Abstract
Transplant recipients have been reported to have an increased risk of solid cancers but most studies are small and have limited ability to evaluate the interaction of host, disease, and treatment-related factors. In the largest study to date to evaluate risk factors for solid cancers, we studied a multi-institutional cohort of 28 874 allogeneic transplant recipients with 189 solid malignancies. Overall, patients developed new solid cancers at twice the rate expected based on general population rates (observed-to-expected ratio 2.1; 95% confidence interval 1.8-2.5), with the risk increasing over time (P trend < .001); the risk reached 3-fold among patients followed for 15 years or more after transplantation. New findings showed that the risk of developing a non–squamous cell carcinoma (non-SCC) following conditioning radiation was highly dependent on age at exposure. Among patients irradiated at ages under 30 years, the relative risk of non-SCC was 9 times that of nonirradiated patients, while the comparable risk for older patients was 1.1 (P interaction < .01). Chronic graft-versus-host disease and male sex were the main determinants for risk of SCC. These data indicate that allogeneic transplant survivors, particularly those irradiated at young ages, face increased risks of solid cancers, supporting strategies to promote lifelong surveillance among these patients.
Introduction
Hematopoietic cell transplantation (HCT) is an effective treatment for malignant and nonmalignant diseases. Over the last 3 decades, advances in treatment and supportive care have translated into steady improvements in survival after allogeneic HCT. With larger numbers of long-term survivors, quantifying the late effects of transplantation has been a research priority. Previous studies have reported that survivors of HCT have an increased risk of developing new solid cancers with the risk rising among long-term survivors from 2% to 6% at 10 years after transplantation.1–11 Several factors contributed to this increase, including total body irradiation (TBI), which has been a mainstay of the preparative regimens for allogeneic HCT until recently,2–12 primary disease, male sex, and pretransplantation therapy. Chronic graft-versus-host disease (GHVD) and immunosuppressive therapy have also been shown to contribute to excess risk, particularly for squamous cell carcinomas (SCCs).1,11 Young age at transplantation has been reported to be a strong risk factor in some,2,6,7,10,12 but not all, previous studies.3,5,8,9,13 However, prior investigations have often been limited by small numbers of solid cancer cases (typically 20-50 cases of non-skin solid cancers), the combining of data from allogeneic and autologous cohorts, and small variation in conditioning regimens within single institutions. In particular, there is little information on the joint effects of age at transplantation, radiation conditioning regimens, and other factors on the risk of solid cancers, and whether these risks differ by type of solid tumor.
The current study expands and updates our previously assembled multi-institutional cohort of HCT survivors from more than 234 centers worldwide.6 We have doubled the number of people who have survived 5 or more years after HCT, from 3234 to 6641, and increased the number of solid cancers to 189 cases (including carcinomas in situ and invasive SCCs of the skin), making this the largest study to date to assess risk factors for solid cancers following HCT. The purpose of this analysis is to further our understanding of the effects of age at and time since HCT, the role of radiation and other risk factors for subsequent solid cancers, and to evaluate differences in risk by type of solid cancer (SCC vs non-SCC) and by the site of the solid cancer.
Methods
Patients
We assembled an international cohort of 28 874 patients who received allogeneic HCT using bone marrow as the source of stem cells between 1964 and 1994 at 271 participating Center for International Blood and Marrow Transplant Research (CIBMTR) transplantation centers (n = 23 542), and between 1969 and 1996 at the Fred Hutchinson Cancer Research Center (FHCRC) in Seattle (n = 5332). Overall 92% of patients were followed through the respective study end dates (90.1% from the CIBMTR were followed through 1995; 98.8% from FHCRC were followed through 1996). Patients who received transplants for Fanconi anemia or primary immunodeficiency diseases were excluded due to their inherent susceptibility to cancer. A total of 6641 patients were followed for more than 5 years after HCT, 1985 patients for more than 10 years, and 378 patients for 15 or more years. The previous analysis6 included 19 229 patients and included CIBMTR patients who received transplants between 1964 and 1990 and FHCRC patients between 1969 and 1992; other publications have included data from selected transplantation teams participating in this study.
Participating CIBMTR centers are required to report all transplantations consecutively with compliance monitored by on-site audits; patients are followed yearly for survival and second malignancies. Computerized checks for errors, physicians' review of submitted data, and on-site audits of participating centers ensure high quality data. FHCRC has had a long-term follow-up program in place for more than 30 years, which records delayed complications including second malignancies. FHCRC patients and their physicians are contacted annually for information on the recipient's overall condition and to capture late events. Second cancer studies at the CIBMTR and FHCRC were conducted with a waiver of informed consent at their respective institutions and at the National Cancer Institute (NCI), in accordance with the Declaration of Helsinki, during the time period of this study. Pathology and physician reports of second cancers were reviewed centrally (by W.D.T.), and, if necessary, tumors were reclassified. Carcinoma in situ of the skin and basal cell skin cancers were excluded from all analyses.
Statistical analysis
Comparisons with the general population.
For each transplant recipient, the number of person-years at risk was calculated from the date of transplantation until the date of last contact, death, diagnosis of a new cancer, or completion of the study, whichever occurred first. Age-, sex-, calendar year–, and region-specific incidence rates for all invasive solid cancers combined and for cancers of specific anatomical sites were applied to the appropriate person-years at risk to compute the expected numbers of cancers. Incidence rates for all invasive cancers (excluding nonmelanoma skin cancers) were obtained from selected registries in the United States, England and Wales, Europe, and Asia.14–16 Observed-to-expected (O/E) ratios, also called standardized incidence ratios, were calculated based on 153 cases of invasive solid cancers. Analyses excluded carcinoma in situ (n = 17) and invasive SCC of the skin (n = 19) for which population-based incidence rates were not available. The exact Poisson distribution was used to calculate 95% confidence intervals (CIs).17 The excess absolute risk (EAR) is frequently used as an absolute measure of the overall burden due to second cancers and is calculated as the observed number of cancers minus the expected number of cancers, divided by the person-years at risk, and expressed per 10 000 person-years at risk. Tests of heterogeneity and of linear trends were conducted using methods of Breslow.18
Comparisons within the cohort.
Multivariate analyses were used to compare risks of second solid cancers for various subgroups of transplant recipients who survived for at least 1 year after transplantation, using Poisson regression methods for grouped survival data.17,19 These within-cohort comparisons were based on 165 cases of solid cancers (including 19 invasive SCCs of the skin and 13 nonskin carcinomas in situ). Based on our previous studies demonstrating differences in risk patterns by type of new solid tumors after HCT,1,11 we evaluated risk factors for all solid cancers combined and separately for 55 SCCs and 110 non-SCCs. Since the risk of cancer may differ according to the disease for which the transplant was performed, the data were stratified according to the primary disease in 5 categories: acute lymphoblastic leukemia (ALL), acute myelogenous leukemia, chronic myelogenous leukemia (CML), severe aplastic anemia (SAA), and other (including lymphoma). The data were also stratified according to the interval since transplantation (1 to < 5, 5 to < 10, 10 to < 15, and 15 or more years), cohort (FHCRC or CIBMTR), and age at the time of transplantation (< 10, 10-19, 20-29, 30-39, 40-49, 50 y or older). Variables tested in the multivariate models included donor type (twin, sibling, or unrelated person) and match status (1 antigen mismatch, ≥ 2 antigen mismatch), use of radiation conditioning regimens (including TBI or limited field irradiation only [LFI]), TBI dose, use of T-cell depletion of the bone marrow, T cell–depletion method, antithymocyte or antilymphocyte globulin (ATG) for acute GVHD prophylaxis or treatment, age at transplantation, year of transplantation, and occurrence of acute or chronic GVHD by grade. Occurrences of acute GVHD grades II to IV and chronic GVHD (moderate or severe in the CIBMTR cohort and clinically extensive disease in the FHCRC cohort) were entered as time-dependent covariates.
