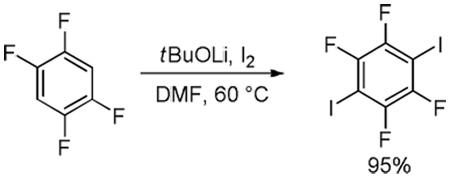
Abstract
A new method has been developed for in situ halogenation of acidic sp2 carbon-hydrogen bonds in heterocycles and electron-deficient arenes. Either selective monohalogenation or one-step exhaustive polyhalogenation is possible for substrates possessing several C-H bonds that are flanked by electron-withdrawing groups. For the most acidic arenes, such as pentafluorobenzene, K3PO4 base can be employed instead of BuLi for metalation/halogenation sequences.
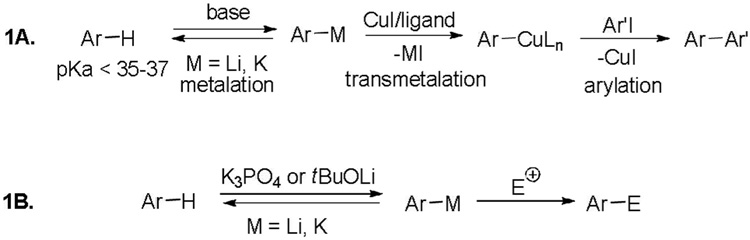
We have recently developed a method for copper-catalyzed arylation of sp2 C-H bonds possessing DMSO pKa’s below 35.1 The reaction was shown to proceed via an aryllithium or arylpotassium intermediate that is formed in situ by the reaction of the arene with either K3PO4 or tBuOLi metalating agent (Scheme 1A). It occurred to us that halogenation of arenes that are reactive under the copper-catalyzed arylation conditions should be possible under mild nucleophilic conditions by employing relatively weak bases such as K3PO4 or tBuOLi (Scheme 1B). Current methods for halogenation of electron-deficient arenes such as polyfluoro-or polychlorobenzenes involve either harsh electrophilic conditions or the use of strong alkyllithium bases and cryogenic conditions.2 If the positive halogen source would form an active halogenating agent in the reaction with base, an in situ trapping of the arylmetal species should be possible. Martin has reported use of a strong LiTMP base for in situ deprotonation/silylation of aromatic nitriles, esters, and pyridine derivatives.3 Other relevant examples include trifluorobenzene metalation by tBuLi in the presence of TMSCl first reported by Gilman and reinvestigated by Schlosser as well as Kondo’s report on functionalizing electron-rich and electron-deficient arenes by employing t-Bu-P4 base/electrophile combinations.4 Several other groups have described similar methodologies with either silyl or boryl-derived electrophiles.5 It appears, however, that in situ trapping has not been widely applied to arene halogenation.3d,e Furthermore, potassium phosphate and tBuOLi have not been employed as metalating agents for such reactions. We report here a simple method for in situ halogenation of sp2 C-H bonds in heterocycles as well as electron-deficient arenes under non-cryogenic conditions.
Scheme 1.
Functionalization of Acidic sp2 C-H Bonds.
Minor modifications of the copper-catalyzed arylation conditions allowed the development of an efficient process for in situ sp2 C-H bond halogenation. The scope of the halogenation reaction is presented in Table 1. Electron-rich heterocycles such as benzothiazole, butylimidazole, and phenyloxazole can be chlorinated and brominated (entries 1–4). The halogenating reagent must be optimized for each case. Either carbon tetrahalides (entries 1–3) or dibromotetrafluoroethylene (entry 4) may be employed for electron-rich heterocycle halogenation.
Table 1.
