Abstract
OBJECTIVE
This population study examines the relationship between LDL density and persistent albuminuria in subjects with type 1 diabetes at the end of the Diabetes Control and Complications Trial (DCCT).
RESEARCH DESIGN AND METHODS
Subjects were classified as persistently normoalbuminuric (albumin excretion rate [AER] <30 mg/d, n = 1,056), microalbuminuric (AER ≥30–299 mg/day, n = 80), and macroalbuminuric (AER = 300 mg/day, n = 24) based on the last two AER measures.
RESULTS
Triglyceride (P <0.01) and LDL cholesterol (P <0.01) levels were higher in macroalbuminuric subjects compared with normoalbuminuric subjects. Cholesterol distribution by density-gradient ultracentrifugation showed an increase in intermediate-density lipoprotein (IDL) and a shift in peak LDL from buoyant toward more dense particles with progressive albuminuria. In the entire group, there was a significant negative correlation between the peak buoyancy of LDL particles and albuminuria (r = −0.238, P <0.001, n = 1,160). This correlation persisted in the normoalbuminuric DCCT group (r = −0.138, P<0.001, n = 1,056).
CONCLUSIONS
As albuminuria increases in subjects with type 1 diabetes, dyslipidemia occurs with an increase in IDL and dense LDL that may lead to increased cardiovascular disease.
Lipid abnormalities often develop with proteinuria even in the absence of renal insufficiency in patients with and without diabetes (1–6). This dyslipidemia is characterized by higher total, VLDL, intermediate-density lipoprotein (IDL), and LDL cholesterol levels as well as increased triglyceride and apolipoproteinB (apoB) levels. HDL cholesterol was decreased in some studies. Winocour et al. (7) found higher amounts of free and total cholesterol content of IDL1, an IDL subspecies. More recently, Groop et al. (8) found increased IDL mass and a decrease in the lipid:apoB ratio of the VLDL and IDL subfractions. Deighen et al. (9) found higher amounts of small dense LDL in subjects with nondiabetic glomerular disease and AERs >2.5 g/day compared with normal subjects.
The lipid abnormalities seen with proteinuria, both with and without diabetes, have been associated with an increase in cardiovascular disease (CVD). Elevated total and LDL cholesterol levels are well-established risk factors for CVD. In addition, elevated IDL (10), apoB (11), and triglyceride levels (12) and decreased HDL (13) levels have been associated with CVD and are frequently seen in concert with small dense LDL (14).
CVD is a leading cause of morbidity and mortality among diabetic subjects, especially those who are proteinuric (15–18) or microalbuminuric (19,20). Even nondia-betic subjects at the microalbuminuric level may have an increased prevalence of CVD (1). The dyslipidemia that accompanies albuminuria may possibly contribute to the increased risk of CVD in type 1 diabetes.
The Diabetes Control and Complications Trial (DCCT), a multicenter randomized clinical trial, was designed to compare the effects of standard and intensive diabetes therapy on microvascular complications. DCCT subjects were followed for a mean of 6.2 years. This study examines the relationship between persistently albuminuric levels and small dense LDL as well as the dyslipidemia associated with small dense LDL in a large unselected population of subjects with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Study population
The DCCT was a clinical trial involving 1,441 subjects with type 1 diabetes, aged 13–39 years at baseline, who had a 1- to 15-year history of diabetes. Subjects under-went randomization to intensive versus conventional diabetes treatment (21). The average follow-up time was 6.2 years. Subjects in the primary prevention cohort had an AER of <40 mg/day and no evidence of retinopathy. Subjects in the secondary prevention cohort had an AER of <200 mg/day and minimal to moderate nonproliferative diabetic retinopathy at baseline (22). There were no baseline differences in triglyceride, total cholesterol, HDL cholesterol, or calculated LDL cholesterol levels between the two cohorts (23).
Subjects were excluded from the study if they had a total cholesterol level >3 SDs above the mean for sex and age as defined by The Lipid Research Clinics Population Studies Data Book (24), a calculated LDL level >190 mg/dl, cardiovascular abnormalities such as major electro cardiogram abnormalities, symptoms of peripheral vascular disease, a clinical history of CVD, or a body weight >30% above ideal body weight as d e fined by the 1983 Metropolitan Life Insurance Company norms (25). At the time of the final follow-up visit for the DCCT, 1,378 subjects had samples available for density-gradient ultracentrifugation (DGUC) measurement. Of these, 1,299 were Caucasian with complete kidney function (albumin excretion, serum creatinine, and creatinine clearance [CCr]), body measurement (BMI and waist-to-hip ratio), and fasting lipid (triglyceride, total cholesterol, and HDL cholesterol) data. Data from that visit are examined cross-sectionally in this article.
