Abstract
Expression of hepatic drug metabolizing enzymes (DMEs) is altered in infection and inflammation. However, the role of Gram+ve bacterial components and their receptor, Toll-like receptor (TLR) 2 in regulation of hepatic DMEs is unknown. Gene expression of DMEs is regulated by members of the nuclear receptor superfamily (PXR, CAR and RXRα). The TLR2 ligand, lipoteichoic acid (LTA) reduced RNA levels of CAR and its target genes, Cyp2b10, Cyp2a4 and Sultn in mouse liver (~60-80% reduction). Hepatic genes regulated by PXR and CAR, Cyp3a11 and Mrp2 were moderately reduced by LTA, along with ~50% reduction of PXR RNA and nuclear protein levels of RXRα. The effects of LTA were significantly attenuated by pre-treatment with the Kupffer cell inhibitor, gadolinium chloride, indicating that Kupffer cells contribute to LTA-mediated down-regulation of hepatic genes. These results indicate that treatment with Gram+ve bacterial components preferentially down-regulate CAR and its target genes in the liver.
Keywords: drug-metabolizing enzymes, liver, nuclear receptors, Toll-like receptors, inflammation, Lipoteichoic acid
During infection and inflammation, expression and activity of key phase I and phase II drug metabolizing enzymes (DMEs) and drug transporters are altered in the liver, leading to impaired drug metabolism and clearance [1; 2]. Gene expression of these enzymes and transporters are regulated by members of the nuclear receptor (NR) superfamily, pregnane X receptor (PXR), constitutive androstane receptor (CAR) and retinoid X receptor (RXRα) [3; 4; 5]. Inflammation-mediated alterations in DME and transporter gene expression is associated with reduced expression and activity of the regulatory NRs [1; 6; 7; 8; 9; 10; 11; 12]. However, the mechanism of suppression of hepatic DME and transporter genes in inflammation is not fully understood. Inflammatory responses in the liver are mediated by Toll-like receptors (TLRs) present on Kupffer cells (KCs) which recognize microbial components and endogenous ligands from damaged or stressed cells [13; 14; 15; 16; 17]. TLR2 and TLR4 are activated by components of Gram+ve (lipoteichoic acid (LTA)), and Gram-ve (lipopolysaccaharide (LPS)) bacteria, respectively [15; 16; 18; 19]. We have recently reported that the Gram-ve bacterial endotoxin, lipopolysaccharide (LPS) can modulate expression of hepatic DMEs by Toll-like receptor (TLR) 4-dependent mechanism [8]. However, the role of Gram+ve bacterial components and their receptor, TLR2 in regulation of hepatic gene expression has never been investigated. In addition to infections by Gram-ve bacteria, there is also a high incidence of infection by Gram+ve bacteria which can induce inflammatory responses leading to potential alterations in metabolism, distribution and elimination of drugs and toxins. In fact, it has been reported that Gram+ve organisms account for ~50% cases of sepsis from 1979-2000 [20]. LTA, derived from the cell-wall components of Gram+ve bacteria like Staphylococcus aureus are known to cause systemic inflammation [21]. LTA has been shown to induce release of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF) α and other inflammatory mediators from immune cells, including macrophages [22; 23].
It has been previously shown that LPS-mediated induction of pro-inflammatory cytokines results in the activation of a variety of cell-signaling kinases including c-Jun N-terminal kinase (JNK) and NF-κB [8; 9; 24; 25; 26]. These cell-signaling components are involved in regulation of DMEs and transporters by modulating the activity of some of their regulatory NRs [26; 27; 28]. It has been shown that curcumin, a known inhibitor of JNK, can block LPS-mediated down-regulation of Cytochrome P450 (Cyp) enzymes, although the underlying mechanism is not known [29]. Furthermore, we have shown that activation of JNK by LPS or IL-1β results in modification and nuclear export of RXRα, leading to suppression of RXRα-dependent hepatic genes [9; 25]. JNK inhibits Glucocorticoid receptor (GR) activity, resulting in suppression of CAR gene expression [26]. Reduction in CAR gene expression in inflammation has also been attributed to the disruption of GR-mediated transactivation of the CAR gene by NF-κB [24; 30]. Recent work has demonstrated that NF-κB can interact with PXR-RXR complex, leading to the suppression of Cyp3a4 gene expression by LPS [31].
In this study we sought to determine whether the Gram+ve bacterial component, LTA has a role in regulating gene expression of key phase I and II DMEs and drug transporters in the liver. Our results indicate that LTA treatment resulted in altered gene expression of DMEs, transporters and their regulatory nuclear receptors in the liver. Expression of TLR2 and the pro-inflammatory cytokines were significantly induced by LTA, and the cell-signaling components, JNK and NF-κB were activated in the liver. Expression of the NR, CAR, and its target genes were rapidly and profoundly repressed by LTA, indicating that CAR is preferentially targeted by TLR2-dependent mechanisms in inflammation. The effects of LTA on hepatic genes were attenuated by the Kupffer cell inhibitor, gadolinium chloride (GdCl3), which indicates that cytokines released by KCs may play a role in mediating the effects of LTA.
Materials and methods
Materials
Lipoteichoic acid (S. aureus) was purchased from InvivoGen (San Diego, CA) and freshly diluted to the desired concentration in pyrogen-free 0.9% saline before injection. Anti-JNK (#9252), anti-phospho-JNK (#9251) (Cell Signaling, Beverly, MA), anti-RXRα (D-20) (#sc-553) and anti-TLR2 (H-175) (#sc-10739) (Santa Cruz Biotechnology, CA) were used according to manufacturer’s instructions. Oligonucleotides were obtained from Sigma Genosys, Houston, TX. All reagents for real-time PCR were purchased from Applied Biosystems (Foster City, CA).