Cumulative incidence.
The cumulative incidence of solid cancer (including non–skin carcinoma in situ and invasive SCC of the skin) was estimated taking into account the competing risk of death among patients who did not develop a second malignancy.20 To provide comparison to earlier studies (including our previous cohort analysis),6 we also calculated the incidence of solid cancers using the Kaplan-Meier method, which does not account for competing risks.21
Results
Characteristics of the 28 874 transplant recipients included in the study are shown in Table 1. The median age at HCT was 27 years with 58% of the cohort undergoing HCT at ages younger than 30 years. Most (74%) of the patients received transplants for leukemia and most received a bone marrow graft from an HLA-identically matched sibling. TBI was part of the conditioning regimen for 67% of the patients with administered doses varying widely over the study period. Nearly 30% of patients were conditioned with cyclophosphamide (with or without other drugs) without radiation, most frequently in the later calendar years. The grafts of 13% of transplant recipients were T cell–depleted; among those receiving T cell–replete grafts, cyclosporine and methotrexate were used as GVHD prophylaxis in 45%, while cyclosporine-based prophylaxis without methotrexate was used in 23%. The cumulative incidence of grades II to IV acute GVHD was 38% at 100 days after HCT, and the incidence of chronic GVHD by 1 year after transplantation was nearly 30%. Rates of chronic GVHD varied widely by transplantation center and calendar year with the cumulative incidence reaching nearly 40% for patients treated during 1991 through 1996 at large research-based centers.
Table 1.
Characteristics of 28 874 patients undergoing HCT
| Characteristic | No. of patients | % |
|---|---|---|
| All patients | 28 874 | 100.0 |
| CIBMTR | 23 542 | 81.5 |
| FHCRC | 5 332 | 18.5 |
| Sex | ||
| Male | 17 122 | 59.3 |
| Female | 11 752 | 40.7 |
| Age at transplantation, y | ||
| 0-9 | 4 058 | 14.1 |
| 10-19 | 5 773 | 20.0 |
| 20-29 | 6 812 | 23.6 |
| 30-39 | 6 796 | 23.5 |
| 40-49 | 4 379 | 15.2 |
| 50 or older | 1 056 | 3.7 |
| Median | 27 | |
| (Min-Max) Range | (0.08-72.41) | |
| Geographic region of transplantation team | ||
| United States, Canada | 13 745 | 47.6 |
| Europe | 11 340 | 39.3 |
| Other | 3 789 | 13.1 |
| Primary disease | ||
| ALL | 5 916 | 20.5 |
| ANLL | 7 461 | 25.8 |
| CML | 7 594 | 26.3 |
| Other leukemia | 452 | 1.6 |
| NHL | 1 152 | 4.0 |
| HL | 242 | 0.8 |
| Myeloma | 507 | 1.8 |
| Other malignancies* | 158 | 0.5 |
| SAA | 2 842 | 9.8 |
| MDS, MPD | 1 298 | 4.5 |
| Hemoglobinopathies | 906 | 3.1 |
| Other | 346 | 1.2 |
| Donor-recipient relationship and histocompatibility | ||
| Identical twin | 631 | 2.2 |
| HLA-identical sibling | 22 030 | 76.3 |
| HLA-mismatched sibling, other relative | 2 939 | 10.2 |
| Unrelated donor | 3 066 | 10.6 |
| Other, uncertain (not autologous) | 208 | 0.7 |
| Transplant-conditioning regimens | ||
| TBI + Cy ± other drugs | 16 934 | 58.6 |
| TBI + other drugs (no Cy) | 2 409 | 8.3 |
| LFI ± Cy ± other drugs | 809 | 2.8 |
| Busulfan + Cy ± other drugs | 6 510 | 22.5 |
| Cy ± other drugs | 1 881 | 6.5 |
| Other | 331 | 1.1 |
| Preventative GVHD therapy | ||
| No T-cell depletion | ||
| CsA (no MTX) | 6 540 | 22.7 |
| MTX (no CsA) | 4 552 | 15.8 |
| CsA + MTX | 12 986 | 45.0 |
| Other | 1 023 | 3.5 |
| T-cell depletion | 3 726 | 12.9 |
| Unknown | 47 | 0.2 |
| Cumulative incidence of acute GVHD (grades II-IV), % | ||
| 100 d after transplantation | 38.2 | |
| 1 y after transplantation | 38.4 | |
| Cumulative incidence of chronic GVHD, %† | ||
| 1 y after transplantation | 28.9 | |
| 3 y after transplantation | 31.0 | |
| TBI dose | ||
| No TBI or LFI | 8 592 | 29.8 |
| Single dose | ||
| Less than 10 Gy | 1 934 | 6.7 |
| 10 Gy or more | 2 387 | 8.3 |
| Fractionated dose | ||
| Less than 12 Gy | 2 710 | 9.4 |
| 12 to less than 13 Gy | 7 122 | 24.7 |
| 13 to less than 14 Gy | 2 329 | 8.1 |
| 14 to less than 15 Gy | 1 528 | 5.3 |
| 15 Gy or more | 1 161 | 4.0 |
| Limited field irradiation, no TBI | 809 | 2.8 |
| Unknown TBI dose, other radiation | 302 | 1.0 |
Percentages do not always add up to 100% due to rounding.
ANLL indicates acute nonlymphocytic leukemia; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; MDS, myelodysplastic syndromes; MPD, myeloproliferative disorders; Cy, cyclophosphamide; CsA, cyclosporine; and MTX, methotrexate.
Other malignancies include 121 solid (nonbreast) cancers (primarily neuroblastoma and sarcomas), 3 breast cancers, and 34 other malignancies. See Table 2 for second malignancies among these patients.
Chronic GVHD is defined as clinical extensive disease for FHCRC patients and includes all grades of chronic GVHD for CIBMTR patients.
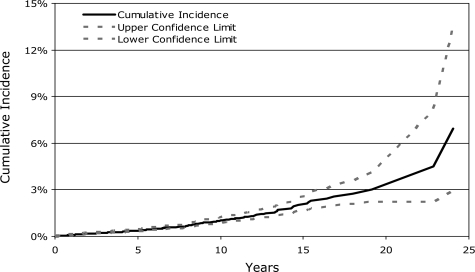
The cumulative incidence of developing a solid malignancy (including nonskin carcinoma in situ) using competing risk analyses was 1% (95% CI, 0.9-1.3%) at 10 years, 2.2% (95% CI, 1.7-2.7%) at 15 years, and 3.3% (95% CI, 2.4-4.5%) at 20 years after HCT (Figure 1). The comparable cumulative incidence estimates using the Kaplan-Meier method were 2.5% (95% CI, 2.0-3.0%), 5.8% (95% CI, 4.3-7.0%), and 8.8% (95% CI, 6.2-12.3%) at 10, 15, and 20 years after transplantation, respectively. The latter results are similar to our earlier reported solid cancer incidence estimates of 2.2% (95% CI, 1.5-3.0%) at 10 years and 6.7% (95% CI, 3.7-9.6%) at 15 years after transplantation.6
Figure 1.
Cumulative incidence of second solid malignancies after allogeneic HCT.