Subjects were classified based on AER as follows: persistent normoalbuminuria (<30 mg/day, n = 1,056), persistent micro albuminuria (30–299 mg/day, n = 80), and persistent macroalbuminuria (≥300 mg/day, n = 24) based on the last two albuminuria levels, and their end-study characteristics were examined (Table 1). A total of 139 subjects could not be classified at a persistent level of albuminuria and were not included in this analysis.
Table1.
Follow-up characteristics of subjects
| Normoalbuminuria (AER <30 mg/day) | Microalbuminuria (AER = 30–299 mg/day) | Macroalbuminuria (AER ≥300 mg/day) | |
|---|---|---|---|
| n | 1,056 | 80 | 24 |
| Intensive/standard treatment | 554/502 | 31/49* | 6/18* |
| M/F | 580/476 | 51/29 | 18/6 |
| 1°/2° | 570/486 | 18/62† | 2/22† |
| Age (years) | 33.6 (33.2–34.0) | 32.8 (31.1–34.6) | 33.0 (30.3–35.6) |
| BMI (kg/m2) | 25.8 (25.6–26.1) | 25.5 (24.7–26.2) | 24.6 (23.4–25.7) |
| AER (mg/day) | 10.0 (9.7–10.4) | 93.6 (78–109)† | 1,931 (1,099–2,764)† |
| CCr (ml/s · 1.73 m−2) | 2.02 (1.98–2.05) | 2.09 (1.98–2.20) | 1.72 (1.45–1.98)*‡ |
| HbA1c (%) | 8.01 (7.90–8.12) | 8.66 (8.12–9.21)* | 8.93 (7.96–9.90)† |
| Serum creatinine (µmol/l) | 76.3 (75.5–77.0) | 77.6 (75.0–80.1) | 107.2 (89.5–124.9)†§ |
| Systolic blood pressure (mm Hg) | 115 (114–116) | 122 (119–125)† | 131 (127–135)†‡ |
| Diastolic blood pressure (mm Hg) | 73.6 (73.1–74.2) | 78.2 (76.6–79.8)† | 84.5 (80.7–88.3)†‡ |
| Triglyceride (mmol/l) | 0.91 (0.88–0.94) | 1.11 (0.98–1.25)* | 1.65 (1.27–2.03)† |
| Total cholesterol (mmol/l) | 4.63 (4.58–4.68) | 4.76 (4.55–4.97) | 5.54 (4.99–6.09)† |
| LDL cholesterol (mmol/l) | 2.89 (2.84–2.93) | 3.01 (2.84–3.18) | 3.64 (3.18–4.09)† |
| HDL cholesterol (mmol/l) | 1.33 (1.31–1.34) | 1.25 (1.18–1.31) | 1.15 (1.05–1.25) |
| ApoAI (mg/dl) | 138 (137–139) | 138 (133–143) | 140 (133–147) |
| ApoB (mg/dl) | 83 (82–84) | 89 (84–94) | 110 (99–121) |
| Lp(a) (mg/dl) | 21 (19–22) | 20.8 (16.1–25.6) | 20.9 (9.0–32.9) |
Data are means (95% CI).
P = 0.05 for comparison with the normoalbuminuric value
P = 0.01 for comparison with the normoalbuminuric value
P = 0.05 for comparison with the microalbuminuric value
P = 0.01 for comparison with the microalbuminuric value.
Chemical analyses
Laboratory parameters measured at the final follow-up of the DCCT after an overnight fast of at least 8 h included total cholesterol, triglyceride, lipoprotein cholesterol distribution by DGUC, apoAI, apoB, lipoprotein( a) [Lp(a)], HbA1c, urinary AER, and CCr. After the serum was separated and stored briefly at −20°C, it was placed on dryice and sent immediately to the DCCT Central Biochemistry Laboratory. Total cholesterol and triglyceride levels were measured by enzymatic methods. HbA1c was measured by high-performance liquid chromatography. AER and CCr were measure d with methods described previously (21,26). The plasma was stored at −70°C before being shipped on dry ice to the Northwest Lipid Research Laboratories (NWLRL, Seattle, WA). At the NWLRL, Lp(a) mass was measured with a monoclonal antibody-based enzyme-linked immunosorbent assay (27). apoB and apoAI were measured by a highly standardized nephelometric system (28,29). Cholesterol distribution was measured by DGUC with a Beckman VTI-65 rotor (Palo Alto, CA) (30,31). In this procedure, 1.0 ml of plasma was mixed with 1.5 ml of NaCl solution (specific gravity [sp.gr.] = 1.006) and 1.5 ml of KBr solution (sp.gr. = 1.21) for a final density of 1.080 g/ml and then overlaid with an additional 9.5 ml of NaCl solution. Samples were ultracentrifuged for 70 min at 65,000 rpm at 10°C to separate the lipoproteins by flotation characteristics. A Brandel tube piercer (BR-184, Gaithersburg, MD) was used to drain the tube from the bottom at a flow rate of 1.0 ml/min. Thirty-eight 0.35-ml fractions were collected with a Pharmacia fraction collector (Piscataway, NJ). This procedure gives a continuous profile of lipoprotein distribution based on flotation characteristics (32). This is the same principle described by Winocour et al. (7) in their study of dyslipidemia in microalbuminuric subjects with type 1 diabetes, although Winocour et al. (7) analyzed pooled sequentially sampled fractions. A cholesterol assay kit (Boehringer Mannheim, Indianapolis, IN) was used to measure the cholesterol content in each fraction. The cholesterol content contained in each fraction was expressed as a percentage of total cholesterol across all fractions and was calculated by dividing each fraction by the sum of all fractions and then multiplying by 100%. Relative flotation rate (Rf), a measure of buoyancy, was determined by dividing the fraction number containing the peak LDL cholesterol level among fractions 7–19 by the total number of fractions collected (n = 38).