Animals and treatments
Adult male C57BL/6 mice were obtained from The Jackson Laboratory, Maine. The animals were maintained in a temperature- and humidity-controlled environment and were provided with water and rodent chow ad lib. Mice were given intraperitoneal (IP) injection with 6 mg/kg body wt. LTA in saline or saline alone. Livers were removed at the time indicated in the figure legends (0 to 16 hours) after treatment. For 0h treatment, mice were IP-injected with LTA, and sacrificed immediately.
To inactivate Kupffer cells, mice were given a single dose of GdCl3 intravenously (10 mg/kg), followed by IP injection of LTA (6 mg/kg) 24h after GdCl3 treatment. This concentration of GdCl3 has been previously shown to inhibit and deplete Kupffer cells from the liver [6; 32]. All animal protocols were approved by the Institutional Animal Care and Use Committee. Experiments were performed in triplicate and repeated three to four times.
Preparation and analysis of nuclear and whole cell extracts and membrane fractions
Nuclear and whole cell extracts were prepared as described previously [9; 33]. Membrane fractions were prepared from mouse liver using the Mem-PER® Eukaryotic Membrane Protein Extraction Reagent kit according to the manufacturer’s protocol (Pierce, Rockford, IL). The samples were analyzed by immunoblotting, and signals were developed by a standard enhanced chemiluminescence method following the manufacturer’s protocol (Perkin Elmer Life Sciences, Boston, MA).
Real time quantitative PCR analysis
Total RNA was isolated from mouse liver tissues using TRIzol Reagent (Sigma) according to manufacturer’s instructions. cDNA synthesis was done using “High capacity Reverse transcription kit” from Applied Biosystems. Real time quantitative PCR was performed using an ABI PRISM 7300 Sequence Detection System instrument and software (Applied Biosystems, Inc., Foster City, CA). Briefly, each amplification reaction (25 μl) contained 50-100 ng of cDNA, 300 nM of forward primer, 300 nM of reverse primer, 200 nM of fluorogenic probe and 15 μl of TaqMan® Universal PCR master mix. Quantitative expression values were extrapolated from standard curves and were normalized to cyclophilin. The sequences of the primers and probes were obtained from the literature or purchased from Applied Biosystems, as reported previously [9; 33].
Serum cytokine analysis
Serum IL-1β, TNFα and IL-6 levels were determined by enzyme-linked immunosorbent assay (ELISA; BD OptEIA™ Mouse IL-1β, TNFα and IL-6 ELISA kits from BD Biosciences, San Diego, CA) according to the manufacturer’s directions.
Statistical analysis
Statistical analysis of the results was performed using SigmaStat (Systat, Inc, Point Richmond, CA). Data are reported as mean ± S.D. Differences between experimental groups were assessed for statistical significance by the Mann-Whitney test. P-values <0.05 were considered significant.
Results
Regulation of gene expression of DMEs by lipoteichoic acid
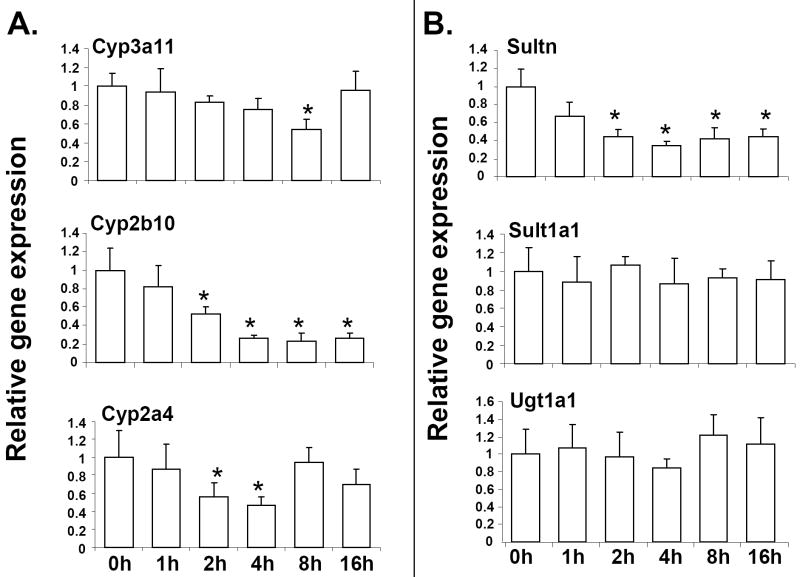
Hepatic expression of DME genes is altered during infection and inflammation; however, the molecular mechanism by which this suppression occurs remains to be fully elucidated [1; 2]. To investigate the role of the Gram+ve bacterial components in the regulation of DME genes, mice were injected with the LTA, which is derived from Gram+ve bacteria, and is a known ligand for TLR2. RNA was isolated from livers harvested at 0 to 16 h after LTA injection and was analyzed by real-time PCR (Fig. 1). Interestingly, maximal suppression of RNA levels occurred for Cyp2b10; ~50% reduction as early as 2h after LTA administration, followed by further reduction to ~80% from 2-16h (Fig. 1A). RNA levels of the key murine phase I DME, Cyp3a11 was reduced ~40% after 8h of LTA treatment, and Cyp2a4 RNA was reduced ~50% after 2-4h of LTA treatment. The reduction in RNA levels of both Cyp3a11 and Cyp2a4 were not detected by 16h. In case of the phase II enzymes, Amine N-sulfotransferase (Sultn) was significantly reduced by LTA as early as 2h after treatment, and this reduction in RNA levels was detected till 16h of treatment (~60-70% reduction) (Fig. 1B). However, Sult1a1 and Ugt1a1 were not affected by LTA treatment. For all the genes tested, no effect of LTA was detected at 24 or 48 hours (data not shown). These results show that there is a time-dependent effect of LTA on gene expression, depending on the target genes. Previous reports indicate that the Cyp genes are regulated by circadian clock [34; 35] We find that RNA levels of the hepatic DME genes tested were unaltered in saline-injected mice from 0-16h, indicating that circadian clock had no effect on the expression of these genes (data not shown).