Overall, transplant recipients developed an invasive solid cancer at twice the rate expected based on population incidence rates (Observed, 153; observed/expected [O/E], 2.09; 95% CI, 1.77-2.45, EAR, 9 excess cancers per 10 000 person-years; Table 2). Risk of solid tumors was not significantly different for the CIBMTR compared with the FHCRC cohorts (O/E, 2.01 vs 2.29, respectively; P = .44). Males appeared to have slightly higher overall O/E ratios than females (O/E, 2.39 vs 1.83, respectively; P = .10). Significantly elevated risks were observed for tumors of the oral cavity (O/E, 7.01), liver (O/E, 6.32), brain and central nervous system (CNS; O/E, 5.94), thyroid (O/E, 5.79), bone (O/E, 8.5), soft tissue (O/E, 6.5), and for melanoma of the skin (O/E, 3.47). In an unusual pattern, several sarcomas occurred at sites other than the bone and soft tissue, including sarcomas of the liver (n = 4), small intestine (n = 1), lung (n = 1), uterus (n = 1), and cervix (n = 1); 2 patients developed Kaposi sarcoma (details in Table 2). The majority (27 of 40 cases) of second solid cancers of the brain/CNS, thyroid, bone, and soft tissues occurred among children (age < 17 years at HCT).
Table 2.
Ratio of observed (O) to expected (E) cases and excess absolute risk of new invasive solid cancers, according to time since HCT
| Time since transplantation |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 1 y |
1-4 y |
5-9 y |
10 y or longer |
15 y or longer |
Total | |||||||||
| No. of patients | 28 874 | 15 170 | 6 641 | 1985 | 378 | 28 874 | ||||||||
| Person-years at risk | 19 335 | 40 520 | 19 674 | 6054 | 1047 | 85 583 | ||||||||
| Subsequent cancer site | O | O/E | O | O/E | O | O/E | O | O/E | O | O/E | O | E | O/E (95% CI) | EAR‡ |
| All solid cancers* | 19 | 1.3 | 52 | 1.56§ | 51 | 2.78§ | 31 | 4.55§ | 5 | 3.28§ | 153 | 73.25 | 2.09§ (1.77-2.45) | 9.32 |
| Oral cavity and pharynx | 0 | 0 | 4 | 2.23 | 15 | 15.50§ | 8 | 25.69§ | 0 | 0 | 27 | 3.85 | 7.01§ (4.62-10.20) | 2.70 |
| Lip | 0 | 0 | 2 | 19.53§ | 2 | 37.34§ | 2 | 102.53§ | 0 | 0 | 6 | 0.22 | 26.78§ (10.01-59.36) | 0.68 |
| Tongue | 0 | 0 | 1 | 2.87 | 5 | 26.90§ | 4 | 63.12§ | 0 | 0 | 10 | 0.75 | 13.34§ (6.40-24.52) | 1.08 |
| Salivary gland | 0 | 0 | 0 | 0 | 4 | 58.37§ | 0 | 0 | 0 | 0 | 4 | 0.28 | 14.16§ (3.89-36.58) | 0.43 |
| Gum, other mouth | 0 | 0 | 1 | 2.18 | 2 | 8.08 | 2 | 24.76§ | 0 | 0 | 5 | 0.99 | 5.06§ (1.64-11.79) | 0.47 |
| Pharynx | 0 | 0 | 0 | 0 | 2 | 4.85 | 0 | 0 | 0 | 0 | 2 | 1.61 | 1.24 (0.15-4.49) | 0.05 |
| Esophagus | 0 | 0 | 2 | 4.63 | 0 | 0 | 1 | 13.58 | 1 | 69.5 | 3 | 0.93 | 3.24 (0.67-9.43) | 0.24 |
| Colon | 0 | 0 | 2 | 1.15 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3.83 | 0.52 (0.06-1.89) | −0.21 |
| Rectum, rectosigmoid jct, anus | 0 | 0 | 3 | 2.75 | 0 | 0 | 1 | 4.49 | 0 | 0 | 4 | 2.39 | 1.67 (0.46-4.29) | 0.19 |
| Liver | 1 | 4.49 | 2 | 3.87 | 1 | 3.66 | 3 | 31.41§ | 0 | 0 | 7 | 1.11 | 6.32§ (2.54-12.99) | 0.69 |
| Bronchus and lung | 1 | 0.69 | 4 | 1.22 | 3 | 1.68 | 0 | 0 | 0 | 0 | 8 | 7.18 | 1.11 (0.48-2.20) | 0.10 |
| Female breast | 1 | 0.32 | 5 | 0.67 | 2 | 0.47 | 5 | 3.19§ | 2 | 5.82 | 13 | 16.4 | 0.79 (0.42-1.36) | −0.94 |
| Female genital system | 1 | 0.69 | 3 | 0.88 | 4 | 2.05 | 1 | 1.35 | 1 | 6.17 | 9 | 7.56 | 1.19 (0.54-2.26) | 0.40 |
| Cervix | 1 | 1.64 | 3 | 2.16 | 0 | 0 | 1 | 3.74 | 1 | 18.9 | 5 | 3.03 | 1.65 (0.54-3.85) | 0.54 |
| Uterine corpus | 0 | 0 | 0 | 0 | 2 | 3.96 | 0 | 0 | 0 | 0 | 2 | 1.9 | 1.05 (0.13-3.80) | 0.03 |
| Testis | 0 | 0 | 2 | 1.33 | 0 | 0 | 1 | 3.19 | 0 | 0 | 3 | 3.35 | 0.90 (0.18-2.62) | −0.07 |
| Melanoma, skin | 4 | 3.75§ | 9 | 3.86§ | 2 | 1.58 | 3 | 5.79§ | 0 | 0 | 18 | 5.18 | 3.47§ (2.06-5.49) | 1.5 |
| Brain, CNS | 3 | 4.47 | 3 | 2.11 | 11 | 15.54§ | 1 | 4.30 | 0 | 0 | 18 | 3.03 | 5.94§ (3.52-9.39) | 1.75 |
| Thyroid | 3 | 5.42§ | 4 | 3.23 | 4 | 5.82§ | 5 | 17.76§ | 0 | 0 | 16 | 2.76 | 5.79§ (3.31-9.41) | 1.55 |
| Bones, joints | 0 | 0 | 3 | 8.98§ | 2 | 11.95§ | 1 | 20.46 | 1 | 126.46§ | 6 | 0.71 | 8.50§ (3.10-18.39) | 0.62 |
| Soft tissue | 1 | 4.25 | 2 | 4.03 | 4 | 15.72§ | 0 | 0 | 0 | 0 | 7 | 1.08 | 6.50§ (2.61-13.36) | 0.69 |
| Other solid cancers† | 4 | 1.42 | 4 | 0.63 | 3 | 0.88 | 1 | 0.78 | 0 | 0 | 12 | 13.9 | 0.86 (0.45-1.51) | −0.22 |
Includes all invasive second solid cancers excluding nonmelanoma skins; excludes 17 carcinomas in situ (lip [n = 1], rectum [n = 2], lung [n = 1], melanoma [n = 5], breast [n = 2], cervix [n = 3], vulva [n = 2], penis [n = 1]), and 19 invasive squamous cell skin cancers. Histologies of special interest: leiomyosarcoma of the small intestine (n = 1), angiosarcomas of the liver (n = 2) and fibrous histiocytomas of the liver (n = 2), high-grade sarcoma of the lung (n = 1), desmoplastic round cell tumor of the uterus (n = 1), and rhabdomyosarcoma of the cervix (n = 1). Two patients developed Kaposi sarcoma (1 of the digestive organs and the other of skin of the leg). The 4 cancers of the salivary gland were mucoepidermoid carcinomas. Two patients whose indication for HCT was neuroblastoma developed new second malignancies (1 bladder cancer, 1 thyroid cancer). The number of patients (person-years at risk [PYR]) for males was 17 122 (49 331 PYR) and for females was 11 752 (36 252 PYR).