Halogenation scope.a
 | ||||
|---|---|---|---|---|
| entry | arene | reagent base |
product | yield |
| 1 |  |
CCl4 tBuOLi |
80% | |
| 2 | 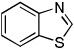 |
CBr4 K3PO4 |
82% | |
| 3 | CBr4 tBuOLi |
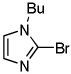 |
91% | |
| 4 | 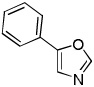 |
(BrCF2)2 tBuOLi |
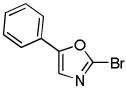 |
80% |
| 5 | 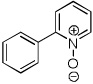 |
CBr4 tBuOLi |
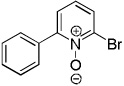 |
56% |
| 6b | CBr4 tBuOLi |
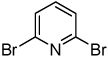 |
30% | |
| 7 | C6Cl5H | ICl tBuOLi |
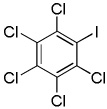 |
90% |
| 8 | 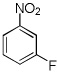 |
CBr4 tBuOLi |
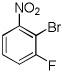 |
55% |
| 9 | 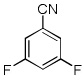 |
CBr4 tBuOLi |
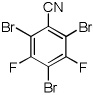 |
40% |
| 10 | C6F5H | I2 K3PO4 |
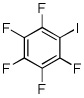 |
85% |
| 11 | 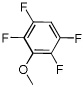 |
I2 tBuOLi |
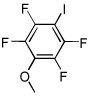 |
98% |
| 12 | 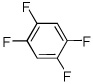 |
I2 tBuOLi |
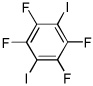 |
95% |
| 13c | 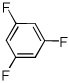 |
ICl tBuOLi |
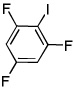 |
58% |
| 14d | 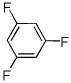 |
ICl tBuOLi |
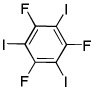 |
39% |
Substrate (1 equiv), base (2–4 equiv), halogenating reagent (1.5-4 equiv). Yields are isolated yields. See the Supporting Information for details.
m-Xylene solvent.
1,3,5-Trifluorobenzene (3 equiv), ICl (1 equiv).
1,3,5-Trifluorobenzene (1 equiv), ICl (4 equiv).
Electron-deficient heterocycles such as pyridine Noxide and 2-phenylpyridine N-oxide are brominated by employing a mixture of tBuOLi base and carbon tetrabromide (entries 5 and 6). For pyridine N-oxide, dihalogenation/reduction to the dibromopyridine product is obtained in a moderate yield (entry 6). Selective monohalogenation of pyridine N-oxide was not achieved under a variety of conditions. In contrast with the copper-catalyzed arylation process, halogenation is unsuccessful for pyridazine and pyrimidine, presumably due to their insufficient acidity.6 Consequently, higher acidity is required for the halogenation reaction if it is compared to arylation.7
Electron-deficient arenes can also be efficiently halogenated (entries 7–14). Pentachlorobenzene is iodinated in excellent yield by using a combination of iodine(I) chloride and tBuOLi in DMF (entry 7). Nitro and cyano groups are tolerated as shown in bromination of 3-nitrofluorobenzene (entry 8) and one-step tribromination of 3,5-difluorobenzonitrile (entry 9). For the latter substrate, selective monohalogenation could not be achieved. If acidic pentafluorobenzene is halogenated, even the weak K3PO4 base can be employed (entry 10), affording iodopentafluorobenzene in excellent yield. Previously, butyllithium has been used for pentafluorobenzene deprotonation-reaction with electrophiles sequences.8 Monoiodination of methoxytetrafluorobenzene and one-step diiodination of 1,2,4,5-tetrafluorobenzene affords the corresponding halides in excellent yields (entries 11 and 12). 1,3,5-Trifluorobenzene can be either monoiodinated or triiodinated in acceptable yields depending on the ratio of arene to iodine(I) chloride (entries 13 and 14). 1,3- Dihalobenzenes are insufficiently acidic and are unreactive under these conditions. Attempted bromination of 1,3-dinitrobenzene resulted in about 10% conversion to the product. 1,3,5-Trinitrobenzene decomposes under the reaction conditions.
The halogenation of electron-deficient arenes most likely proceeds by an initial generation of equilibrium concentration of the aryl anion followed by reaction with an electrophile.3d,e The lifetime of aryllithium and arylpotassium species must be short since significant amounts of benzyne-derived products are not formed.
At this point we are unsure about the exact structure of the electrophilic halogenating agent. It is conceivable that the reaction of tBuOLi with the halogen electrophile may produce a t-butyl hypohalide.9 However, it is known that, at least for iododerivative, the structure of the hypohalide varies with the method of preparation.10
In conclusion, we have developed a new method for in situ halogenation of acidic sp2 carbon-hydrogen bonds in heterocycles and electron-deficient arenes. Either monohalogenation or one-step polyhalogenation is possible for substrates possessing several C-H bonds that are flanked by electron-withdrawing groups. For the most acidic substrates, such as pentafluorobenzene, K3PO4 base can be employed instead of the currently used BuLi for metalation/functionalization sequences.
Supplementary Material
Experimental details, data and spectra for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We thank the Welch Foundation (Grant No. E-1571), the NIGMS (Grant No. R01GM077635), A. P. Sloan Foundation, and Camille and Henry Dreyfus Foundation for supporting this research. We also thank Prof. Hans J. Reich (University of Wisconsin-Madison) for helpful comments.