Statistical analysis
DGUC fractional differences between groups were compared with Student’s t test (Fig. 1–Fig 3). Analysis of variance on ranks was used to compare the serum lipid, lipoprotein, and other follow-up laboratory tests among the study groups by using the Kruskal-Wall is test to compare all three groups simultaneously, followed by Dunn’s test to isolate the groups that differed. Significance levels of 0.05 and 0.01 were used for assessment with Dunn’s test. A χ2 analysis was used to compare categorical characteristics of the subjects such as sex, treatment group, and intervention cohort (primary versus secondary) with albuminuria categories. Multiple linear regression was used to analyze the entire population for the relationship of log10AER with log10triglyceride and total, LDL, and HDL cholesterol levels and Rf independent of treatment group assignment, sex, follow-up HbA1c level, CCr, and intervention cohort. The correlation coefficient was also generated for Rf as a function of follow-up log10AER values. SigmaStat (Jandel Scientific, Version 2.0, San Rafael, CA) was used for these determinations.
Figure 1.
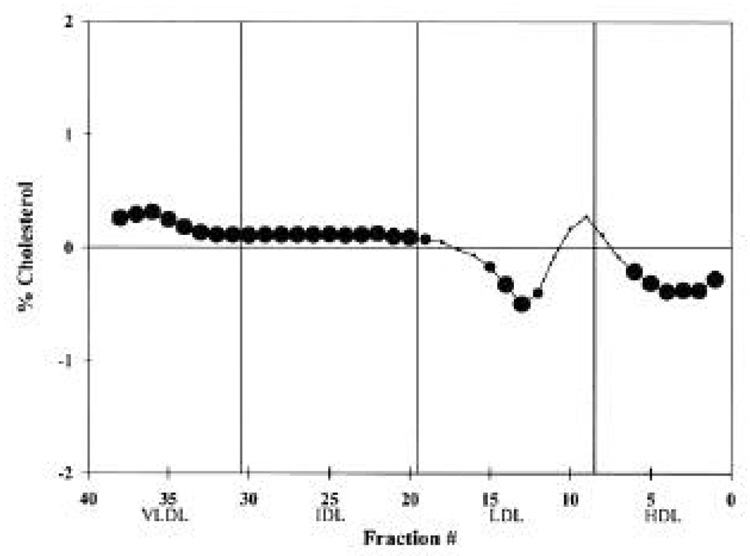
Difference plot comparing the mean lipoprotein levels of each normalized fraction from the DGUC for the microalbuminuric group (n = 80) versus the normoalbuminuric group (n = 1,056). If the difference is positive, the microalbuminuric cholesterol value is greater than the normoalbuminuric value. │, standard density-gradient fractions with HDL located in fractions 1–8, LDL in fractions 9–19, IDL in fractions 20–30, and VLDL in fractions 31–38 (36). For a fractional difference, , P ≤ 0.05;
, P ≤ 0.05;  , 0.05 < P ≤ 0.10; ●, not significant.
, 0.05 < P ≤ 0.10; ●, not significant.
Figure 3.