Other solid cancers included leiomyosarcoma of the small intestine (n = 1), Kaposi sarcoma (n = 2), neuroendocrine cancer of the pancreas/duodenum (n = 1), neuroblastoma of nose/nasal cavities (n = 1), cancers of the prostate (n = 1), penis (n = 1), bladder (n = 1), kidney (n = 1), and metastatic carcinoma of unknown primary site (n = 3).
EAR is calculated by the number of observed cases minus the expected cases per 10 000 person-years at risk.
P < .05.
Time since transplantation played a significant role in the development of second invasive solid malignancies, as shown in Table 2. Risks increased from 1.3-1.6 times that expected in the first 5 years of follow-up to reach O/E = 4.55 (95% CI, 3.09-6.46) among survivors of 10 or more years (P trend < .001). Risks remained high, at a 3-fold level, among the 378 patients who were followed for 15 or more years after transplantation (O/E, 3.28; 95% CI, 1.06-7.68). EARs increased from 2.3 excess solid cancers per 10 000 person-years for the first year of follow-up to 4.6, 16.6, and 40.0 excess solid cancers per 10 000 person-years for the intervals of 1 to 4, 5 to 9, and 10 or more years after transplantation, respectively (P trend < .001; data not shown). Although numbers were often small, risks tended to rise over time for cancers of the oral cavity, liver, and thyroid and for sarcomas of the bone and soft tissue; however, risks for melanoma of the skin appeared nearly constant over the follow-up intervals. Particularly noteworthy was the 3-fold increase in breast cancer risk among survivors for 10 or more years, based on 5 cases. Long-term survivors also had significantly elevated (20- to 30-fold) risks of bone sarcomas and cancers of the oral cavity and liver, and a 6-fold excess risk of melanoma during 10 or more years of follow-up.
Risk of invasive solid cancer was strongly related to both age of the recipient at HCT and exposure to radiation (TBI or LFI) as part of the conditioning regimen (Table 3). Overall, irradiated patients who survived at least 1 year after transplantation had a significantly higher risk of developing a second invasive solid cancer than nonirradiated patients (O/E, 2.68 vs 1.26, respectively; P = .001). Children irradiated at ages younger than 10 years had a particularly high 55-fold increase in the risk of developing a solid cancer, based on 30 observed tumors. Risk remained significantly elevated at 4- to 6-fold for recipients irradiated at ages 10 to 19 and 20 to 29 years, however, no excess risk was evident for those conditioned with radiation at ages of 30 years or older (P < .001 for the trend in O/E by age at HCT). A similar decreasing trend with older age at irradiation was observed when risk was measured in absolute terms (P < .001 for trend in EAR by age). In contrast, among nonirradiated patients there was no similarly strong evidence of a declining trend in O/E ratios with increasing age (P = .11 for trend in O/E by age at HCT), based on a small numbers of cases. Only a marginal evidence of difference by primary disease was detected among those not receiving radiation (P = .06 for heterogeneity of O/Es). After adjusting for latency, primary disease and transplant registry, the decline in risk of solid malignancies with increasing age was significantly steeper among those who were irradiated than those not irradiated (P < .001 for difference in age trends). Similar patterns in risk by age and radiation exposure were found for long-term survivors (> 5 years), although comparisons were limited by sparse numbers in the nonirradiated group.
Table 3.
Risk of invasive solid cancers according to age at transplantation, conditioning with radiation (TBI or LFI), and time since HCT
| Age at HCT, y | No conditioning radiation |
Conditioning radiation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of pts | O | E | O/E | 95% CI | EAR* | No. of pts | O | E | O/E | 95% CI | EAR* | |
| ≥ 1-y survivors | ||||||||||||
| < 10 | 1092 | 1 | 0.39 | 2.59 | 0.06-14.29 | 1.22 | 1 401 | 30 | 0.54 | 55.29‡ | 37.5-79.31 | 43.2 |
| 10-19 | 1092 | 4 | 0.98 | 4.10‡ | 1.11-10.45 | 5.86 | 2 167 | 13 | 2.09 | 6.23‡ | 3.31-10.64 | 9.70 |
| 20-29 | 1048 | 2 | 2.10 | 0.95 | 0.12-3.44 | −0.24 | 2 539 | 30 | 6.23 | 4.81‡ | 3.25-6.87 | 19.05 |
| 30-39 | 939 | 5 | 3.99 | 1.25 | 0.41-2.92 | 3.25 | 2 399 | 20 | 13.70 | 1.46 | 0.89-2.25 | 6.09 |
| 40-49 | 681 | 4 | 5.22 | 0.77 | 0.21-1.96 | −6.94 | 1 335 | 18 | 15.80 | 1.14 | 0.68-1.80 | 4.45 |
| ≥ 50 | 189 | 4 | 3.20 | 1.25 | 0.34-3.20 | 17.79 | 224 | 3 | 4.18 | 0.72 | 0.15-2.10 | −20.36 |
| All ages | 5041 | 20 | 15.88 | 1.26 | 0.77-1.95 | 2.10 | 10 065 | 114 | 42.53 | 2.68‡ | 2.21-3.22 | 15.39 |
| P trend† | .11 | .79 | < .001 | < .001 | ||||||||
| ≥ 5-y survivors | ||||||||||||
| < 10 | 524 | 1 | 0.17 | 5.73 | 0.15-32.77 | 4.42 | 676 | 23 | 0.29 | 80.28‡ | 50.3-119 | 76.40 |
| 10-19 | 482 | 3 | 0.63 | 4.75 | 0.98-13.92 | 10.91 | 1 114 | 11 | 1.33 | 8.25‡ | 4.13-14.8 | 18.81 |
| 20-29 | 405 | 1 | 1.13 | 0.88 | 0.02-4.93 | −0.87 | 1 260 | 18 | 3.63 | 4.96‡ | 2.94-7.84 | 26.86 |
| 30-39 | 291 | 2 | 1.57 | 1.27 | 0.15-4.60 | 5.29 | 1 079 | 13 | 6.99 | 1.86 | 0.99-3.18 | 15.53 |
| 40-49 | 171 | 0 | 1.08 | 0 | 0-3.42 | −40.14 | 528 | 8 | 6.51 | 1.23 | 0.53-2.42 | 9.63 |
| ≥ 50 | 41 | 1 | 0.56 | 1.8 | 0.05-9.95 | 75.38 | 52 | 1 | 1.19 | 0.84 | 0.02-4.68 | −16.29 |
| All ages | 1914 | 8 | 5.15 | 1.55 | 0.67-3.06 | 4.26 | 4 709 | 74 | 19.9 | 3.71‡ | 2.91-4.66 | 28.46 |
| P trend† | .06 | .53 | < .001 | < .001 | ||||||||
pts indicates patients.
EAR was calculated as [(O-E)/PYR] × 10 000. (Analyses excluded patients with unknown radiation).
P for trend of increasing risk with increasing age at transplantation. P for test of difference in age trends for radiation versus no radiation, adjusted for primary disease, registry, and time since transplantation: P < .001 for 1+ year survivors, and P = .09 for 5+ year survivors.
P < .05.
Additional radiation exposure may also have contributed to the occurrence of a new malignancy, since 22 of the 134 second, invasive, solid cancers developing among survivors for 1 year or more were known to have received additional radiation to a field that was at or near to the site of the solid tumor. Among the 114 patients with second solid cancers who had received radiation as part of the conditioning regimen, 16% also had pretransplantation radiation exposure to the site of second cancer, most frequently these were head and neck cancers following cranial irradiation for acute leukemia (second cancers: 9 brain/CNS, 3 thyroid, 3 oral cavity). In the transplant cohort that did not receive conditioning radiation, 3 patients with second solid cancers (of the esophagus, tongue, and bone/hip, respectively) had pretransplantation radiotherapy to the sites involved by secondary tumor, and one additional patient received posttransplantation thoracoabdominal irradiation for chronic GVHD before the diagnosis of second colon cancer.
