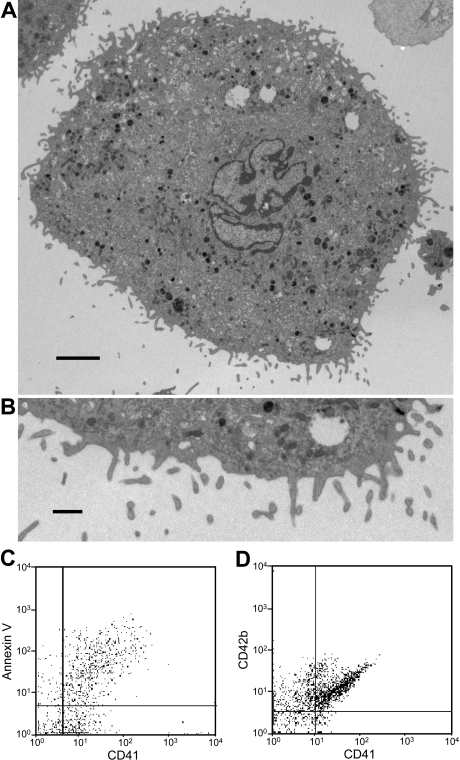
Although DIC microscopy provided sufficient resolution to visualize microparticles forming as beads along slender micropodia, it was difficult to assess using this technique whether microparticles also formed along the surface of the megakaryocyte plasma membrane. We used the Richardson Technology Microscopy, an optical imaging system capable of particle resolution below 150 nm, to reveal the spatial and temporal dynamics of microparticle formation at the surface of megakaryocytes in culture. These studies showed that day-3 megakaryocytes formed blebs along their surface (Figure 1B; Video S4) and emphasized the highly dynamic nature of this process. The blebs observed in these cells involved the entire surface of the cell. Peripheral organelle exclusion from blebs and microparticles was apparent. Microparticles formed without the addition of any platelet agonists to the culture medium. Consistent with our video microscopy analysis, electron microscopy of cultured mouse megakaryocytes revealed particles of 0.2 to 0.5 μm emanating from the mouse megakaryocyte surface (Figure 1C,D). These structures did not appear to contain any organelles and lacked any recognizable cytoskeletal structures such as microtubules or actin filaments. Together, these microscopy studies demonstrate generation of submicron particles by megakaryocytes.
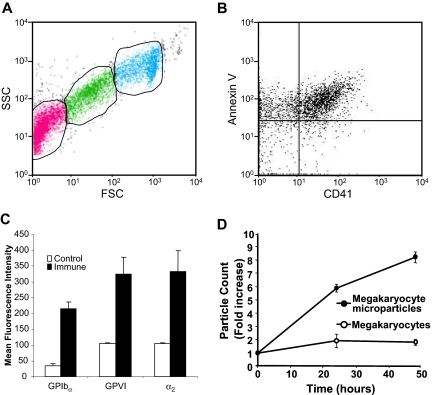
The fact that the submicron particles are released from mouse megakaryocytes enabled us to isolate and characterize these particles from megakaryocyte suspensions. Platelet microparticles are typically identified by their small size (< 1 μm), expression of platelet markers such as CD41 or CD42b, and expression of PS on their extracellular surface. Analysis of day-3 megakaryocyte suspensions by flow cytometry demonstrated 3 populations that could be distinguished on the basis of their forward and side scatter characteristics. First, we identified a cell population with forward and side scatter characteristics identical to those of mouse platelets (Figure 2A, green). Populations of particles with forward and side scatter characteristics consistent with microparticles (Figure 2A, pink) and megakaryocytes (Figure 2A, aqua) were also observed. Following removal of megakaryocytes and platelets by low-speed centrifugation, the microparticle population was isolated by ultracentrifugation. Double staining of the microparticle population with FITC-labeled annexin V and a phycoerythrin-labeled antibody directed at CD41 demonstrated that the majority of these particles expressed surface PS and CD41 (Figure 2B). Analysis for additional platelet surface proteins demonstrated that binding of antibodies directed again CD42b was increased 6.3-fold for CD42b (P = .001), 3.1-fold for GPVI (P = .003), and 3.2-fold for α2 integrin (P = .005), compared with isotype-matched control antibodies (Figure 2C). The population of CD41+ microparticles increased as a function of time in culture (Figure 2D). These data show that megakaryocytes produce PS+, CD41+, CD42b+, GPVI+, α2 integrin+ microparticles.
Figure 2.