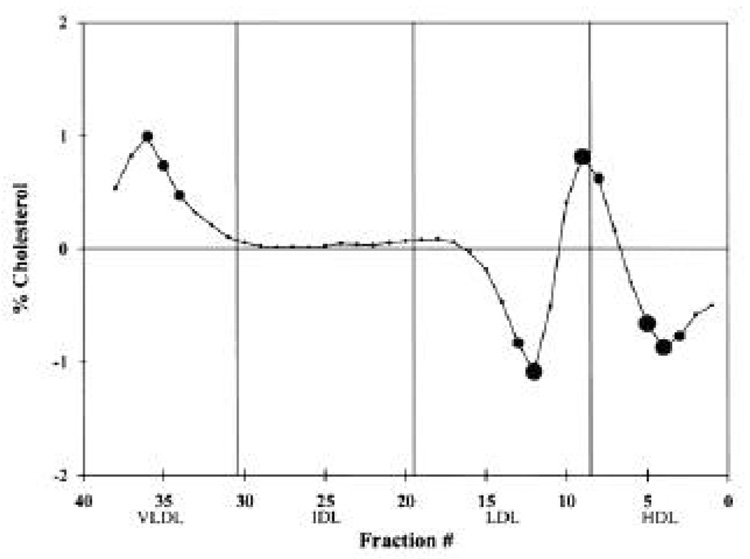
Difference plot comparing the mean cholesterol levels of each normalized fraction from the DGUC for the macroalbuminuric group (n = 24) versus the microalbuminuric group (n = 80). If the difference is positive, the macroalbuminuric cholesterol value is greater than the microalbuminuric value. │, Standard fractions. For a fractional difference,  , P ≤ 0.05;
, P ≤ 0.05;  0.05 < P ≤ 0.10; ●, not significant.
0.05 < P ≤ 0.10; ●, not significant.
RESULTS
Differences between the group with microalbuminuria and the group with normoalbuminuria were seen in both the quantity and distribution of lipids and lipoproteins. Among the lipid measurements, triglyceride differed significantly between these two groups with higher values for the group with microalbuminuria (Table 1). DGUC differences between these two groups indicated that there was a higher percentage of dense VLDL and IDL cholesterol in the group with microalbuminuria (Fig. 1). Also, there was a lower percentage of LDL cholesterol in buoyant fractions for the microalbuminuric subjects and no change in the more dense LDL cholesterol in fractions 7–11.
When comparing the chemical measurements of the macroalbuminuric group with those of the normoalbuminuric group, the macroalbuminuric subjects had triglyceride, total cholesterol, LDL cholesterol, HbA1c, and apoB levels that were significantly higher and an HDL cholesterol level that was significantly lower (Table 1). With DGUC, significantly higher percentages of VLDL cholesterol and marginally higher percentages of cholesterol in some IDL fractions were seen in subjects with macroalbuminuria (Fig. 2). Also, there was a lower percentage of buoyant LDL, a marginally higher percentage of dense LDL, and a marginally lower percentage of HDL cholesterol in the macroalbuminuric group.
Figure 2.
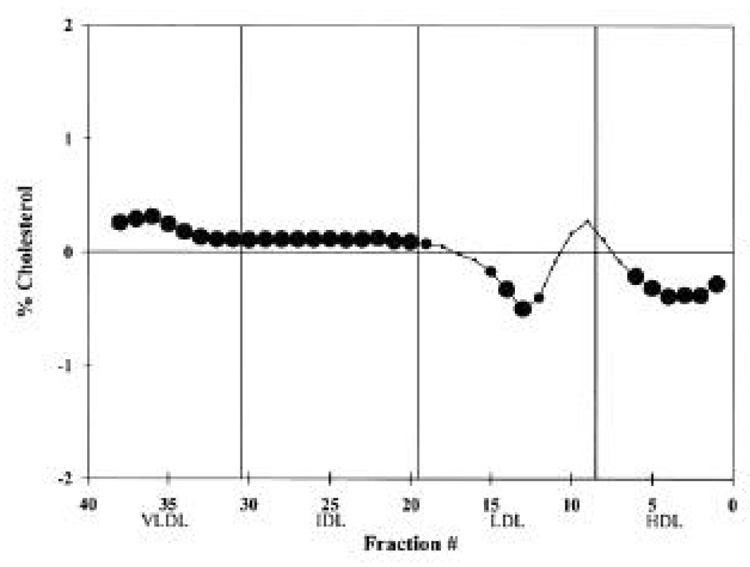
Difference plot comparing the mean cholesterol levels of each normalized fraction from the DGUC for the macroalbuminuric group (n = 24) versus the normoalbuminuric group (n = 1,056). If the difference is positive, the macroalbuminuric cholesterol value is greater than the normoalbuminuric value. │, Standard fractions. For a fractional difference,  , P ≤0.05;
, P ≤0.05;  0.05 < P ≤ 0.10; ●, not significant.
0.05 < P ≤ 0.10; ●, not significant.
When comparing the lipid and lipoprotein measurements of the macroalbuminuric group with those of the microalbuminuric group, the macroalbuminuric subjects had higher mean triglyceride, total cholesterol, LDL cholesterol, and apoB levels (Table 1). Although the percentage of cholesterol in some VLDL fractions was marginally higher among those with macroalbuminuria when using DGUC, there was no difference in the percentage of IDL cholesterol (Fig. 3). Also, there was a higher percentage of dense LDL cholesterol and a lower percentage of HDL cholesterol in subjects with macroalbuminuria. There were no significant differences in the Lp(a) and apoAI levels among these three groups.