Multivariate models were used to identify risk factors for solid cancers among patients who survived at least 1 year after HCT (Table 4). Comparisons within the cohort were based on 165 cases of solid cancers, including 13 cases of non-skin carcinomas in situ and 18 invasive SCCs of the skin. For all solid tumors combined, TBI conditioning regimens increased the risk of second solid malignancies by 1.8-fold compared with nonradiation regimens. Significant elevations in risk were also found for conditioning with LFI (relative risk [RR], 2.95), and the development of moderate to severe chronic GVHD (RR, 1.55). There was no apparent effect on risk of all solid tumors combined by the use of grafts from donors with 2 or more mismatched HLA, the occurrence of or therapy for acute GVHD grades II to IV, T-cell depletion of the donor bone marrow, or chemotherapy (nonradiation) conditioning regimens (busulfan and cyclophosphamide [BUCY] vs non-BUCY regimens).
Table 4.
Risk factors for second solid cancers, according to time since transplantantion and type of solid cancer, among ≥ 1 year survivors of HCT
| Variable | All solid cancers |
Non-SCC cancers |
SCC cancers |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. cases | RR | 95% CI | No. cases | RR | 95% CI | No. cases | RR | 95% CI | |
| General risk factors, ≥ 1-y survivors | n = 165 cases | n = 110 cases | n = 55 cases | ||||||
| TBI | 125 | 1.75† | 1.06-3.00 | 93 | 2.29† | 1.18-4.88 | 32 | 1.11 | 0.51-2.53 |
| LFI, no TBI | 9 | 2.95† | 1.13-7.35 | 4 | 3.94 | 0.92-14.77 | 5 | 2.24 | 0.62-7.95 |
| Chronic GVHD* | 56 | 1.55† | 1.10-2.16 | 21 | 0.70 | 0.41-1.12 | 35 | 5.04† | 2.90-9.00 |
| Risk factors by time after HCT | |||||||||
| 1-4 years after HCT | n = 65 cases | n = 48 cases | n = 17 cases | ||||||
| TBI | 47 | 1.37 | 0.75-2.66 | 37 | 1.63 | 0.78-3.81 | 10 | 0.96 | 0.33-3.09 |
| LFI, no TBI | 2 | 2.08 | 0.25-12.40 | 1 | 1.38 | 0.06-11.91 | 1 | 6.52 | 0.13-150 |
| Chronic GVHD* | 25 | 1.81† | 1.06-3.03 | 13 | 1.06 | 0.52-2.01 | 12 | 6.79† | 2.44-21.78 |
| ≥ 5 y after HCT | n = 100 cases | n = 62 cases | n = 38 cases | ||||||
| TBI | 78 | 2.74† | 1.17-6.82 | 56 | 6.33† | 1.58-35.52 | 22 | 1.33 | 0.43-4.33 |
| LFI, no TBI | 7 | 3.80† | 1.24-11.57 | 3 | 11.14† | 1.62-76.57 | 4 | 2.17 | 0.52-8.53 |
| Chronic GVHD* | 31 | 1.38 | 0.87-2.12 | 8 | 0.45† | 0.20-0.91 | 23 | 4.38† | 2.26-8.5.72 |
Multivariate Poisson regression models were based on 165 solid cancers, including 18 invasive SCCs of the skin and 13 nonskin carcinomas in situ; analyses were stratified by registry (FHCRC, CIBMTR), age (<10, 20-29, 30-39, 40-49, ≥ 50 y), latency (1 to < 5, 5 to < 10, 10 to < 15, ≥ 15 y after HCT), and primary disease (ALL, ANLL, CML, SAA, other). Analyses exclude patients with unknown radiation/unknown radiation dose.
Risk factors for three groups of second cancers are formed based on results from Curtis et al.1 (1) All solid cancers as defined in the previous note, (2) nonsquamous cell invasive solid cancers and nonskin carcinomas in situ, and (3) SCCs (including invasive SCCs of the skin but excluding in situ carcinomas of the skin). Male sex was an additional risk factor for the SCCs (data not shown above): survivors for 1 year or longer, RR was 2.32 (95% CI, 1.29-4.46); 1-4 years after HCT, RR, 1.16 (95% CI, 0.44-3.20); 5 or more years after HCT, RR, 3.52 (95% CI, 1.61-8.80).
Chronic GVHD was moderate or severe grade in CIBMTR patients, or clinically extensive in FHCRC patients.
P < .05.
Risk factors for developing a solid tumor differed substantially by solid tumor type. Radiation (TBI or LFI) given for the conditioning regimen was a key risk factor only for non-SCCs (TBI: RR, 2.29; LFI: RR, 3.94), while the increased risk associated with chronic GVHD was limited to SCCs (RR, 5.04). Male sex was a significant risk factor only for SCC when added to the model shown in Table 4 (RR, 2.32, 95% CI, 1.25-4.31, data not shown). There was no significant elevation in risk related to conditioning radiation in the interval of 1 to 4 years after transplantation, however, the use of TBI and LFI was strongly associated with non-SCCs among survivors for 5 or more years (TBI: RR, 6.33, LFI: RR, 11.14). Significant elevations in risk of SCC following chronic GVHD were seen in both the early and late follow-up intervals (1-4 years: RR, 6.79; ≥ 5 years: RR, 4.38).
While use of TBI was associated with an increased risk of non-SCCs after HCT, there was no clear association of increasing risk with increasing TBI dose. Compared with nonirradiated patients surviving 1 year, the RR for single dose TBI was 3.35 (95% CI, 1.46-7.69) for 10 Gy or more and 1.22 (95% CI, 0.41-3.70) for less than 10 Gy; for fractionated TBI this RR was 2.37 (95% CI, 0.98-5.77), 2.45 (95% CI, 1.14-5.28), and 2.18 (95% CI, 0.91-5.17) for less than 12 Gy, 12 Gy, and 13 Gy or more, respectively (data not shown). In separate analyses limited to patients receiving TBI, there was no evidence that those treated with higher radiation doses had greater risks than patients receiving lower doses (single dose: P trend = .34; fractionated dose: P trend > .50). The TBI dose-response relationships followed the same general patterns when the analysis was limited to survivors for 5 or more years.
Additional analyses assessed the interaction between various risk factors for new solid cancers and age at HCT (Table 5). Patients who underwent either TBI or LFI at young ages (< 30 years) had a 9- to 10-fold increase in risk of non-SCCs compared with nonirradiated patients. On the other hand, there was little evidence of an elevated risk associated with radiation treatments given at older ages (RR, 1.08 for TBI at ages ≥ 30 years), indicating a significant interaction in risk between age at transplantation and TBI exposure (P = .01). In a markedly different pattern, there was no association between SCC risk and radiation exposure for either younger or older patients (TBI: RR, 1.12 for ages < 30 years vs RR, 1.08 for ages ≥ 30 years at HCT). Further, risk of SCC associated with chronic GVHD did not vary significantly by age at HCT (RR, 6.34 for ages < 30 years vs RR, 4.07 for ages ≥ 30 years; P = .50 for interaction of age at transplantation and chronic GVHD). Although the radiation effect was higher for patients who were young at HCT, the main effect of age followed the reverse pattern with the highest risks for older patients. In an analysis where a continuous age variable replaced the age strata (see “Methods”), the risk of solid cancer increased with increasing age at HCT (P trend < .001).
Table 5.