Mouse megakaryocytes produce microparticles that express phosphatidylserine and multiple platelet markers on their surface. (A) Mouse megakaryocytes were cultured for 3 days and analyzed by flow cytometry. The middle gate (green) indicates particles with FSC and SSC characteristics of mouse platelets. The gate to the left (pink) indicates microparticles. The gate on the right (aqua) indicates megakaryocytes. (B) Microparticles isolated from megakaryocyte cultures were stained using anti-CD41 antibody and annexin V. (C) Microparticles isolated from megakaryocyte cultures were stained with either isotype-matched nonimmune IgG (control) or IgG directed at CD42b, GPVI, or α2 integrin (immune). Error bars represent the standard deviation of 3 independent assays. (D) Megakaryocyte suspensions were analyzed for megakaryocytes (○) and microparticles (•) on days 3, 4, and 5 of culture. Values relative to the number of megakaryocytes and microparticles, respectively, on day 3 are indicated. Error bars indicate the standard deviation of 4 independent assays.
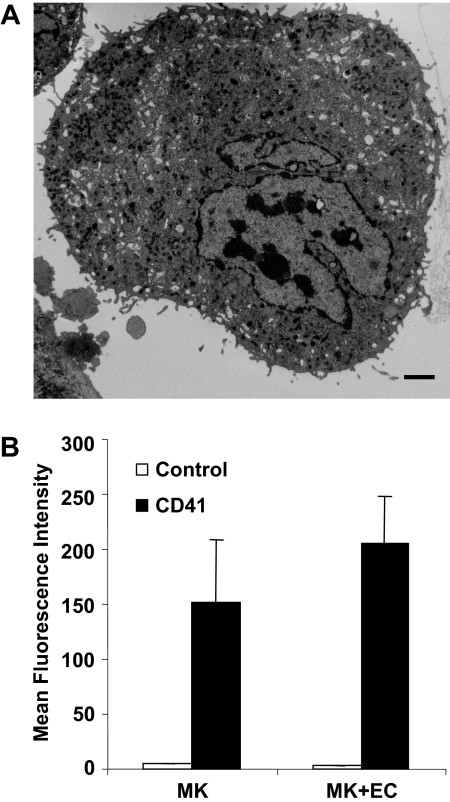
Megakaryocytes in vivo are confined within the subendothelial layer of the bone marrow sinuses where development is regulated at multiple levels by several cytokines.35,38–40 Since the development of megakaryocytes occurs within a complex bone marrow environment where both cytokines and adhesive interactions play an essential role, we analyzed microparticle formation using an in vitro system that recapitulates some aspects of the in vivo environment.35 Megakaryocytes were cocultured with bone marrow endothelial cell monolayers in the presence of the chemokines SDF-1 and FGF-4. Electron microscopy of these cocultures revealed particles of 0.2 to 0.5 μM emanating from the megakaryocyte surface (Figure 3A). We next compared the degree of megakaryocyte-derived microparticle generation from mouse megakaryocytes cultured alone versus mouse megakaryocytes cocultured with bone marrow endothelial cells. Quantification of CD41+ microparticle production demonstrated no statistical difference between microparticle generation under these 2 conditions (P = .2). Similarly, we did not observe differences in microparticle formation when megakaryocytes were incubated in the presence versus the absence of TPO, with 1% versus 10% fetal calf serum, or in the presence versus the absence of VEGF165, insulin-like growth factor, SDF-1, and FGF-4 (data not shown). These data do not rule out an effect of bone marrow endothelial cells or specific cytokines on megakaryocyte microparticle production, but suggest that these factors are not specifically required for stimulating megakaryocyte microparticle formation.
Figure 3.
Mouse megakaryocytes cocultured with bone marrow endothelial cells produce microparticles. (A) Thin-section electron micrograph showing small projections and vesicles emanating from the surface of a megakaryocyte that has been cocultured with bone marrow–derived endothelial cells. Scale bar represents 2 μm. (B) Mouse megakaryocytes were incubated for 24 hours in the presence or absence of bone marrow endothelial cells. CD41+ microparticles within the supernatants were quantified by flow cytometry. Error bars indicate the standard deviation of 3 independent assays.
Disruption of the actin-based cytoskeleton enhances microparticle generation from megakaryocytes
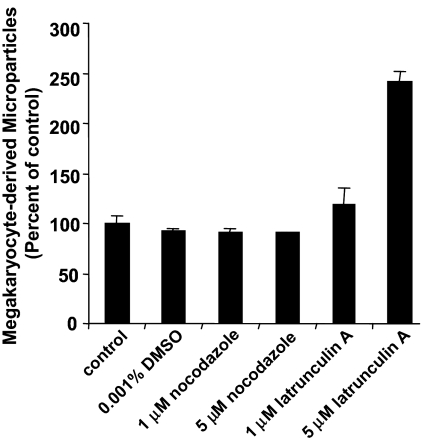
Since platelet production is powered by remodeling of the megakaryocyte cytoskeleton,29–32 we determined whether microparticle formation from megakaryocytes also requires microtubule formation or actin cytoskeletal reorganization. Megakaryocytes were incubated overnight in the presence of cytoskeleton inhibitors and the number of CD41+ microparticles in megakaryocyte supernatants was determined by flow cytometry. We used nocodazole to evaluate the role of microtubules in microparticle formation. At concentrations 1 μM or higher, nocodazole blocks proplatelet formation.30–32 However, incubation of cultured megakaryocytes with 1 μM or 5 μM nocodazole failed to inhibit generation of microparticles from megakaryocyte suspensions (Figure 4). In contrast, proplatelet formation and platelet production were markedly inhibited in these cultures as observed previously.31 We next used latrunculin A to evaluate the role of actin polymerization in microparticle formation. Latrunculin A binds to G-actin with a Kd of 0.2 μM, thereby inhibiting actin polymerization and eliciting actin depolymerization. Incubation of megakaryocyte suspensions with latrunculin A reduced F-actin content by 40% plus or minus 5%, but failed to inhibit megakaryocyte-derived microparticle formation. At a concentration of 5 μM, latrunculin A actually augmented microparticle formation by 2.4-fold (P = .003; Figure 4). Consistent with the flow cytometry data, incubation with latrunculin A enhanced the production of microparticles and resulted in more extensive blebbing as visualized by video microscopy (data not shown). Megakaryocyte suspensions that produced microparticles retained their ability to generate proplatelets. These results demonstrate that platelet microparticles are generated from megakaryocytes by an active process that is distinct from platelet formation.