There was a significant inverse correlation between Rf and log10AER among all subjects (n = 1,160) in this study (r = −0.238, P<0.001). In addition, log10AER remained significantly inversely correlated among all 1,056 normoalbuminuric subjects in this study (r = −0.138, P < 0.001).
The relationships between the lipid abnormalities and albuminuric levels were independent of other effects. When DGUC difference plots between albuminuric groups were generated separately for male and female subjects, the same trends in cholesterol distribution existed for each sex (data not shown). When DGUC difference plots between intensive and standard treatment groups within the same albuminuric level were generated, there were no significant differences. Multiple linear regression showed a significant independent relationship of log10AER to log10triglyceride, total, LDL, and HDL cholesterol levels; and Rf when linearly modeled with sex, treatment group (intensive versus conventional), CCr, intervention cohort (primary versus secondary), BMI, and HbA1c as other independent variables (Table 2). In addition, a statistically significant relationship between follow-up HbA1c and these lipids also existed.
Table 2.
Multiple linear regression relating independent variables (x1–x7) to five models with dependent variables (y1–y5)
| Dependent variables |
|||||
|---|---|---|---|---|---|
| Independent variables | y1. Total cholesterol (mmol/l) | y2. LDL cholesterol (mmol/l) | y3. log10triglyceride (mmol/l) | y4. HDL cholesterol (mmol/l) | y5. Rf |
| x1. log10AER (mg/day) | 0.128 (<0.001) | 0.110 (<0.001) | 0.179 (<0.001) | −0.063 (0.024) | −0.185 (<0.001) |
| x2. HbA1c (%) | 0.106 (<0.001) | 0.109 (<0.001) | 0.125 (<0.001) | −0.047 (0.130) | −0.082 (<0.001) |
| x3. BMI (kg/m2) | 0.192 (<0.001) | 0.213 (<0.001) | 0.238 (<0.001) | −0.155 (<0.001) | −0.055 (0.054) |
| x4. CCr (ml/s · 1.73 m−2) | −0.101 (<0.001) | −0.097 (<0.001) | −0.075 (0.008) | −0.014 (0.615) | −0.015 (0.608) |
| x5. Intervention (0 = 1°, 1 = 2°) | 0.030 (0.301) | 0.048 (0.096) | 0.033 (0.239) | −0.058 (0.034) | 0.015 (0.614) |
| x6. Sex (0 = female, 1 = male) | −0.054 (0.063) | 0.050 (0.084) | 0.101 (<0.001) | −0.365 (<0.001) | −0.191 (<0.001) |
| x7. Group (0 = intensive, 1 = standard) | 0.046 (0.164) | 0.032 (0.331) | 0.028 (0.378) | 0.024 (0.443) | −0.032 (0.322) |
| Model multiple r | 0.285 | 0.292 | 0.357 | 0.421 | 0.312 |
Data for independent variables are the β-coefficients normalized by dividing by SD to allow for direct comparison of the strength of the independent variables (P value). Data for the model multiple r are for each dependent variable and represent how well the multiple linear model accounts for the effect of the set of independent variables on each dependent variable (equation form: y1–5 = β0 + β1x1 + β2x2 + β3x3 + β4x4 + β5x5 + β6x6+ β7x7, where β0 is the y-intercept of the regression line).
CONCLUSIONS
This large unselected study of subjects with type 1 diabetes at progressive stages of albuminuria shows that a constellation of atherogenic abnormalities is present within the same population, characterizes how those abnormalities evolve at progressive stages of albuminuria, and indicates how prevalent such abnormalities may be. The LDL cholesterol elevation with progressive albuminuria was characterized first by an increase in IDL subfractions, then by an increase in the dense LDL subfractions with a decrease in bouyant LDL. These differences associated with albuminuria were independent of HbA1c.
Among serum lipids and lipoproteins, there was a significantly higher triglyceride level with microalbuminuria. In subjects with persistent macroalbuminuria, there was a further elevation in triglyceride, total cholesterol, LDL cholesterol, and apoB levels when compared with subjects with normoalbuminuria and microalbuminuria. Serum HDL cholesterol levels were lower in the persistently macroalbuminuric group compared with the normoalbuminuric group. Other studies of subjects with type 1 diabetes showed similar results. Watts et al. (4) found higher total and LDL cholesterol and lower HDL cholesterol levels in subjects with type 1 diabetes and proteinuria when compared with normal control subjects. When Dullaart et al. (5) analyzed the lipids and lipoproteins of a group of male subjects with type 1 diabetes and microabuminuria, higher LDL cholesterol and apoB levels were found in these subjects compared with the normoalbuminuric subjects with and without diabetes, although triglyceride and HDL cholesterol levels did not differ from the control subjects. Vannini et al. (6) found higher mean total and LDL cholesterol, higher triglyceride and apoB, and lower HDL cholesterol levels in dipstick-positive albuminuric patients with type 1 diabetes. Winocour et al. (7) showed higher IDL cholesterol levels in pooled fractions of sera in subjects with type 1 diabetes and persistent microalbuminuria compared with normoalbuminuric subjects. Deighen et al. (9) showed greater amounts of small dense LDL in subjects with nondiabetic glomerular disease and nephrotic-range proteinuria.