Fig. 1.
Regulation of DME RNA levels in mouse liver following LTA treatment. Mice were IP-injected with 6 mg/kg LTA and livers were harvested from 0 to 16 hours (n=6-8 per group). RNA was isolated from the livers and analyzed by TaqMan real-time PCR as described in Materials and methods. RNA levels of phase I (A) and phase II (B) enzymes were determined. All data were presented as ± SD and standardized for cyclophilin RNA levels. Expression in 0h LTA-injected mice was set to 1, fold change after LTA treatment from 1-16h was compared to the 0h LTA-injected controls. The asterisks indicate significant difference (p < 0.05).
Regulation of gene expression of drug transporters by lipoteichoic acid
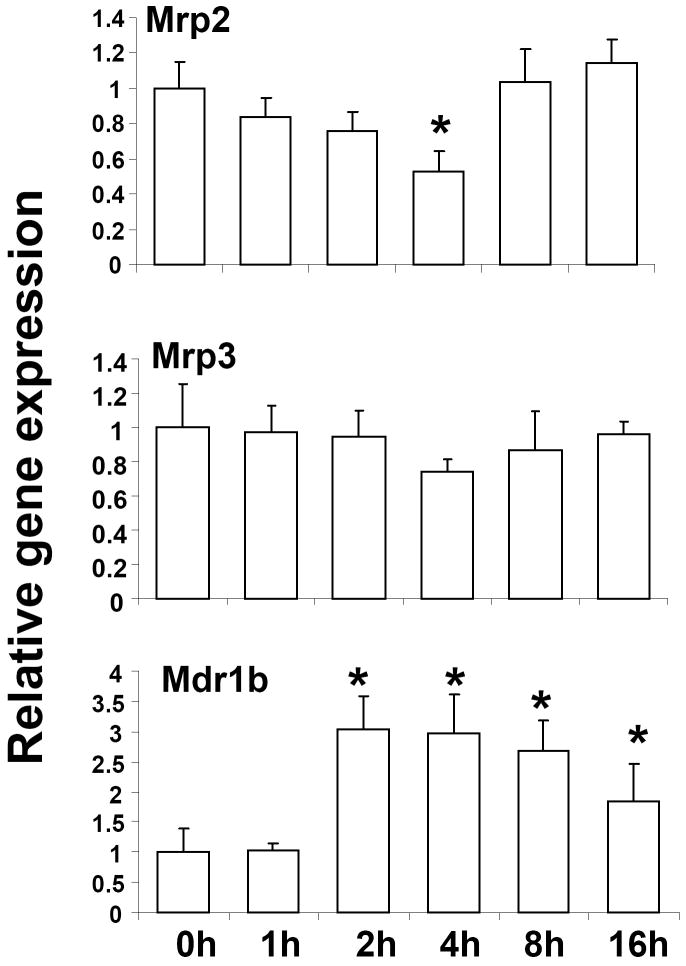
In order to determine the effect of LTA on the expression of hepatic transporters, RNA levels of multidrug resistance-associated proteins (Mrps) 2 and 3 were determined by real-time PCR. LTA treatment suppressed Mrp2 RNA levels after 4h (~50%), whereas Mrp3 levels were not significantly affected (Fig. 2). Mdr1b, one of two rodent homologues of the human Mdr1 gene, was induced ~3-fold by LTA, likely via an NF-κB dependent mechanism as reported previously for lipopolysachcharide treatment [36]. There were no time-dependent changes in RNA levels of the hepatic transporters in saline-injected mice (data not shown).
Fig. 2.
Regulation of transporter RNA levels in mouse liver following LTA treatment. Mice were IP-injected with 6 mg/kg LTA and livers were harvested at various time-points (n=6 per group). RNA was isolated from the livers and analyzed by TaqMan real-time PCR as described in Materials and methods. All data were presented as ± SD and standardized for cyclophilin RNA levels. Expression in 0h LTA-injected mice was set to 1, fold change after LTA treatment from 1-16h was compared to the 0h LTA-injected controls. The asterisks indicate significant difference (p < 0.05).