References
- 1.(a) Do H-Q, Daugulis O. J. Am. Chem. Soc. 2007;129:12404. doi: 10.1021/ja075802+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Do H-Q, Daugulis O. J. Am. Chem. Soc. 2008;130:1128. doi: 10.1021/ja077862l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Do H-Q, Khan RMK, Daugulis O. J. Am. Chem. Soc. 130:15185. doi: 10.1021/ja805688p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ReviewSlocum DW, Shelton P, Moran KM. Synthesis. 2005:3477.; (b) Prakash GKS, Mathew T, Hoole D, Esteves PM, Wang Q, Rasul G, Olah GA. J. Am. Chem. Soc. 2004;126:15770. doi: 10.1021/ja0465247. [DOI] [PubMed] [Google Scholar]; (c) Deacon GB, Smith RNM. Austr. J. Chem. 1982;35:1587. [Google Scholar]; (d) Hellmann M, Bilbo AJ, Pummer WJ. J. Am. Chem. Soc. 1955;77:3650. [Google Scholar]; (e) Mongin F, Marzi E, Schlosser M. Eur. J. Org. Chem. 2001:2771. [Google Scholar]; (f) Burukin AS, Vasil'ev AA, Struchkova MI, Kachala VV, Zlotin SG. Russ. Chem. Bull. 2005;54:964. [Google Scholar]; (g) Boga C, Del Vecchio E, Forlani L, Todesco PE. J. Organomet. Chem. 2000;601:233. [Google Scholar]
- 3.Krizan TD, Martin JC. J. Am. Chem. Soc. 1983;105:6155.Taylor SL, Lee DY, Martin JC. J. Org. Chem. 1983;48:4156.Marsais F, Laperdrix B, Güngör T, Mallet M, Quéguiner G. J. Chem. Res. Miniprint. 1982:2863.In situ exhaustive halogenation of cyclopentadieneStraus F, Kollex L, Heyn W, Kuhnel R. Chem. Ber. 1930;63B:1868.Tribromobenzene bromination by a mixture of tBuOK and tBuOBr:Mach MH, Bunnett JF. J. Am. Chem. Soc. 1974;96:936.
- 4.(a) Dua SS, Gilman H. J. Organomet. Chem. 1974;64:C1–C2. [Google Scholar]; (b) Howells RD, Gilman H. Tetrahedron Lett. 1974;15:1319. [Google Scholar]; (c) Schlosser M, Guio L, Leroux F. J. Am. Chem. Soc. 2001;123:3822. doi: 10.1021/ja0032733. [DOI] [PubMed] [Google Scholar]; (d) Imahori T, Kondo Y. J. Am. Chem. Soc. 2003;125:8082. doi: 10.1021/ja0342300. [DOI] [PubMed] [Google Scholar]; (e) Eaton PE, Cunkle GT, Marchioro G, Martin RM. J. Am. Chem. Soc. 1987;109:948. [Google Scholar]
- 5.(a) Vazquez E, Davies IW, Payack JF. J. Org. Chem. 2002;67:7551. doi: 10.1021/jo026087j. [DOI] [PubMed] [Google Scholar]; (b) Caron S, Hawkins JM. J. Org. Chem. 1998;63:2054. [Google Scholar]; (c) Kristensen J, Lysén M, Vedsø P, Begtrup M. Org. Lett. 2001;3:1435. doi: 10.1021/ol015598+. [DOI] [PubMed] [Google Scholar]; (d) Black WC, Guay B, Scheuermeyer F. J. Org. Chem. 1997;62:758. doi: 10.1021/jo961839t. [DOI] [PubMed] [Google Scholar]
- 6.(a) Shen K, Fu Y, Li J-N, Liu L, Guo Q-X. Tetrahedron. 2007;63:1568. [Google Scholar]; (b) Bordwell FG. Acc. Chem. Res. 1988;21:456. [Google Scholar]
- 7.A figure listing substrates not halogenated under these conditions is included in Supporting Information.
- 8.ReviewBurton DJ, Yang Z, Morken P. A. Tetrahedron. 1994;50:2993.
- 9.(a) Glover SA, Goosen A. Tetrahedron Lett. 1980;21:2005. [Google Scholar]; (b) Goosen A, McCleland CW. J. Chem. Soc. Perkin Trans. 1976;1:383. [Google Scholar]
- 10.Tanner DD, Gidley GC, Das N, Rowe JE, Potter A. J. Am. Chem. Soc. 1984;106:5261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details, data and spectra for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.