Risk factors for second solid cancers, according to age at transplantation and type of solid cancer, among survivors for 1 or more years after HCT
| Variable | All solid cancers |
Non-SCCs |
SCCs |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. cases | RR | 95% CI | No. cases | RR | 95% CI | No. cases | RR | 95% CI | |
| Age < 30 y at HCT | n = 98 | n = 67 | n = 31 | ||||||
| No radiation | 14 | 3 | 11 | ||||||
| TBI | 78 | 3.34† | 1.51-7.93 | 62 | 8.87† | 2.51-45.25 | 16 | 1.12 | 0.33-3.83 |
| LFI, no TBI | 6 | 3.63† | 1.11-11.42 | 2 | 10.40† | 1.27-85.02 | 4 | 1.80 | 0.43-6.97 |
| Chronic GVHD* | 28 | 1.39 | 0.87-2.16 | 8 | 0.46† | 0.20-0.93 | 20 | 6.34† | 3.04-13.92 |
| Age ≥ 30 y at HCT | n = 67 | n = 43 | n = 24 | ||||||
| No radiation | 17 | 10 | 7 | ||||||
| TBI | 47 | 1.09 | 0.58-2.15 | 31 | 1.08 | 0.49-2.60 | 16 | 1.08 | 0.40-3.40 |
| LFI, no TBI | 3 | 3.11 | 0.53-16.06 | 2 | 2.58 | 0.31-15.74 | 1 | 7.21 | 0.15-164 |
| Chronic GVHD* | 28 | 1.72† | 1.02-2.86 | 13 | 1.01 | 0.49-1.97 | 15 | 4.07† | 1.75-9.96 |
Multivariate Poisson regression models were based 165 solid cancers including 18 invasive SCCs of the skin and 13 nonskin carcinomas in situ; analyses were stratified by registry (FHCRC, CIBMTR), age (< 10, 20-29, 30-39, 40-49, ≥ 50 y), latency (1 to < 5, 5 to < 10, 10 to < 15, ≥ 15 y after HCT), and primary disease (ALL, ANLL, CML, SAA, other). Analyses excluded patients with unknown radiation or unknown radiation dose.
Chronic GVHD was moderate or severe grade in CIBMTR patients, or clinically extensive in FHCRC patients.
P < .05.
Risk of solid malignancies varied by disease for which HCT was performed. Patients transplanted for CML had significantly lower risks of solid cancers than those with acute leukemia (RR, 0.56; 95% CI, 0.36-0.84), while patients with SAA had marginally lower risks (RR, 0.83; 95% CI, 0.43-1.56), as did those with other primary diseases (RR, 0.66; 95% CI, 0.37-1.13). Risk of SCC following chronic GVHD was significantly lower for patients with acute leukemia (RR, 2.16; 95% CI, 0.84-5.28) than those with other primary diseases (RR, 9.51; 95% CI, 4.44-22.75; P = .015 for interaction). There was no evidence of a statistically significant interaction in risk of solid cancers (either overall or for non-SCC) between conditioning radiation (TBI or TLI) and primary disease.
Table 6 provides the results for risk factors for specific solid cancer sites. Although caution in interpretation is required for RR based on small numbers (< 5 cases), some broad patterns were evident. Radiation (TBI or TLI) given as part of the conditioning regimen was strongly associated with a significantly increased risk of cancers of the thyroid, bone and connective tissue, brain, and female breast as well as melanoma of the skin; conditioning with LFI alone heightened the risk of SCC of the oral cavity. Depletion of T cells in the donor marrow was related to a significantly elevated risk of cutaneous melanoma. Male sex and chronic GVHD were strong risk factors for SCC of the skin and SCC of the oral cavity, while female sex was associated with melanoma.
Table 6.
Risk factors for second solid cancers, by site of second cancer, among survivors for 1 or more years after HCT
| Variable | Thyroid (n = 13) |
Bone/connective tissue (n = 12) |
Brain, CNS (n = 15) |
Female breast (n = 13) |
Melanoma of the skin (n = 18) |
SCC skin (n = 18) |
SCC oral cavity (n = 24) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | RR | No. | RR | No. | RR | No. | RR | No. | RR | No. | RR | No. | RR | |
| Radiation conditioning | 12 | 5.7‡ | 11 | 5.9‡ | 15 | ∞‡§ | 12 | 5.8‡ | 18 | ∞‡§ | 8 | 0.4 | 17 | 0.8 |
| LFI | 1 | 1.7 | 0 | 0.0 | 0 | 0.0 | 1 | 4.6 | 0 | 0.0 | 1 | 1.8 | 4 | 4.7‡ |
| T-cell dep. | 1 | 1.2 | 2 | 2.3 | 0 | 0.0 | 0 | 0.0 | 6 | 3.5‡ | 1 | 0.7 | 2 | 1.0 |
| Male sex | 4 | 0.3 | 6 | 0.8 | 12 | 3.0 | NA | NA | 5 | 0.3‡ | 17 | 11.9‡ | 19 | 2.8‡ |
| Acute GVHD* | 4 | 1.0 | 2 | 0.5 | 6 | 1.7 | 1 | 0.2‡ | 5 | 0.9 | 8 | 2.0 | 8 | 1.1 |
| Chronic GVHD† | 1 | 0.3 | 1 | 0.3 | 1 | 0.3 | 3 | 0.8 | 2 | 0.4 | 14 | 11.0‡ | 16 | 5.3‡ |
| HLA mis/unrel | 1 | 0.6 | 1 | 0.7 | 3 | 1.6 | 1 | 0.9 | 2 | 1.0 | 1 | 0.6 | 1 | 0.5 |
| ATG | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 | 2 | 3.3 | 1 | 1.0 | 1 | 0.7 | 2 | 1.3 |
Analyses were based 165 solid cancers including 18 invasive squamous cell carcinomas (SCC) of the skin and 13 nonskin carcinomas in situ. Poisson regression models were stratified by age (< 10, 20-29, 30-39, 40-49, ≥ 50 y), and latency (1 to < 5, 5 to < 10, 10 to < 15, ≥ 15 y after HCT); patients with unknown radiation or unknown radiation dose are excluded. The table presents univariate relative risks for variables with P > .05, and multivariate relative risks, adjusted for other significant risk factors, for variables that are statistically significant. No. indicates number of observed cases of second cancers; dep, depletion; HLA mis/unrel, 2 or more HLA antigen mismatches with a sibling, related, or unrelated donor; ATG, antithymocyte globulin to prevent or to treat acute GVHD; and NA, not applicable.
Acute GVHD was grades II-IV.
Chronic GVHD was moderate or severe grade in CIBMTR patients, or clinically extensive in FHCRC patients.
P < .05.
An RR of ∞ indicates a confidence interval with a lower limit greater than 1.0 but an upper limit that is unbounded or indeterminate (∞), because all cases were exposed to the risk factor of interest.
Discussion
This is the largest study to date to evaluate the risk of all solid cancers after allogeneic HCT and included more than 28 000 patients and 189 cases of new cancers. The expansion of our earlier study6 increased our numbers for 5-year HCT survivors to more than 6000, enabling us to better characterize the risk of cancer and the joint effects of risk factors in a population of patients who are fortunate enough to enjoy long-term survival after HCT. Our results showed that the risk of non-SCCs was strongly linked to radiation conditioning regimens received at a young age (particularly those younger than 10 years old), and that this risk increased with time from HCT. In contrast, the risk of SCCs was related to chronic GVHD and male sex, with little variation in risk by age at HCT and follow-up time. Because of the multi-institutional design of the cohort and the large number of transplantation teams included worldwide, our results are broadly representative of the second solid cancer experience of HCT patients from this period.