Figure 4.
Microparticle production by megakaryocytes is a microtubule-independent process that is regulated by actin. Mouse megakaryocytes were cultured in the presence of vehicle alone (control), 0.001% DMSO, 1 μM nocodazole, 5 μM nocodazole, 1 μM latrunculin A, or 5 μM latrunculin A. Following an overnight incubation, supernatants were analyzed for CD41+ microparticles by flow cytometry. Values are reported as percentage of control compared with the number of microparticles detected in supernatants of megakaryocytes exposed to buffer alone. Error bars indicate the standard deviation of 4 independent assays.
Circulating microparticles manifest features of megakaryocyte-derived microparticles
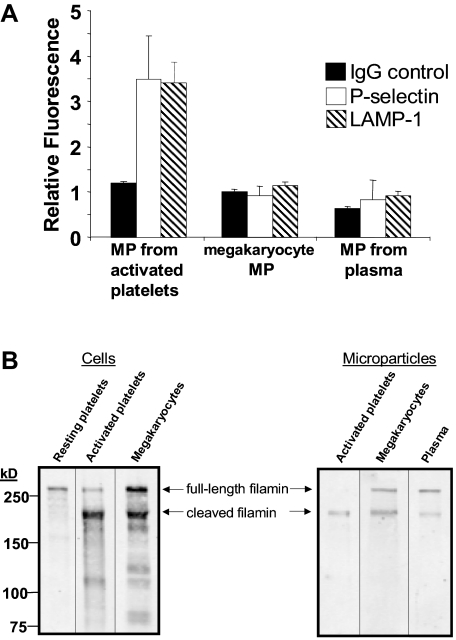
To test whether circulating microparticles are derived from megakaryocytes, we identified surface markers to help distinguish megakaryocyte-derived microparticles from microparticles generated by activated platelets. Three sets of mouse microparticles were analyzed for surface expression of the platelet activation markers CD62P and LAMP-1. Microparticles generated from activated platelets exposed to thrombin plus collagen demonstrated a 3.5-fold increase in anti-CD62P antibody binding (P = .02) and a 3.4-fold increase in anti–LAMP-1 antibody (P = .003) binding compared with nonimmune IgG (Figure 5A). In contrast, megakaryocyte-derived microparticles isolated from cultured fetal megakaryocytes did not express CD62P or LAMP-1 on their surface. Similarly, neither CD62P nor LAMP-1 was identified on circulating mouse microparticles isolated from the plasma of untreated, wild-type mice. These studies demonstrate that neither megakaryocyte-derived microparticles nor microparticles isolated from the circulation express platelet activation markers.
Figure 5.
Megakaryocyte-derived microparticles resemble plasma-derived microparticles with regard to surface markers and expression of full-length filamin A. (A) Microparticles were isolated from mouse platelets incubated with thrombin and collagen, cultured mouse megakaryocytes, or platelet-free plasma. Microparticles were subsequently labeled with nonimmune IgG, anti-CD62P antibody, or anti–LAMP-1 antibody as indicated. Error bars indicate the standard deviation of 4 independent assays. (B) Filamin A was assayed by immunoblot analysis in resting mouse platelets, mouse platelets exposed to thrombin plus collagen, and cultured megakaryocytes. Filamin A was also assayed by immunoblot analysis in microparticles isolated from activated platelets, cultured mouse megakaryocytes, or platelet-free mouse plasma. Full-length filamin A is found in megakaryocyte-derived microparticles and circulating microparticles, but not microparticles from activated platelets.