Regulation of expression of nuclear receptors by lipoteichoic acid
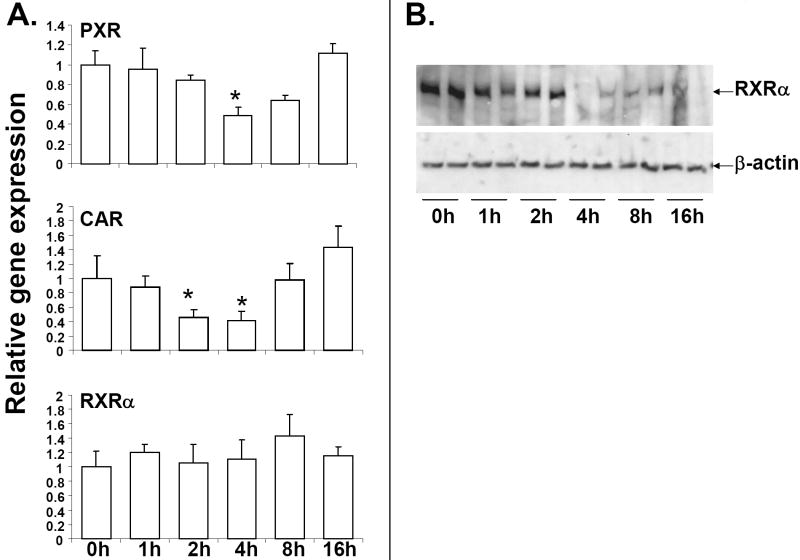
It is well-established that the xenobiotic NRs, PXR and CAR heterodimerize with the central nuclear receptor, RXRα to bind to conserved sequences in the promoter regions of DME and transporter genes resulting in activation of these genes [4; 5; 37; 38]. RNA levels of CAR were reduced ~50% as early as 2h of LTA treatment, and remained low at 4h, but was restored to basal levels at 8h of LTA treatment (Fig. 3A). PXR RNA levels were reduced ~40% by LTA at 4h, and was back to basal levels at 8h. RNA levels of RXRα were not affected by LTA treatment; however, nuclear protein levels of RXRα were significantly reduced by LTA treatment from 4 to 16h (Fig. 3B). RXRα protein levels were also found to be reduced from 4-16h in whole cell extracts prepared from livers of saline or LTA-treated mice (data not shown). Reduction in expression of the regulatory NRs likely contributes to down-regulation of DME and transporter genes by LTA. Expression of the NRs are not altered at different time-points in saline-injected mice (data not shown), indicating that alterations in NR RNA levels from 0 to 16h was due to LTA treatment.
Fig. 3.
Regulation of NR expression in mouse liver by LTA. Mice were IP-injected with 6 mg/kg LTA and livers were harvested at various time-points (n=6 per group). RNA was isolated from the livers and analyzed by TaqMan real-time PCR as described in Materials and methods. All data were presented as ± SD and standardized for cyclophilin RNA levels. Expression in 0h LTA-injected mice was set to 1, fold change after LTA treatment from 1-16h was compared to the 0h LTA-injected controls. The asterisks indicate significant difference (p < 0.05) (A). Nuclear extracts were prepared from livers from saline and LTA -injected mouse livers RXRα protein levels were measured by western blotting (B).
Regulation of expression of cytokines and Toll-like receptor 2 by lipoteichoic acid
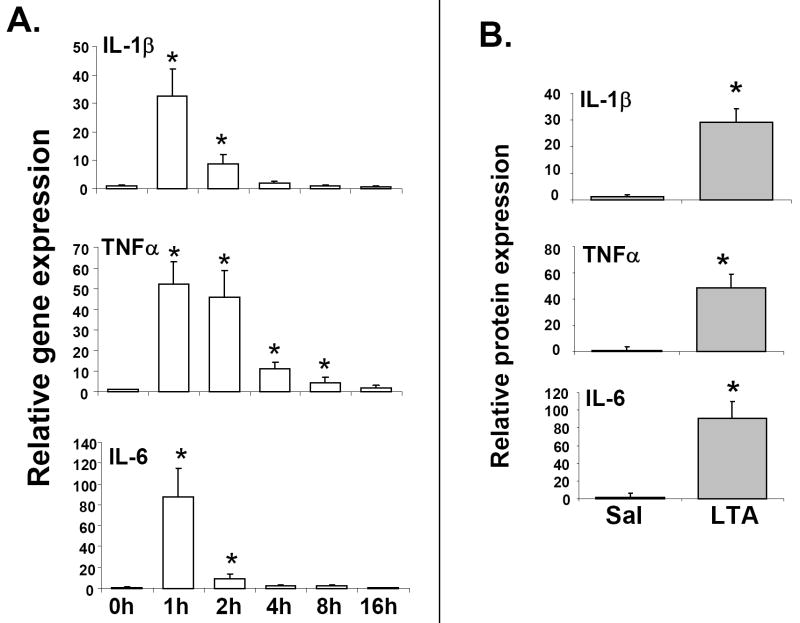
It has been previously shown that the TLR4 ligand, LPS results in the activation of pro-inflammatory cytokines in several cell types in liver, including the resident liver macrophages or Kupffer cells, which are the primary sites of hepatic cytokine production [39]. These cytokines play important roles in the modulation of gene expression in hepatocytes [1]. To determine the immunostimulatory potential of LTA, RNA levels of the pro-inflammatory cytokines were measured in the livers of LTA-injected mice. RNA levels of the pro-inflammatory cytokines, IL-1β, TNFα and IL-6 were significantly induced as early as 1-2h by LTA treatment (Fig. 4A), and were reduced thereafter. TNFα RNA remained significantly induced at 2h, and was detectable at 4h, compared to IL-1β or IL-6. In accordance with the RNA levels, serum IL-1β, TNFα and IL-6 levels were found to be significantly induced at 1h after LTA treatment (Fig. 4B).
Fig. 4.
Regulation of cytokine expression by LTA. Mice were IP-injected with 6 mg/kg LTA and livers were harvested at various time-points (n=6 per group). RNA was isolated from the livers and analyzed by TaqMan real-time PCR as described previously in Materials and methods (A). Serum cytokine levels were measured by enzyme-linked immunosorbent assay (n=6 per group). Error bars denote S.D. (B).