In new findings, the risk of developing a non-SCC solid tumor was found to be highly dependent on both age at transplant and the use of radiation in the conditioning regimen. Using multivariate analyses we found that the risk among patients irradiated at ages under 30 years was nearly 10-times that of nonirradiated patients, while the comparable radiation-related risk for older patients (ages ≥ 30 years) was 1.1. The interaction of age at HCT and TBI exposure was highly statistically significant, emphasizing the heightened susceptibility of children and young adults to the carcinogenic effects of radiation. More detailed comparisons using stable general population rates (O/E ratios), showed a steep declining trend in radiation-related solid cancer risk with increasing age among irradiated patients, with a much less downward trend observed among nonirradiated patients. Further, a highly significant difference between the age trends was found in a model adjusting for follow-up time and primary disease. To our knowledge, this is the first study of risk of solid cancers following HCT to assess the role of radiation exposure by age at irradiation in multivariate analyses accounting for confounding variables. Earlier reports6,9 found sharply higher O/E ratios for patients who received transplants at young ages; however, there were few long-term survivors receiving conditioning regimens without radiation. Reflecting the typical rise in cancer background rates with increasing age, our results from multivariate analyses confirmed previous investigations3,5,8,9 in finding that the risk of solid tumors after HCT was highest among those transplanted at older ages.
Radiation was a significant risk factor for the development of several non-SCCs, particularly cancers of the breast, thyroid, brain and CNS, bone and connective tissue, and melanoma. Conditioning with limited field irradiation was associated with an increased risk of SCC of the oral cavity, with most of the excess attributed to thoracoabdominal irradiation among patients who were treated for SAA at a single center. For the majority of these sites, risks were greater among those who survived 5 or more years after initial radiotherapy, in keeping with the latent period typical for radiation-related solid cancers.22 These findings are consistent with prior studies that evaluated the risks of radiation-induced solid cancers among cancer survivors23–38 as well as atomic bomb survivors and other radiation-exposed populations.22,39,40 Our finding of a nearly 6-fold increased risk for radiation-associated breast cancer after HCT confirms a recent report linking breast cancer excesses with young age at transplantation (younger than 18 years) and use of TBI, with the risk increasing sharply among those followed for 10 or more years.7 Radiation-related thyroid cancers have been described in cohorts of HCT survivors, with an elevated risk associated with young age, radiation exposure, female sex, and chronic GVHD.12 We also found a strong association of radiation exposure with increased risk of CNS and bone and soft tissue sarcomas, confirming our previous study6 and investigations of childhood cancer survivors treated with radiotherapy,27,32–34 which reported younger age at initial diagnosis (CNS, sarcomas) and higher doses of anthracyclines or alkylating agents (sarcomas) as strong risk factors. Four cases of rare liver sarcomas (2 angiosarcomas, 2 fibrous histiocytomas) occurred in patients exposed to radiation, 2 with latency periods longer than 10 years after HCT, further underscoring the risk of radiation. Increased risks of cutaneous melanoma in the current study were associated with radiation, T-cell depletion, and a short latent period (< 1 year), suggesting a dual role for immunosuppression and radiation for this skin cancer.
Both the relative risk and absolute excess risk of developing a new solid tumor increased significantly with increasing follow-up in our current study (P for trend < .001), with O/E ratios remaining increased at a 3-fold level among survivors for 15 years or longer. Some of this excess risk, particularly in the early follow-up years, may be related to the increased medical surveillance received by transplant recipients. Our estimate of cumulative incidence (3.3% at 20 years) using newer competing risk methods was similar to that of an earlier University of Minnesota study (3.8% at 20 years) based on both allogeneic and autologous transplant recipients,9 but lower than previous incidence estimates based on Kaplan-Meier statistics.3,8,10 Our report provided new information on the latency patterns for radiation-related new solid cancers after HCT in analyses adjusting for confounding variables. We found little evidence that conditioning radiation increased the risk of non-SCC in the early follow-up period, but radiation-related risk rose to more than 6-fold among 5-year survivors.
Chronic GVHD and immunosuppression appear to be important risk factors only for certain types of second solid malignancies. Overall, development of chronic GVHD was associated with a 5-fold increase in risk of SCC, confirming our earlier findings.1,6 A particularly strong risk was observed in the 1- to 4-year interval after HCT, which remained elevated at high levels among long-term survivors. Our expanded study demonstrated a significant interaction of GVHD and primary disease affecting SCC risk, with lower risks for those transplant recipients with acute leukemia. TBI did not heighten the risk of SCCs, for either younger or for older transplant recipients. Duration of immunosuppression (including therapy for chronic GVHD), and particular immunosuppressant agents have been correlated with second cancers in previous analyses, especially squamous cell malignancies.1,4,6,10–12
In contrast to our previous study6 and an earlier FHCRC investigation,5 no clear dose effect of radiation was demonstrated in the current cohort among those who received fractionated TBI as part of the conditioning regimen. Compared with nonirradiated patients, patients who underwent fractionated TBI had a relatively constant 2-fold higher risk of solid cancer with radiation doses ranging from less than 12 Gy to more than 15 Gy, providing little evidence of a substantial upturn in risk at higher doses.
Important strengths of our study include its large size, the high completeness of follow-up, and the diverse international cohort including more than 270 centers. However, our investigation is limited by not including patients undergoing transplantation in recent calendar years. Our study period reflects an era when radiation was a mainstay of conditioning (< 30% not exposed to radiation conditioning), and the graft source was bone marrow. Risk of radiation-related malignancies and other long-term adverse effects of radiation, particularly effects on growth, mental development, and fertility in children have led clinicians to the increasing use of nonradiation conditioning regimens, and reduced doses of radiation for myeloablative transplantation.41 Other changes include widespread use of reduced-intensity preparative regimens,41 in which radiation doses are less than 5 Gy or are avoided altogether, and fractionation of myeloablative TBI dosing. In addition, use of peripheral blood stem cells as the graft source has been increasing, despite their association with increased risk of chronic GVHD.42 The impact of such changes on development of second cancers in HCT recipients is yet to be determined since reduction in exposure to radiation as part of the preparative regimen may have a less-than-anticipated reduction in risk of second solid cancers. A recent case report of skin cancers following nonmyeloablative HCT suggests that the risk of second malignancies persists in this group of patients.43 Future studies should evaluate contributions to the risk of second malignancies among transplant conditioning regimens that do not include radiation, and where peripheral blood is the source of hematopoietic stem cells.
Several of the patients in our study who subsequently developed second solid cancers were known to have been exposed to local field radiation and other carcinogenic therapies before transplantation. Changes to the treatment regimens for malignant diseases over the last decade may have diminished the excess cancer risk experienced by transplant recipients.38 The impact of such changes on development of second cancers in HCT recipients has yet to be determined, and future studies will need to evaluate the late effects of therapy with these new treatment approaches.
HCT can be curative for hematologic malignancies and nonmalignant disorders, and the benefits for patients with these conditions generally outweigh the risk of second malignancies. However, these data substantiate the significant risk of developing second solid malignancies in HCT survivors, with emergence of additional types of cancer such as breast cancer occurring 10 or more years after transplantation, a time period when the full effect of radiation-induced cancers would be emerging. Our study shows that when feasible, efforts to reduce radiation exposure in children and young adults, either during conventional therapy or pretransplantation conditioning should continue. In addition, transplant physicians and community practitioners must remain vigilant for the development of second malignancies in transplant recipients and should encourage high compliance with age-appropriate cancer screening and enhanced early detection strategies, and avoidance of carcinogenic exposures. Physicians should continually reassess patients' requirements for immunosuppression, given the risk of second cancers and other complications associated with long-term immunosuppression. The role of community physicians should not be underestimated, as they are increasingly called upon to provide long-term follow-up care of transplantation survivors. Whether changes in transplant care over the last decade—including increasing use of peripheral blood as the source of hematopoietic cells, decreasing use and doses of radiation, increasing transplantation in older patients, changes in posttransplantation immunosuppression and exposure to new chemotherapeutics—will modify the overall risk of second cancers faced by transplantation survivors remains an important research question.