Results using activation markers are consistent with the supposition that a population of circulating microparticles is derived from megakaryocytes. However, surface markers could potentially be shed in the circulation41 or during megakaryopoiesis and still be found in the circulation. Thus, we evaluated megakaryocyte-derived microparticles and microparticles isolated from plasma for cleavage of a cytoplasmic protein, filamin A. Filamin A is a 280-kDa actin cross-linking protein that is cleaved by calpain following platelet activation.21,42 As expected, resting mouse platelets contained full-length filamin A, whereas activated platelets expressed mostly cleaved filamin A (Figure 5B). In contrast, mouse megakaryocytes contained both full-length and cleaved filamin A. Megakaryocyte-derived microparticles demonstrated a ratio of full-length to cleaved filamin A similar to that of megakaryocytes (Figure 5B). Filamin A in microparticles generated from activated platelets was almost entirely cleaved. Circulating microparticles isolated from plasma demonstrated a ratio of full-length cleaved filamin A similar to that of microparticles from megakaryocytes. Together with the surface marker results, these data indicate that CD41+ microparticles circulating in the plasma of healthy wild-type mice share several markers with megakaryocyte-derived microparticles that they do not share with microparticles generated from activated platelets.
Generation of microparticles from human megakaryocytes
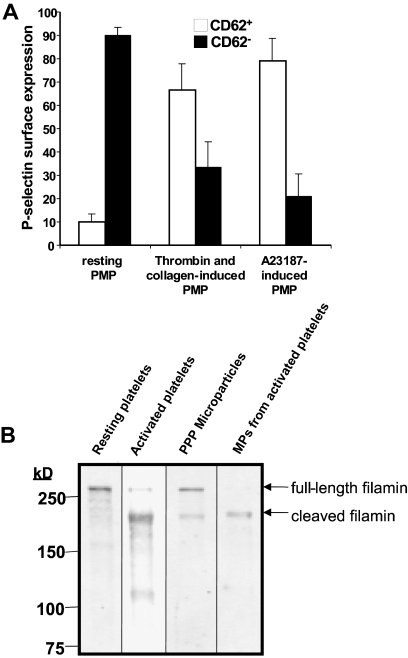
To assess the derivation of circulating platelet microparticles in humans, we isolated microparticles from the plasma of healthy volunteers. These samples were assessed for coexpression of the platelet-specific antigens CD41 and CD62P. This evaluation demonstrated that only a small portion of CD41+ microparticles express CD62P on their surface (Figure 6A). In contrast, the majority of CD41+ microparticles derived from platelets exposed to either collagen plus thrombin or calcium ionophore A23187 express CD62P (Figure 6A). These results raise the possibility that platelet microparticles are not derived from activated platelets. Consistent with these findings, we observed that resting platelets isolated from the plasma of healthy individuals contained full-length filamin A (Figure 6B). In contrast, activated platelets showed primarily cleaved filamin A. Microparticles derived from plasma contained both full-length and cleaved filamin A, whereas microparticles derived from activated platelets contained only cleaved filamin A. To assess whether microparticles are generated by unactivated platelets over extended incubations, we incubated platelet-rich plasma with lepirudin to maintain platelets in a resting state. Lack of P-selectin surface expression over a 72-hour period confirmed that platelets were not activated (Figure S1A). Consistent with this observation, filamin A remained uncleaved in platelets incubated under these conditions (Figure S1B). Under conditions in which no platelet activation occurred, no platelet microparticle formation was observed over a 72-hour period (Figure S1C). These data are inconsistent with the premise that platelet microparticles circulating in healthy individuals arise primarily from resting or activated platelets, supporting the possibility that such microparticles derive from an additional or alternative source, such as megakaryocytes.
Figure 6.
Analysis of CD41+ microparticles from human plasma demonstrates that the majority are CD62P− and contain full-length filamin A. (A) Microparticles were isolated from human plasma, platelets activated with thrombin and collagen, or platelets activated by Ca2+-ionophore A23187 and analyzed for CD62P expression by flow cytometry. The percentage of CD62P+ (□) and CD62P− (■) microparticles is indicated. Error bars indicate the standard deviation of 4 independent assays. (B) Filamin A was assayed by immunoblot analysis in resting human platelets, human platelets exposed to thrombin and collagen, microparticles isolated from platelet-free plasma, or microparticles isolated from activated platelets.
To directly test whether human megakaryocytes can generate microparticles, we cultured megakaryocytes from human cord blood CD34+ cells and analyzed the supernatants for microparticles. As observed with mouse megakaryocytes, electron microscopy of cultured mouse megakaryocytes revealed a population of cells with particles of 0.2 to 0.5 μM emanating from the megakaryocyte surface (Figure 7A). Flow cytometry of CD41+ microparticles from cultured, human megakaryocytes demonstrates that the majority of CD41+ particles also stain with annexin V and anti-CD42b antibody (Figure 7B). These results confirm that human megakaryocytes produce microparticles.
Figure 7.