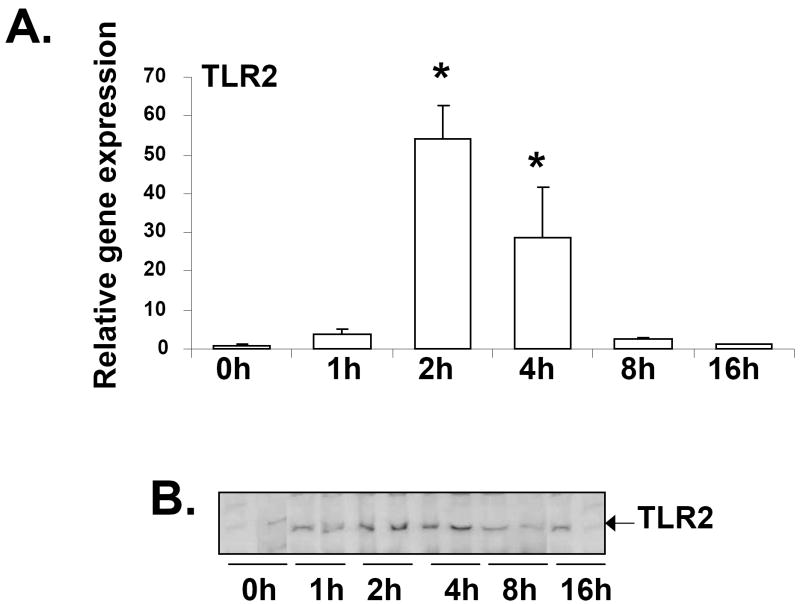
It is known that LTA can stimulate cytokine production in immune cells by TLR2-dependent pathway [40], and LTA-mediated induction of TLR2 gene expression had been previously reported in human odonoblasts [41]. LTA treatment resulted in ~50-fold induction of TLR2 RNA levels in mouse liver at 2 hours, followed by a reduction after 4 hours (Fig. 5A). Western blot analysis demonstrated a similar increase of TLR2 protein expression in the membrane fractions prepared from the livers of mice injected with LTA (Fig. 5B).
Fig. 5.
Regulation of TLR2 expression by LTA. Mice were IP-injected with 6 mg/kg LTA and livers were harvested at various time-points (n=6 per group). RNA was isolated from the livers and analyzed by TaqMan real-time PCR as described previously in Materials and methods (A). TLR2 expression was analyzed by western blot analysis of membrane fractions prepared from livers of mice injected with LTA at various time-points (B).
Activation of cell-signaling pathways by lipoteichoic acid
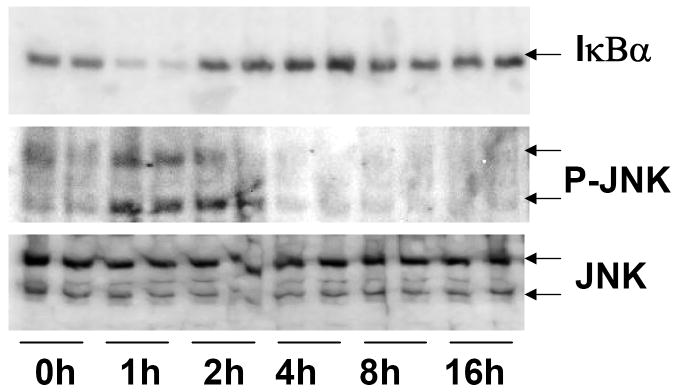
The cell-signaling components, JNK and NF-κB are critical components of the TLR signaling pathway [15], and the mechanism of suppression of key hepatic genes in LPS-induced inflammation involves cross-talk between cell-signaling and NRs; [25; 26; 29; 42; 43; 44]. Activation of JNK by LTA was maximal at 1-2h after LTA treatment as indicated by increased levels of phosphorylated JNK (P-JNK) in whole cell extracts (Fig. 6). At higher time-points (from 4-16h), P-JNK levels were not detectable. The levels of total JNK protein remained constant at all time-points. Activation of NF-κB was determined by degradation of the inhibitory subunit, IκBα after LTA treatment (Fig. 6). Activation of NF-κB was maximal at 1h after LTA treatment, and decreased thereafter.
Fig. 6.
Regulation of cell-signaling pathways by LTA. Whole cell extracts were prepared from the livers of saline and LTA-injected mice, and the samples were analyzed by immunoblotting. Phosphorylation of JNK (P-JNK) and degradation IκBα of were measured as markers of JNK and NF-κB activation, respectively.
Role of Kupffer cells in mediating the effects of LTA on expression of hepatic genes
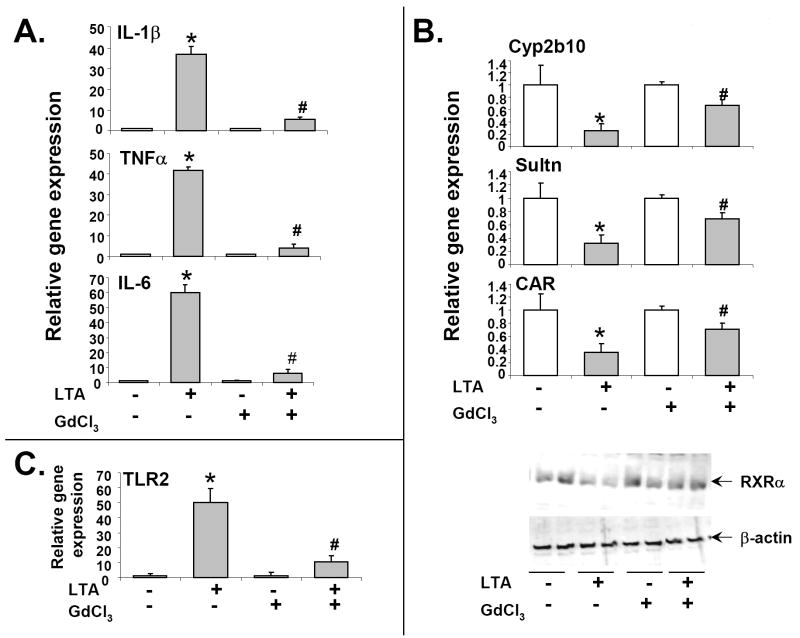
Our results have shown that LTA treatment of mice leads to the induction of the pro-inflammatory cytokines, IL-1β, TNFα and IL-6 (Fig. 4). The resident liver macrophages or Kupffer cells are the main source of cytokine production in the liver [39], and previous studies have shown that treatment with GdCl3 can inhibit the Kupffer cells [6; 32]. We have previously shown that cytokines can affect DME and nuclear receptor expression in the hepatocytes [33; 45], raising the possibility that cytokines may be involved in mediating the effects of LTA on hepatic genes. We find that LTA-mediated induction of cytokine RNA levels was attenuated by GdCl3 pre-treatment (Fig. 7A), indicating that this treatment was effective in inhibiting the Kupffer cells. The effect of GdCl3 was tested on representative DMEs and NRs, Cyp2b10, Sultn, CAR and RXRα, based on the observation that LTA had maximal effects on their expression (Figs 1 and 3). GdCl3 pre-treatment ameliorated the effects of LTA on these NRs and DMEs (~50-60% attenuation), indicating that Kupffer cells are involved in mediating the effects of LTA (Fig. 7B). Interestingly, TLR2 induction by LTA was reversed by GdCl3, indicating that Kupffer cells play important roles in regulating the expression of TLR2 by LTA (Fig. 7C).