Acknowledgments
We thank all the dedicated physicians and data professionals at participating transplantation centers who contributed data to this study. We thank the Long-Term Follow-Up staff and Gary Schoch from FHCRC, Seattle, WA and Diane Knutson and Sharon Nell from the CIBMTR, Milwaukee, WI for support in data collection. We acknowledge Linda Kaufman, Kathy Chimes, and Diane Fuchs from Westat for coordination of field studies, and Nathan Appel, David Hacker, Heather Bath, and George Geise from Information Management Services for computing support. We give special thanks to Sandy Sobotka for her able assistance in manuscript preparation.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen; an anonymous donation to the Medical College of Wisconsin; the Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US; Baxter International; Bayer HealthCare Pharmaceuticals; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; the Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene Corporation; CellGenix; the Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex; CytoTherm; DOR BioPharma; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals; the European Group for Blood and Marrow Transplantation; Gambro BCT; Gamida Cell; Genzyme Corporation; Histogenetics; HKS Medical Information Systems; Hospira; the Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co.; Merck & Company; The Medical College of Wisconsin; MGI Pharma; Michigan Community Blood Centers; Millennium Pharmaceuticals; Miller Pharmacal Group; Milliman USA; Miltenyi Biotec; the National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; the Oncology Nursing Society; Osiris Therapeutics; Otsuka Pharmaceutical Development & Commercialization; Pall Life Sciences; PDL BioPharma; Pfizer; Pharmion Corporation; Saladax Biomedical; Schering Plough Corporation; the Society for Healthcare Epidemiology of America; StemCyte; StemSoft Software; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS; Vidacare Corporation; Vion Pharmaceuticals; ViraCor Laboratories; ViroPharma; and Wellpoint.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.E.C., H.J.D., K.A.S., M.M.H., and G.S. designed research; R.E.C., K.A.S., M.M.H., H.J.D., and M.E.D.F. collected data; J.D.R., R.E.C., E.G., K.A.S., and O.L. performed statistical analysis; all the authors interpreted data; R.E.C. and J.D.R. drafted the manuscript; and all the authors critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Douglas Rizzo, Center for International Blood and Marrow Transplantation Research, Medical College of Wisconsin, 9200 W Wisconsin Avenue, Suite C5500, Milwaukee, WI 53226; e-mail: rizzo@mcw.edu.
References
- 1.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socie G, Curtis RE, Deeg HJ, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18:348–357. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher G, Forrest DL. Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. 2007;109:84–92. doi: 10.1002/cncr.22375. [DOI] [PubMed] [Google Scholar]
- 4.Shimada K, Yokozawa T, Atsuta Y, et al. Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant. 2005;36:115–121. doi: 10.1038/sj.bmt.1705020. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DL, Leisenring W, Schwartz JL, Deeg HJ. Second malignant neoplasms following hematopoietic stem cell transplantation. Int J Hematol. 2004;79:229–234. doi: 10.1532/ijh97.03178. [DOI] [PubMed] [Google Scholar]
- 6.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DL, Rovo A, Leisenring W, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: a report from the FHCRC and the EBMT-Late Effect Working Party. Blood. 2008;111:939–944. doi: 10.1182/blood-2007-07-099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolb HJ, Socie G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation: Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131:738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 9.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 11.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A, Rovelli A, Merlo DF, et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT Late Effects Working Party Study. J Clin Oncol. 2007;25:2449–2454. doi: 10.1200/JCO.2006.08.9276. [DOI] [PubMed] [Google Scholar]
- 13.Ades L, Mary JY, Robin M, et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004;103:2490–2497. doi: 10.1182/blood-2003-07-2546. [DOI] [PubMed] [Google Scholar]
- 14.Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2003. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 15.Parkin DM, Muir CS, Whelan SL, Gao YT, Ferlay J, Powell J. Cancer Incidence in Five Continents. Vol VI. Lyon, France: International Agency for Research on Cancer; 1992. (IARC scientific publications no. 120) [Google Scholar]
- 16.Muir CS, Waterhouse J, Mack T, Powell J, Whelan S. Cancer Incidence in Five Continents. Vol V. Lyon, France: International Agency for Research on Cancer; 1987. (IARC scientific publilcations no. 88) [Google Scholar]
- 17.Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol II. Lyon, France: International Agency for Research on Cancer; 1987. The design and analysis of cohort studies. (IARC scientific publications no. 82) [PubMed] [Google Scholar]
- 18.Breslow NE. Elementary methods of cohort ana-lysis. Int J Epidemiol. 1984;13:112–115. doi: 10.1093/ije/13.1.112. [DOI] [PubMed] [Google Scholar]
- 19.Preston DL, Lubin JH, Pierce DA. Epicure user's guide. Seattle, WA: HiroSoft International; 1993. [Google Scholar]
- 20.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.National Research Council of the National Academies (Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation) Health risks from exposure to low levels of ionizing radiation: BEIR VII, Phase 2. Washington, D.C.: National Academies Press; 2006. [PubMed] [Google Scholar]
- 23.Curtis RE, Freedman DM, Ron E, et al. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Academy Press; 2006. pp. 1–492. NIH Publication no 05-5302. [Google Scholar]
- 24.van Leeuwen FE, Travis LB. Second cancers. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 2575–2602. [Google Scholar]
- 25.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 26.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 27.Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973-2002. Int J Cancer. 2007;121:2233–2240. doi: 10.1002/ijc.22827. [DOI] [PubMed] [Google Scholar]
- 28.Dores GM, Metayer C, Curtis RE, et al. Second malignant neoplasms among long-term survivors of Hodgkin's disease: a population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- 29.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol. 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 30.Ronckers CM, Sigurdson AJ, Stovall M, et al. Thyroid cancer in childhood cancer survivors: a detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166:618–628. doi: 10.1667/RR3605.1. [DOI] [PubMed] [Google Scholar]
- 31.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 32.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 33.Jenkinson HC, Winter DL, Marsden HB, et al. A study of soft tissue sarcomas after childhood cancer in Britain. Br J Cancer. 2007;97:695–699. doi: 10.1038/sj.bjc.6603908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007;99:300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bassal M, Mertens AC, Taylor L, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 36.Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297:1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 37.Pui CH, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia S, Sather HN, Pabustan OB, Trigg ME, Gaynon PS, Robison LL. Low incidence of second neoplasms among children diagnosed with acute lymphoblastic leukemia after 1983. Blood. 2002;99:4257–4264. doi: 10.1182/blood.v99.12.4257. [DOI] [PubMed] [Google Scholar]
- 39.Boice JD., Jr . Ionizing radiation. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3rd ed. Vol. 2006. New York, NY: Oxford University Press; pp. 259–293. [Google Scholar]
- 40.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 41.Pasquini M. Center for International Blood and Marrow Transplant Research Newsletter. Vol. 12. 2006. CIBMTR summary slides, 2005 Part 1. pp. 5–8. [Google Scholar]
- 42.Stem Cell Trialists' Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavalier M, Shmalo JA, Yu M, Billings SD, Abonour R, Nelson RP., Jr Skin cancer after nonmyeloablative hematopoietic cell transplantation. Bone Marrow Transplant. 2006;37:1103–1108. doi: 10.1038/sj.bmt.1705362. [DOI] [PubMed] [Google Scholar]