This study shows AER to be independently related to triglyceride; total, LDL, and HDL cholesterol; and Rf when modeled with HbA1c, sex, treatment group assignment, and intervention cohort (Table 2). The study also shows that albuminuria has its own unique association with lipid abnormalities.
There was a significant independent effect of HbA1c on follow-up triglyceride, total and LDL, and HDL cholesterol levels consistent with the effects of poorer glycemic control on lipids (33). In addition, this study shows an independent association between HbA1c and Rf.
A significantly higher amount of IDL cholesterol was seen at the persistently microalbuminuric stage compared with the normoalbuminuric stage (Fig. 1). There were lower amounts of some of the more buoyant LDL and marginally higher amounts of some VLDL fractions. At the persistently macroalbuminuric level, there was a significantly higher amount of VLDL and dense LDL, and there was less HDL and buoyant LDL cholesterol than in the normoalbuminuric subjects. Thus, there appears to be a progressive deterioration of lipids at progressive stages of persistent albuminuria.
Groop et al. (8) found that albuminuric subjects with type 1 diabetes have an increase in amount of apoB-containing particles due to apoB enrichment along the delipidation cascade with increased apoB in dense VLDL (VLDL2) and IDL cholesterol, and this reflects a greater number of these particles. That study also found a significant increase in IDL mass (combined lipid and apolipoprotein concentrations) at the microalbuminuric level that was not significantly different at the persistently macroalbuminuric level. By using DGUC, this study, which examines the full spectrum of apoB-containing particles, found more IDL cholesterol in persistently microalbuminuric subjects and more VLDL, IDL, and dense LDL cholesterol in persistently macroalbuminuric subjects. The differences between the persistently macroalbuminuric and normoal-buminuric subjects in the IDL fractions (Fig. 2) do not reach statistical significance because of the smaller group of subjects (n = 25 macroalbuminuric subjects). A larger number of persistently macroalbuminuric subjects might have yielded statistically significant differences like those seen when the persistently microalbuminuric subjects were compared with the normoalbuminuric subjects (Fig. 1).
This study connects the findings of Groop et al. (8), who showed an increase in hepatic lipase (HL) with albuminuria, with the findings of Zambon et al. (34), who showed that an increase in HL is associated with an increase in dense LDL. The increase in HL activity seen with albuminuria by Groop et al. (8) may lead to an accumulation of small dense LDL in subjects with type 1 diabetes. The present study demonstrated higher levels of dense LDL with albuminuria, which would be expected if HL plays an important physiologic role in the formation of small dense LDL. The findings of this study differ from those of Lahdenperä et al. (35), who found no significant differences in LDL density distribution among the three levels of albuminuria in subjects with diabetes. However, serum triglyceride was higher at higher levels of albuminuria in this study, and this study also controls for known sex-related effects on LDL density (36) and particle size (37). These study differences could result in the stronger relationship between small dense LDL and albuminuria that was seen in this study and in Deighen et al. (9).
In conclusion, this analysis of the DCCT end-study cohort found that significant atherogenic lipid and lipoprotein abnormalities were associated with progressive stages of persistent albuminuria. To our knowledge, the increase in small dense LDL at progressive levels of persistent albuminuria together with increased IDL in subjects with type 1 diabetes has not been previously reported in a large population-based study. Direct assessment of the role of HL on the increase in dense LDL seen in subjects with type 1 diabetes and albuminuria must be conducted. The dyslipidemia associated with persistent albuminuria may provide one mechanism for the increase in CVD seen in type 1 diabetic subjects with albuminuria.
Acknowledgments
This work was supported in part by a grant from the Juvenile Diabetes Association International (New York ). Dr. Sibley was supported by an American Diabetes Association Mentor-Based Postdoctoral Fellowship awarded to Dr. Brunzell. The DCCT was supported by the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes, Digestive, and Kidney Diseases, National Institutes of Health Grant DK-02456. This work was also supported in part by a National Institutes of Health Clinical Research Training in Renal Disease Fellowship.
We thank the participants and investigators who participated in the DCCT. Also, we thank Chris Casazza for his time and effort in performing the DGUCs.