Fig. 7.
Role of Kupffer cells. Mice were given a single dose of GdCl3 intravenously (10 mg/kg) or the vehicle, saline, followed by IP injection of LTA (6 mg/kg) 24h after GdCl3 treatment. Livers were harvested and RNA and protein analysis was done as described previously in Materials and methods. RNA levels of cytokines were measured after 24h of GdCl3 treatment, followed by LTA treatment for 1h (A). DMEs and nuclear receptors were measured after 4h of LTA treatment (B), and TLR2 RNA levels were measured after 2h of LTA treatment (C). Error bars denote S.D. *p < 0.05 between saline and LTA-treated samples (without GdCl3 treatment); # p < 0.05, comparing LTA injections with and without GdCl3 pre-treatment.
Discussion
Altered expression of hepatic drug metabolizing enzymes and transporters in inflammation can have deleterious consequences in terms of human health, and the underlying mechanism remains to be fully elucidated. The regulation of hepatic genes in inflammation has been widely studied in animal models injected with the Gram-ve bacterial endotoxin, LPS, however, the role of Gram+ve bacterial components have never been investigated. These studies show for the first time that the Gram+ve bacterial component, LTA can alter the expression of hepatic DME and transporter genes in a time-dependent and gene-specific manner. We find that Cyp2b10, Cyp2a4 and Sultn RNA levels are significantly reduced as early as 2h of LTA treatment, and in case of Cyp2b10 and Sultn, the reduction was detected till 16h. Cyp2b10 is a prototypical CAR target gene, and Cyp2a4 and Sultn are known to be regulated only by CAR, and not PXR in mouse liver [46; 47]. Cyp3a11 and the Mrp transporters were shown to be regulated by both PXR and CAR, while Ugt1a1 is known to be primarily regulated by PXR in mouse liver [47].
RNA levels of the NR, CAR is significantly reduced by LTA at 2h, which coincides with the suppression of its target hepatic genes. CAR target genes were reduced from 8-16h, while CAR RNA levels were back to basal levels by this time-point. It is likely that CAR protein levels were reduced by LTA at higher time-points, resulting in reduced expression of its target genes. The lack of reliable antibodies to CAR is a major impediment to make any definite conclusions. Nuclear protein levels of the central NR, RXRα were reduced from 4-16h, and may contribute to reduced expression of the enzyme and transporter genes. The role of NRs in inflammation-mediated alterations of hepatic gene expression is not fully understood. It has been previously shown that LPS-mediated alterations in DME gene expression is also associated with reduced expression of these NRs [1; 6; 7; 8; 9; 10; 11; 12; 48]; however, studies in nuclear receptor deficient mice have indicated that PXR may not be involved in LPS-mediated down-regulation of cytochrome P450 genes [49]. Although, these experiments show that altered expression of DME and transporter genes by LTA is associated with reduced expression of the PXR, CAR and RXRα, the role of these NRs in regulation of LTA-mediated alterations of hepatic gene expression can be best addressed by transgenic mice.
LTA treatment leads to activation of the pro-inflammatory cytokines in the liver at 1h, followed by significant induction of TLR2 expression at 2h. Inactivation of Kupffer cells by GdCl3 attenuates LTA-mediated induction of TLR2, indicating that cytokines may be involved in mediating the induction of TLR2 expression by LTA (Fig. 7C). Cytokine-mediated up-regulation of TLR2 has been reported previously; it has been shown that the cytokines, IL-1β and TNFα can induce the expression of TLR2 in hepatocytes [50]. It is likely that the effects of LTA on hepatic genes are mediated by TLR2 in the liver, and LTA itself may play a role in up-regulation of this cell-surface receptor. LTA-mediated increase in TLR2 expression has been reported in human odontoblasts [41].
These results show for the first time that the TLR2 ligand, LTA has a higher propensity to suppress CAR and its target genes in the liver, and these observations are different from LPS-injected mice. LPS and LTA bind to TLRs 4 and 2 respectively, which recruit downstream adaptors activating cell-signaling components, including kinases. It will be interesting to determine if different adaptor or kinase specificity by the TLRs can account for the differences in gene regulation by LPS and LTA. Suitable transgenic mice models with defects in TLR signaling will provide valuable information regarding the mechanism of suppression of these hepatic genes by LTA.