Microparticle generation from human megakaryocytes. (A) Thin-section electron micrograph showing the surface of an entire human megakaryocyte. Numerous small projections were observed emanating from the surface. Scale bar represents 10 μm. (B) Thin-section electron micrograph showing the megakaryocyte surface at higher magnification. Blebs at various stages are visualized along the surface of this human megakaryocyte. The surface projections are approximately 300 nm in diameter. Scale bar represents 1 μm. (C,D) Microparticles isolated from human megakaryocyte cultures were stained using anti-CD41 antibody and either annexin V or IgG directed at CD42b. This analysis demonstrates that the majority of human megakaryocyte-derived microparticles express PS on their surface and are CD42b+. Error bars indicate the standard deviation of 4 independent assays.
Discussion
There is substantial evidence that platelet-derived microparticles (PMPs) are generated in response to pathologic processes including immunologic platelet disorders and arterial thrombosis.7–10 PMPs from activated platelets are heterogeneous,43 but a high percentage express surface activation markers such as CD62P.2 Studies evaluating surface expression of CD62P on circulating PMPs demonstrated both PMP CD62P+ and CD62P− PMP populations.44,45 In healthy controls, the majority of PMPs did not express CD62P. What is the origin of CD41+, CD62P− MPs? The possibility that a portion of this population of MPs is derived from megakaryocytes rather than platelets has been proposed.45 Previous studies have shown that human megakaryocytes or megakaryocytoid cells produce numerous particles that are smaller than platelets.46–48 Notably, a study of the ultrastructure of cultured human megakaryocytes demonstrated numerous submicron particles surrounding platelet-producing megakaryocytes and demonstrated CD41+ microparticles in human megakaryocyte culture supernatants.46 The mechanism of their generation, however, and whether such microparticles circulate in plasma were not assessed. We have now characterized microparticles generated by megakaryocytes, demonstrated that the mechanism of their formation is distinct from that of platelets, and have shown that microparticles produced via megakaryocytes manifest features of circulating CD41+ microparticles.
Most studies evaluating circulating platelet microparticles have identified them based on their submicron size, surface expression of CD41 or CD42b, and their ability to bind FITC–annexin V. Megakaryocyte-derived microparticles share these characteristics. FITC–annexin V binding indicates that despite the fact they arise from a cell that is not activated, megakaryocytic microparticles express PS on their surface. This observation may be related to the phenomenon of localized PS exposure observed in cultured megakaryocytes.49 The finding that megakaryocyte-derived microparticles are CD62P−, LAMP-1−, and contain full-length filamin A permits distinction between microparticles formed from activated platelets and those generated directly from megakaryocytes. These marker studies indicate that the majority of circulating microparticles in healthy individuals are not derived from cell activation. Instead, microparticles are formed continuously by megakaryocytes in an active, constitutive process that occurs in the absence of agonist stimulation under the same conditions in which proplatelet-producing megakaryocytes grow and differentiate.
We used video-enhanced light microscopy of cultured, living megakaryocytes to demonstrate the generation of microparticles directly from the parent cell of platelets. Megakaryocytes produce microparticles by a mechanism that is distinct from platelet production. Platelets form at the ends of long tubelike processes called proplatelets that are extended by microtubule-based forces hundreds of micrometers from the megakaryocyte cell body. DIC video microscopy revealed that megakaryocyte-derived microparticles form as beads along the length of slender, unbranched micropodia. High-resolution RTM video microscopy showed that microparticles also form from continuous blebbing of the megakaryocyte surface. Microparticle formation generally occurred prior to proplatelet formation; thus, proplatelet extensions were not visualized in videos of microparticle-producing megakaryocytes. To demonstrate conclusively that microparticles were not derived from proplatelets, we inhibited platelet production with the microtubule inhibitor nocodazole. Concentrations of nocodazole (1-5 μM) that inhibit proplatelet formation50 failed to affect generation of megakaryocyte-derived microparticles (Figure 4). Inhibitors of actin polymerization block bending and branching of proplatelet extensions.31 In contrast, the actin assembly inhibitor latrunculin A augments microparticle formation by megakaryocytes. These observations indicate that these microparticles are not formed from proplatelets or mature platelets produced during megakaryopoiesis. Video microscopy confirms the supposition that platelet microparticles are generated directly from megakaryocytes rather than a platelet intermediate.
Previous studies in mice, rabbits, and dogs have suggested that platelet microparticles are cleared within minutes following introduction into the circulation.51–53 The observation that platelet microparticles are cleared rapidly has led to the proposal that they are produced continuously. Formal possibilities for continuous generation of PMPs include production by resting platelets, production by activated platelets, or production by megakaryocytes. We have not been able to detect platelet microparticle formation over a 72-hour period when resting platelets are incubated in the presence of lepirudin (Figure S1). Similarly, we have never observed microparticle formation during real-time imaging of live resting platelets or in evaluation of electron micrographs of resting platelets (data not shown). Thus, we have found no evidence that resting platelets generate PMPs. PMP production by continuous activation of platelets would require continuous activation of platelets to a degree sufficient to result in microparticle formation. One would expect that the degree of platelet activation required to generate this amount of microparticle formation would result in expression of platelet activation markers in circulating platelets, particularly since activated platelets are not necessarily rapidly cleared from the circulation.41 Surface markers such as P-selectin could be shed from activated platelets.41 However, filamin A would be cleaved in microparticles generated from platelet activation. An alternative explanation for the maintenance of basal levels of CD41+ microparticles despite rapid clearance is that a population of microparticles is generated directly by megakaryocytes. The observations that circulating microparticles from healthy individuals are CD62P− and contain full-length filamin A support this possibility.