Abbreviations
- AER
albumin excretion rate
- apoAI
apolipoproteinAI
- apoB
apolipoproteinB
- CCr
creatinine clearance
- CVD
cardiovascular disease
- DCCT
Diabetes Control and Complications Trial
- DGUC
density-gradient ultracentrifugation
- HL
hepatic lipase
- IDL
intermediate-density lipoprotein
- Lp(a)
lipoprotein(a)
- Rf
relative flotation rate
- sp.gr
specific gravity
Footnotes
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
References
- 1.Winocour P, Harland J, Millar J, Laker M, Alberti G. Microalbuminuria and associated cardiovascular risk factors in the community. Atherosclerosis. 1992;93:71–81. doi: 10.1016/0021-9150(92)90201-q. [DOI] [PubMed] [Google Scholar]
- 2.Joven J, Villabona C, Vilella E, Masana L, Albertí R, Vallés M. Abnormalities of lipoprotein metabolism in patients with the nephritic syndrome. N Engl J Med. 1990;323:579–584. doi: 10.1056/NEJM199008303230905. [DOI] [PubMed] [Google Scholar]
- 3.Joven J, Villabona C, Vilella E. Pattern of hyperlipoproteinemia in human nephroticsyndrome: influence of renal failure and diabetes mellitus. Nephron. 1993;64:565–569. doi: 10.1159/000187401. [DOI] [PubMed] [Google Scholar]
- 4.Watts G, Naumova R, Slavin B, Morris R, Houlston R, Kubal C, Shaw K. Serum lipids and lipoproteins in insulin-dependent diabetic patients with persistent microalbuminuria. Diabet Med. 1989;6:25–30. doi: 10.1111/j.1464-5491.1989.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 5.Dullaart R, Dikkeschei L, Doorenbos H. Alterations in serum lipids and apolipoproteins in male type 1 (insulin-dependent) diabetic patients with micro albuminuria. Diabetologia. 1989;32:685–689. doi: 10.1007/BF00274257. [DOI] [PubMed] [Google Scholar]
- 6.Vannini P, Ciavarella A, Flammini M, Bargossi A, Forlani G, Borgnino L, Orsoni G. Lipid abnormalities in insulin-dependent diabetic patients with albuminuria. Dia-betes Care. 1984;7:151–154. doi: 10.2337/diacare.7.2.151. [DOI] [PubMed] [Google Scholar]
- 7.Winocour P, Durrington P, Bhatnagar D, Ishola M, Mackness M, Arrol S. Influence of early diabetic nephropathy on very low density lipoprotein (VLDL), intermediate density lipoprotein (IDL), and low density lipoprotein (LDL) composition. Atheroscle-rosis. 1991;89:49–57. doi: 10.1016/0021-9150(91)90006-o. [DOI] [PubMed] [Google Scholar]
- 8.Groop P-H, Elliott T, Ekstrand A, Franssila-Kallunki A, Friedman R, Viberti G, Taskinen M. Multiple lipoprotein abnormalities in type 1 diabetic patients with renal disease. Diabetes. 1996;45:974–979. doi: 10.2337/diab.45.7.974. [DOI] [PubMed] [Google Scholar]
- 9.Deighen C, Caslake M, McConnell M, Boulton-Jones M, Packard C. Increased atherogenicity of low-density lipoprotein in heavy proteinuria. Nephrol Dialysis Trans-plant. 1998;13:1183–1188. doi: 10.1093/ndt/13.5.1183. [DOI] [PubMed] [Google Scholar]
- 10.Krauss R, Williams P, Brensike J, Detre K, Lindgren F, Kelsey S, Vranizan K, Levy R. Intermediate-density lipoproteins and progression of coronary artery disease in hypercholesterolemic men. Lancet. 1987;2:62–66. doi: 10.1016/s0140-6736(87)92734-6. [DOI] [PubMed] [Google Scholar]
- 11.Brunzell J, Sniderman A, Albers J, Kwiterovich P. Apoproteins B and A-I and coronary artery disease in humans. Arte-riosclerosis. 1984;4:79–83. doi: 10.1161/01.atv.4.2.79. [DOI] [PubMed] [Google Scholar]
- 12.Hokanson J, Austin M. Plasma triglyceride as a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardio-vascular Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 13.Gordon T, Castelli W, Hjortland M, Kannel W. High density lipoprotein as a protective factor against coronary heart disease: the Framingham Study. Am J Med. 1977;62:704–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 14.Austin M, King M, Vranizan K, Krauss R. Atherogenic lipoprotein phenotype: a proposed genetic marker for coronary heart disease. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 15.Borch-Johnsen K, Andersen P, Deckert T. The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1985;28:590–596. doi: 10.1007/BF00281993. [DOI] [PubMed] [Google Scholar]