Our results show that inactivation of Kupffer cells by GdCl3 treatment attenuates the effects of LTA on DMEs and NRs (Fig. 7). This indicates that the mechanism of suppression of hepatic gene expression by LTA likely involves the activation of pro-inflammatory cytokines in the Kupffer cells by TLR2-dependent pathways. These cytokines contribute to activation of cell-signaling pathways, JNK and NF-κB in the hepatocytes, which cross-talk with the NRs, thereby impairing expression of the target genes. However, the preferred suppression of CAR and its target genes by LTA needs to be further investigated. CAR gene expression is regulated by hepatocyte nuclear factors and other basal transcription factors, which may contribute towards LTA-mediated alterations in CAR expression [51]. It is known that hepatocytes also express TLRs [52], there is a possibility that TLR2 on hepatocyte surface may facilitate direct action of LTA on the hepatocytes, leading to altered expression of hepatic DME and transporter genes.
These results show for the first time that the TLR2 ligand, LTA can alter the expression of hepatic genes involved in drug metabolism and transport, and Kupffer cells are involved in mediating the effects of LTA. Thus, inflammation induced by components of Gram+ve bacteria can have serious medical implications in terms of impaired drug metabolism and transport.
Acknowledgments
This work was supported by grants from the National Institutes of Health K01DK076057-02 to RG.
Footnotes
Portions of this work were presented at Experimental Biology, April 2008 in San Diego CA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aitken AE, Richardson TA, Morgan ET. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–49. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- 2.Renton KW. Regulation of drug metabolism and disposition during inflammation and infection. Expert Opin Drug Metab Toxicol. 2005;1:629–40. doi: 10.1517/17425255.1.4.629. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 4.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15:355–66. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 6.Xu DX, Wei W, Sun MF, Wu CY, Wang JP, Wei LZ, Zhou CF. Kupffer cells and reactive oxygen species partially mediate lipopolysaccharide-induced downregulation of nuclear receptor pregnane x receptor and its target gene CYP3a in mouse liver. Free Radic Biol Med. 2004;37:10–22. doi: 10.1016/j.freeradbiomed.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem Biophys Res Commun. 2002;293:145–9. doi: 10.1016/S0006-291X(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 8.Ghose R, White D, Guo T, Vallejo J, Karpen SJ. Regulation of hepatic drug-metabolizing enzyme genes by Toll-like receptor 4 signaling is independent of Toll-interleukin 1 receptor domain-containing adaptor protein. Drug Metab Dispos. 2008;36:95–101. doi: 10.1124/dmd.107.018051. [DOI] [PubMed] [Google Scholar]
- 9.Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2:4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang C, Yoon S, Tindberg N, Jarvelainen HA, Lindros KO, Ingelman-Sundberg M. Hepatic expression of multiple acute phase proteins and down-regulation of nuclear receptors after acute endotoxin exposure. Biochem Pharmacol. 2004;67:1389–97. doi: 10.1016/j.bcp.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Xu DX, Chen YH, Wang JP, Sun MF, Wang H, Wei LZ, Wei W. Perinatal lipopolysaccharide exposure downregulates pregnane X receptor and Cyp3a11 expression in fetal mouse liver. Toxicol Sci. 2005;87:38–45. doi: 10.1093/toxsci/kfi239. [DOI] [PubMed] [Google Scholar]
- 12.Chen YH, Wang JP, Wang H, Sun MF, Wei LZ, Wei W, Xu DX. Lipopolysaccharide treatment downregulates the expression of the pregnane X receptor, cyp3a11 and mdr1a genes in mouse placenta. Toxicology. 2005;211:242–52. doi: 10.1016/j.tox.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–9. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 15.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–65. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 18.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998;95:588–93. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda K. Evolution and integration of innate immune recognition systems: the Toll-like receptors. J Endotoxin Res. 2005;11:51–5. doi: 10.1179/096805105225006687. [DOI] [PubMed] [Google Scholar]
- 20.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 21.Wang JE, Dahle MK, Yndestad A, Bauer I, McDonald MC, Aukrust P, Foster SJ, Bauer M, Aasen AO, Thiemermann C. Peptidoglycan of Staphylococcus aureus causes inflammation and organ injury in the rat. Crit Care Med. 2004;32:546–52. doi: 10.1097/01.CCM.0000109775.22138.8F. [DOI] [PubMed] [Google Scholar]
- 22.Cleveland MG, Gorham JD, Murphy TL, Tuomanen E, Murphy KM. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–12. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattor Y, Kasai K, Akimoto K, Thiemermann C. Induction of NO synthesis by lipoteichoic acid from Staphylococcus aureus in J774 macrophages: involvement of a CD14-dependent pathway. Biochem Biophys Res Commun. 1997;233:375–9. doi: 10.1006/bbrc.1997.6462. [DOI] [PubMed] [Google Scholar]
- 24.Assenat E, Gerbal-Chaloin S, Larrey D, Saric J, Fabre JM, Maurel P, Vilarem MJ, Pascussi JM. Interleukin 1beta inhibits CAR-induced expression of hepatic genes involved in drug and bilirubin clearance. Hepatology. 2004;40:951–60. doi: 10.1002/hep.20387. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Zimmerman TL, Thevananther S, Lee HY, Kurie JM, Karpen SJ. Interleukin-1 beta-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent. J Biol Chem. 2002;277:31416–22. doi: 10.1074/jbc.M204818200. [DOI] [PubMed] [Google Scholar]