Based on our observations, we propose that circulating CD41+ microparticles can be derived from 2 sources. One source is a regulated mechanism in which microparticles are generated directly from mature platelets by activation. Evidence that platelet microparticles can originate from activated platelets in vivo is derived from studies demonstrating increased platelet microparticle levels in patients with thrombotic conditions.7–9,11 The second mechanism of CD41+ microparticle formation is a constitutive mechanism in which microparticles are produced by megakaryocytes. This population is more accurately defined as megakaryocyte-derived microparticles, rather than platelet microparticles, since they arise directly from megakaryocytes and not platelets. The presence of megakaryocyte-derived microparticles in the circulation is supported by the facts that megakaryocytes constitutively produce microparticles as part of their developmental repertoire and that megakaryocyte-derived microparticles share several pertinent characteristics with microparticles isolated from the plasma of healthy individuals. Both megakaryocyte-derived microparticles and platelet microparticles isolated from plasma are CD41+, PS+, CD62P−, LAMP-1−, and express both full-length and cleaved filamin A. In contrast, microparticles originating from activated platelets express activation markers and little, if any, full-length filamin A. These observations support the premise that a significant population of circulating platelet microparticles in healthy individuals stem directly from megakaryocytes rather than from activated platelets.
Functional differences between megakaryocyte-derived microparticles and microparticles generated from activated platelets may exist. For example, endothelial microparticles have recently been demonstrated to transfer mRNA to endothelium.54 Megakaryocyte-derived microparticles and microparticles generated from activated platelets may differ in their mRNA content and, potentially, their ability to influence endothelial cell function. The 2 microparticle types differ in their expression of platelet activation markers such as P-selectin. Megakaryocyte-derived microparticles may be less capable of supporting inflammatory responses and complement activation compared with those derived from activated platelets.55,56 The ability to distinguish PMPs from megakaryocyte-derived microparticles could be useful in evaluating the effects of disease states on microparticle levels and for monitoring platelet microparticles for diagnostic and prognostic purposes. Future comparisons of the 2 microparticle populations will assess these possibilities.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH, Bethesda, MD) grants HL68130 (J.E.I.) and HL63250 (R.F.). J.E.I. and R.F. are American Society of Hematology Junior Faculty Scholars. R.F. is a recipient of an Established Investigator Award from the American Heart Association (Dallas, TX) and a Special Project Award from Bayer Healthcare (Berkeley, CA).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.F. conceived and designed research, analyzed and interpreted data, and drafted the paper; J.R.D., J.R., E.A., S.R.P.-H., and E.B. performed research; G.L.K. assisted with and interpreted microscopy; M.S.-V. assisted with human megakaryocyte studies; and J.E.I. designed research, performed microscopy, analyzed and interpreted data, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Flaumenhaft, Beth Israel Deaconess Medical Center, Division of Hemostasis and Thrombosis, Department of Medicine, 330 Brookline Avenue, Boston, MA 02215; e-mail: rflaumen@bidmc.harvard.edu.
References
- 1.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- 2.Horstman LL, Ahn YS. Platelet microparticles: a wide-angle perspective. Crit Rev Oncol Hematol. 1999;30:111–142. doi: 10.1016/s1040-8428(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 3.Joop K, Berckmans RJ, Nieuwland R, et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost. 2001;85:810–820. [PubMed] [Google Scholar]
- 4.Berckmans RJ, Nieuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–646. [PubMed] [Google Scholar]
- 5.Castaman G, Yu-Feng L, Rodeghiero F. A bleeding disorder characterised by isolated deficiency of platelet microvesicle generation. Lancet. 1996;347:700–701. doi: 10.1016/s0140-6736(96)91259-3. [DOI] [PubMed] [Google Scholar]
- 6.Castaman G, Yu-Feng L, Battistin E, Rodeghiero F. Characterization of a novel bleeding disorder with isolated prolonged bleeding time and deficiency of platelet microvesicle generation. Br J Haematol. 1997;96:458–463. doi: 10.1046/j.1365-2141.1997.d01-2072.x. [DOI] [PubMed] [Google Scholar]
- 7.Hughes M, Hayward CP, Warkentin TE, Horsewood P, Chorneyko KA, Kelton JG. Morphological analysis of microparticle generation in heparin-induced thrombocytopenia. Blood. 2000;96:188–194. [PubMed] [Google Scholar]