- 16.Borch-Johnsen K, Kreiner S. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J Clin Res Educ. 1987;294:1651–1654. doi: 10.1136/bmj.294.6588.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krolewski A, Kosinski E, Warram J, Leland S, Busick E, Asmal A, Rand L, Christlieb A, Bradley R, Kahn C. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson J, Kenny S, Stevens L, Fuller E, Lee E. WHO Multinational Study Group: Proteinuria and mortality in diabetes: the WHO Multinational Study of Vascular Disease in Diabetes. Diabet Med. 1995;12:149–155. doi: 10.1111/j.1464-5491.1995.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 19.Messent J, Elliott T, Hill R, Jarrett R, Keen H, Viberti G. Prognostic significance of microalbuminuria in insulin-dependent diabetes mellitus: a twenty-three year study. Kidney Int. 1992;41:836–839. doi: 10.1038/ki.1992.128. [DOI] [PubMed] [Google Scholar]
- 20.Rossing P, Hougaard P, Borch-Johnsen K, Hans-Henrik P. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ. 1996;313:779–784. doi: 10.1136/bmj.313.7060.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Diabetes Control and Complications Trial Research Group. The Diabetes Control and Complications Trial (DCCT): design and methodologic considerations for the feasibility phase. Diabetes. 1986;35:530–545. [PubMed] [Google Scholar]
- 22.The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- 23.Molitch M, Steffes M, Cleary P, Nathan D. Baseline analysis of renal function in the Diabetes Control and Complications Trial. Kidney Int. 1993;43:668–674. doi: 10.1038/ki.1993.96. [DOI] [PubMed] [Google Scholar]
- 24.The Lipid Research Clinics Population Studies Data Book. Vol. 1. Washington, DC: U.S. Govt. Printing Office; 1980. Department of Health and Human Services: the prevalence study; pp. 1–115. NIH publ.no. 80-1527. [Google Scholar]
- 25.Metropolitan Life Insurance Company: Metropolitan height and weight tables. Stat Bull Metropolitan Life Insurance Co. 1983;64:2–9. [Google Scholar]
- 26.The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 27.Marcovina S, Albers J, Gabel B, Koschinsky M, Gaur V. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a) Clin Chem. 1995;41:246–255. [PubMed] [Google Scholar]
- 28.Marcovina S, Albers J, Kennedy H, Mei J, Henderson O, Hannon H. International Federation of Clinical Chemistry standardization project for measurements of apolipoproteins A-I and B-IV: comparability of apolipoprotein B values by use of international reference material. Clin Chem. 1994;40:586–592. [PubMed] [Google Scholar]
- 29.Marcovina S, Albers J, Kennedy H, Mei J, Henderson L, Hannon W. International Federation of Clinical Chemistry standardization project for measurements of a polipoproteins A-I and B-III: comparability of apo A-I values by use of international reference material. Clin Chem. 1993;39:773–781. [PubMed] [Google Scholar]
- 30.Auwerx J, Marzetta C, Hokanson J, Brun-zell J. Large buoyant LDL-like particles in hepatic lipase deficiency. Arteriosclerosis. 1989;9:319–325. doi: 10.1161/01.atv.9.3.319. [DOI] [PubMed] [Google Scholar]
- 31.Purnell J, Marcovina S, Hokanson J, Kennedy H, Cleary P, Steffes M, Brunzell J. Levels of lipoprotein (a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM: results from follow-up in the Diabetes Control and Complications Trial. Diabetes. 1995;44:1218–1226. doi: 10.2337/diab.44.10.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindgren F, Jensen L, Hatch F. The isolation and quantitative analysis of serum lipoproteins. In: Nelson GJ, editor. Blood Lipids and Lipoproteins: Quantitation, Composition and Metabolism. New York: Wiley; 1972. pp. 181–274. [Google Scholar]
- 33.The Diabetes Control and Complications Trial (DCCT) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 34.Zambon A, Austin M, Brown B, Hokanson J, Brunzell J. Effect of hepatic lipase on LDL in normal men and those with coronary artery disease. Arterioscler Thromb Vasc Biol. 1993;3:147–153. doi: 10.1161/01.atv.13.2.147. [DOI] [PubMed] [Google Scholar]
- 35.Lahdenperä S, Groop P, Tilly-Kiesi M, Kuusi T, Elliott T, Viberti G, Taskinen M-R. LDL subclasses in IDDM patients: relation to diabetic nephropathy. Diabetologia. 1994;37:681–688. doi: 10.1007/BF00417692. [DOI] [PubMed] [Google Scholar]
- 36.Capell W, Zambon A, Austin M, Brunzell J, Hokanson J. Compositional differences of LDL particles in normal subjects with LDL subclass phenotype A and subclass phenotype B. Arterioscler Thromb Vasc Biol. 1996;16:1040–1046. doi: 10.1161/01.atv.16.8.1040. [DOI] [PubMed] [Google Scholar]
- 37.Austin M, King M-C, Vranizan K, Newman B, Krauss R. Inheritance of low-density lipoprotein subclass patterns: results of complex segregational analysis. Am J Human Genet. 1988;43:838–846. [PMC free article] [PubMed] [Google Scholar]