- 26.Pascussi JM, Dvorak Z, Gerbal-Chaloin S, Assenat E, Maurel P, Vilarem MJ. Pathophysiological factors affecting CAR gene expression. Drug Metab Rev. 2003;35:255–68. doi: 10.1081/dmr-120026394. [DOI] [PubMed] [Google Scholar]
- 27.Tan Z, Huang M, Puga A, Xia Y. A critical role for MAP kinases in the control of Ah receptor complex activity. Toxicol Sci. 2004;82:80–7. doi: 10.1093/toxsci/kfh228. [DOI] [PubMed] [Google Scholar]
- 28.Yu R, Lei W, Mandlekar S, Weber MJ, Der CJ, Wu J, Kong AN. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J Biol Chem. 1999;274:27545–52. doi: 10.1074/jbc.274.39.27545. [DOI] [PubMed] [Google Scholar]
- 29.Cheng PY, Wang M, Morgan ET. Rapid transcriptional suppression of rat cytochrome P450 genes by endotoxin treatment and its inhibition by curcumin. J Pharmacol Exp Ther. 2003;307:1205–12. doi: 10.1124/jpet.103.057174. [DOI] [PubMed] [Google Scholar]
- 30.Assenat E, Gerbal-chaloin S, Maurel P, Vilarem MJ, Pascussi JM. Is nuclear factor kappa-B the missing link between inflammation, cancer and alteration in hepatic drug metabolism in patients with cancer? Eur J Cancer. 2006;42:785–92. doi: 10.1016/j.ejca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281:17882–9. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 32.Hardonk MJ, Dijkhuis FW, Hulstaert CE, Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol. 1992;52:296–302. doi: 10.1002/jlb.52.3.296. [DOI] [PubMed] [Google Scholar]
- 33.Ghose R, Mulder J, von Furstenberg RJ, Thevananther S, Kuipers F, Karpen SJ. Rosiglitazone attenuates suppression of RXRalpha-dependent gene expression in inflamed liver. J Hepatol. 2007;46:115–23. doi: 10.1016/j.jhep.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsunaga N, Ikeda M, Takiguchi T, Koyanagi S, Ohdo S. The molecular mechanism regulating 24-hour rhythm of CYP2E1 expression in the mouse liver. Hepatology. 2008;48:240–51. doi: 10.1002/hep.22304. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa T, Manabe S, Watanabe T, Sehata S, Sharyo S, Okada T, Mori Y. Daily fluctuation of hepatic P450 monooxygenase activities in male rats is controlled by the suprachiasmatic nucleus but remains unaffected by adrenal hormones. Arch Toxicol. 1999;73:367–72. doi: 10.1007/s002040050675. [DOI] [PubMed] [Google Scholar]
- 36.Ros JE, Schuetz JD, Geuken M, Streetz K, Moshage H, Kuipers F, Manns MP, Jansen PL, Trautwein C, Muller M. Induction of Mdr1b expression by tumor necrosis factor-alpha in rat liver cells is independent of p53 but requires NF-kappaB signaling. Hepatology. 2001;33:1425–31. doi: 10.1053/jhep.2001.24667. [DOI] [PubMed] [Google Scholar]
- 37.Karpen SJ. Nuclear receptor regulation of hepatic function. J Hepatol. 2002;36:832–50. doi: 10.1016/s0168-8278(02)00129-0. [DOI] [PubMed] [Google Scholar]
- 38.Chen C, Staudinger JL, Klaassen CD. Nuclear receptor, pregname X receptor, is required for induction of UDP-glucuronosyltranferases in mouse liver by pregnenolone-16 alpha-carbonitrile. Drug Metab Dispos. 2003;31:908–15. doi: 10.1124/dmd.31.7.908. [DOI] [PubMed] [Google Scholar]
- 39.Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995;164:383–9. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- 40.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 41.Durand SH, Flacher V, Romeas A, Carrouel F, Colomb E, Vincent C, Magloire H, Couble ML, Bleicher F, Staquet MJ, Lebecque S, Farges JC. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J Immunol. 2006;176:2880–7. doi: 10.4049/jimmunol.176.5.2880. [DOI] [PubMed] [Google Scholar]
- 42.Oesch-Bartlomowicz B, Oesch F. Phosphorylation of cytochromes P450: first discovery of a posttranslational modification of a drug-metabolizing enzyme. Biochem Biophys Res Commun. 2005;338:446–9. doi: 10.1016/j.bbrc.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 43.Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 2001;276:39638–44. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- 44.Zhou C, Tabb MM, Nelson EL, Grun F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, Blumberg B. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ. Nuclear export of retinoid X receptor alpha in response to interleukin-1beta-mediated cell signaling: roles for JNK and SER260. 2006 doi: 10.1074/jbc.M508277200. [DOI] [PubMed] [Google Scholar]
- 46.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–3. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 47.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–46. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 48.Sachdeva K, Yan B, Chichester CO. Lipopolysaccharide and cecal ligation/puncture differentially affect the subcellular distribution of the pregnane X receptor but consistently cause suppression of its target genes CYP3A. Shock. 2003;19:469–74. doi: 10.1097/01.shk.0000048903.46342.ec. [DOI] [PubMed] [Google Scholar]
- 49.Richardson TA, Morgan ET. Hepatic cytochrome P450 gene regulation during endotoxin-induced inflammation in nuclear receptor knockout mice. J Pharmacol Exp Ther. 2005;314:703–9. doi: 10.1124/jpet.105.085456. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Salyapongse AN, Geller DA, Vodovotz Y, Billiar TR. Hepatocyte toll-like receptor 2 expression in vivo and in vitro: role of cytokines in induction of rat TLR2 gene expression by lipopolysaccharide. Shock. 2000;14:361–5. doi: 10.1097/00024382-200014030-00021. [DOI] [PubMed] [Google Scholar]
- 51.Pascussi JM, Robert A, Moreau A, Ramos J, Bioulac-Sage P, Navarro F, Blanc P, Assenat E, Maurel P, Vilarem MJ. Differential regulation of constitutive androstane receptor expression by hepatocyte nuclear factor4alpha isoforms. Hepatology. 2007;45:1146–53. doi: 10.1002/hep.21592. [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, Gambotto AA, Humphris EL, Vodovotz Y, Billiar TR. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002;70:3433–42. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]