- 8.Mallat Z, Benamer H, Hugel B, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 9.Lee YJ, Jy W, Horstman LL, et al. Elevated platelet microparticles in transient ischemic attacks, lacunar infarcts, and multiinfarct dementias. Thromb Res. 1993;72:295–304. doi: 10.1016/0049-3848(93)90138-e. [DOI] [PubMed] [Google Scholar]
- 10.Galli M, Grassi A, Barbui T. Platelet-derived microvesicles in thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Thromb Haemost. 1996;75:427–431. [PubMed] [Google Scholar]
- 11.Tomer A, Harker LA, Kasey S, Eckman JR. Thrombogenesis in sickle cell disease. J Lab Clin Med. 2001;137:398–407. doi: 10.1067/mlc.2001.115450. [DOI] [PubMed] [Google Scholar]
- 12.Ando M, Iwata A, Ozeki Y, Tsuchiya K, Akiba T, Nihei H. Circulating platelet-derived microparticles with procoagulant activity may be a potential cause of thrombosis in uremic patients. Kidney Int. 2002;62:1757–1763. doi: 10.1046/j.1523-1755.2002.00627.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan KT, Lip GY. The potential role of platelet microparticles in atherosclerosis. Thromb Haemost. 2005;94:488–492. doi: 10.1160/TH05-03-0201. [DOI] [PubMed] [Google Scholar]
- 14.Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 15.Pasquet JM, Dachary-Prigent J, Nurden AT. Calcium influx is a determining factor of calpain activation and microparticle formation in platelets. Eur J Biochem. 1996;239:647–654. doi: 10.1111/j.1432-1033.1996.0647u.x. [DOI] [PubMed] [Google Scholar]
- 16.Wiedmer T, Shattil SJ, Cunningham M, Sims PJ. Role of calcium and calpain in complement-induced vesiculation of the platelet plasma membrane and in the exposure of the platelet factor Va receptor. Biochemistry. 1990;29:623–632. doi: 10.1021/bi00455a005. [DOI] [PubMed] [Google Scholar]
- 17.Dachary-Prigent J, Pasquet JM, Freyssinet JM, Nurden AT. Calcium involvement in aminophospholipid exposure and microparticle formation during platelet activation: a study using Ca2+-ATPase inhibitors. Biochemistry. 1995;34:11625–11634. doi: 10.1021/bi00036a039. [DOI] [PubMed] [Google Scholar]
- 18.Fox JE, Austin CD, Boyles JK, Steffen PK. Role of the membrane skeleton in preventing the shedding of procoagulant-rich microvesicles from the platelet plasma membrane. J Cell Biol. 1990;111:483–493. doi: 10.1083/jcb.111.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raucher D, Stauffer T, Chen W, et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 20.O'Connell DJ, Rozenvayn N, Flaumenhaft R. Phosphatidylinositol 4,5-bisphosphate regulates activation-induced platelet microparticle formation. Biochemistry. 2005;44:6361–6370. doi: 10.1021/bi047344c. [DOI] [PubMed] [Google Scholar]
- 21.Bassé F, Gaffet P, Bienvenue A. Correlation between inhibition of cytoskeleton proteolysis and anti-vesiculation effect of calpeptin during A23187-induced activation of human platelets: are vesicles shed by filopod fragmentation? Biochim Biophys Acta. 1994;1190:217–224. doi: 10.1016/0005-2736(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 22.Fox JE, Austin CD, Reynolds CC, Steffen PK. Evidence that agonist-induced activation of calpain causes the shedding of procoagulant-containing microvesicles from the membrane of aggregating platelets. J Biol Chem. 1991;266:13289–13295. [PubMed] [Google Scholar]
- 23.Shcherbina A, Bretscher A, Kenney DM, Remold-O'Donnell E. Moesin, the major ERM protein of lymphocytes and platelets, differs from ezrin in its insensitivity to calpain. FEBS Lett. 1999;443:31–36. doi: 10.1016/s0014-5793(98)01674-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Litvinov RI, Chen X, et al. Loss of PIP5KIgamma, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J Clin Invest. 2008;118:812–819. doi: 10.1172/JCI34239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquet JM, Dachary-Prigent J, Nurden AT. Microvesicle release is associated with extensive protein tyrosine dephosphorylation in platelets stimulated by A23187 or a mixture of thrombin and collagen. Biochem J. 1998;333:591–599. doi: 10.1042/bj3330591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiedmer T, Sims PJ. Participation of protein kinases in complement C5b-9-induced shedding of platelet plasma membrane vesicles. Blood. 1991;78:2880–2886. [PubMed] [Google Scholar]
- 27.Gemmell CH, Sefton MV, Yeo EL. Platelet-derived microparticle formation involves glycoprotein IIb-IIIa: inhibition by RGDS and a Glanzmann's thrombasthenia defect. J Biol Chem. 1993;268:14586–14589. [PubMed] [Google Scholar]
- 28.Radley JM, Haller CJ. The demarcation membrane system of the megakaryocyte: a misnomer? Blood. 1982;60:213–219. [PubMed] [Google Scholar]
- 29.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tablin F, Castro M, Leven RM. Blood platelet formation in vitro. The role of the cytoskeleton in megakaryocyte fragmentation. J Cell Sci. 1990;97(pt 1):59–70. doi: 10.1242/jcs.97.1.59. [DOI] [PubMed] [Google Scholar]
- 31.Italiano JE, Jr, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147:1299–1312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handagama PJ, Feldman BF, Jain NC, Farver TB, Kono CS. In vitro platelet release by rat megakaryocytes: effect of metabolic inhibitors and cytoskeletal disrupting agents. Am J Vet Res. 1987;48:1142–1146. [PubMed] [Google Scholar]
- 33.Schwer HD, Lecine P, Tiwari S, Italiano JE, Jr, Hartwig JH, Shivdasani RA. A lineage-restricted and divergent beta-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr Biol. 2001;11:579–586. doi: 10.1016/s0960-9822(01)00153-1. [DOI] [PubMed] [Google Scholar]
- 34.Lecine P, Villeval JL, Vyas P, Swencki B, Xu Y, Shivdasani RA. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood. 1998;92:1608–1616. [PubMed] [Google Scholar]
- 35.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 36.Mattia G, Vulcano F, Milazzo L, et al. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99:888–897. doi: 10.1182/blood.v99.3.888. [DOI] [PubMed] [Google Scholar]
- 37.Pastos KM, Slayton WB, Rimsza LM, Young L, Sola-Visner MC. Differential effects of recombinant thrombopoietin and bone marrow stromal-conditioned media on neonatal versus adult megakaryocytes. Blood. 2006;108:3360–3362. doi: 10.1182/blood-2006-04-018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafii S, Shapiro F, Rimarachin J, et al. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–19. [PubMed] [Google Scholar]
- 39.Rafii S, Shapiro F, Pettengell R, et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–3363. [PubMed] [Google Scholar]
- 40.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 41.Michelson AD, Barnard MR, Hechtman HB, et al. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci U S A. 1996;93:11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallach D, Davies PJ, Pastan I. Purification of mammalian filamin. Similarity to high molecular weight actin-binding protein in macrophages, platelets, fibroblasts, and other tissues. J Biol Chem. 1978;253:3228–3235. [PubMed] [Google Scholar]
- 43.Perez-Pujol S, Marker PH, Key NS. Platelet microparticles are heterogeneous and highly dependent on the activation mechanism: studies using a new digital flow cytometer. Cytometry A. 2007;71:38–45. doi: 10.1002/cyto.a.20354. [DOI] [PubMed] [Google Scholar]
- 44.Nomura S, Yanabu M, Miyake T, et al. Relationship of microparticles with beta 2-glycoprotein I and P-selectin positivity to anticardiolipin antibodies in immune thrombocytopenic purpura. Ann Hematol. 1995;70:25–30. doi: 10.1007/BF01715378. [DOI] [PubMed] [Google Scholar]
- 45.van der Zee PM, Biro E, Ko Y, et al. P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem. 2006;52:657–664. doi: 10.1373/clinchem.2005.057414. [DOI] [PubMed] [Google Scholar]
- 46.Cramer EM, Norol F, Guichard J, et al. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood. 1997;89:2336–2346. [PubMed] [Google Scholar]
- 47.Rozmyslowicz T, Majka M, Kijowski J, et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. Aids. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- 48.Battinelli E, Willoughby SR, Foxall T, Valeri CR, Loscalzo J. Induction of platelet formation from megakaryocytoid cells by nitric oxide. Proc Natl Acad Sci U S A. 2001;98:14458–14463. doi: 10.1073/pnas.241427398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Botton S, Sabri S, Daugas E, et al. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 50.Patel SR, Richardson JL, Schulze H, et al. Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood. 2005;106:4076–4085. doi: 10.1182/blood-2005-06-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flaumenhaft R. Formation and fate of platelet microparticles. Blood Cells Mol Dis. 2006;36:182–187. doi: 10.1016/j.bcmd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Gemmell CH, Yeo EL, Sefton MV. Flow cytometric analysis of material-induced platelet activation in a canine model: elevated microparticle levels and reduced platelet life span. J Biomed Mater Res. 1997;37:176–181. doi: 10.1002/(sici)1097-4636(199711)37:2<176::aid-jbm5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 53.Rand ML, Wang H, Bang KW, Packham MA, Freedman J. Rapid clearance of procoagulant platelet-derived microparticles from the circulation of rabbits. J Thromb Haemost. 2006;4:1621–1623. doi: 10.1111/j.1538-7836.2006.02011.x. [DOI] [PubMed] [Google Scholar]
- 54.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 55.Del Conde I, Cruz MA, Zhang H, Lopez JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201:871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin W, Ghebrehiwet B, Peerschke EI. Expression of complement components and inhibitors on platelet microparticles. Platelets. 2008;19:225–233. doi: 10.1080/09537100701777311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.