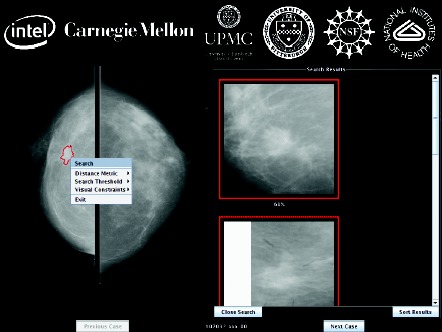
 Screenshot of the MassFind application built on the OpenDiamond platform (Figure provided by M. Satyanarayanan, PhD).
Screenshot of the MassFind application built on the OpenDiamond platform (Figure provided by M. Satyanarayanan, PhD).
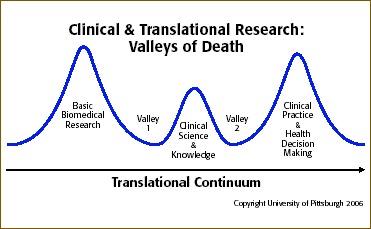
Bridging the valley of death” is a phrase that has been used in nonhealth‐related disciplines to describe the fundamental challenge of applying research and development advances to operations. 1 However, the “valley of death” metaphor is equally appropriate to describe the two major gaps in the translational research continuum: (1) the translation of laboratory discoveries to human subjects and (2) the translation of the resulting evidence to clinical and public health practice and policy. 2 , 3 As one of the initial 12 academic health centers to receive a Clinical and Translational Science Award (CTSA) from the National Institutes of Health (NIH) in 2006, the University of Pittsburgh is part of a growing national consortium (24 institutions in 18 states as of September 2007) on the forefront of developing and evaluating new research models as a means of bridging these valleys.
The intent of the CTSA program—an outgrowth of NIH's well‐publicized Roadmap for Medical Research—is to transform how clinical and translational research is done and, thus, to improve how efficiently and effectively biomedical advances reach the individual patient and the population as a whole. NIH has expressed the significance and urgency of this plan: “Over the years, clinical research that helps discover mechanisms of disease, prevention, diagnosis, or treatment has become more difficult to conduct. Yet the exciting discoveries we are currently making require us to conduct even more efficiently the complex clinical studies needed to make rapid medical progress and to further inform our basic science efforts. This is undoubtedly the most challenging, but critically important, area identified through the NIH roadmap process.” 4
Strategies employed by industry to meet the “valley of death” challenge include the development of interdisciplinary research programs, infrastructure, interfaces with user communities, observation and data access partnerships, and continuous evaluation processes. 1 In addition to adopting these strategies, the biomedical research community must develop strategies to overcome other barriers in order to more effectively translate research findings into healthcare practice. 5 Among these barriers are the complex organizational structures of traditional academic health centers, cultures that do not foster collaboration, a shortage of translational investigators to bridge the two major gaps, the absence of mechanisms to facilitate transdisciplinary research, inadequate financial support, and regulatory impediments to clinical research.
With $83.5 million in CTSA funding over a 5‐year period, the University of Pittsburgh has established a Clinical and Translational Science Institute (CTSI) to serve the dual purposes of: (1) integrating existing programs with innovative new clinical and translational science initiatives under a common umbrella and (2) creating an awareness and understanding—initially among members of the biomedical research/healthcare community but eventually among the general public as well—of the tangible benefits to health practice that can be realized from clinical and translational research.
A primary CTSI focus is to develop, nurture, and support a cadre of highly trained clinical and translational scientists by implementing comprehensive educational and training programs. Other goals include (1) fostering a multidisciplinary, team‐based approach to research (such as through an online resource for linking investigators and clinicians from throughout the University's six health sciences schools and the University of Pittsburgh Medical Center (UPMC)); (2) providing needed resources (for example, research facilitators to serve as a single point of access for investigators to tap into CTSI's growing array of programs and services); and (3) developing community and industry partnerships not only to draw patients, particularly those from underserved communities who might benefit most from the experience, into clinical research but also to foster the development of novel technologies that could result from such efforts. In all, CTSI embraces 10 core areas of development covering aspects of research from the basics of study design to technology transfer and entrepreneurship.
During the recently completed first year of funding, two initiatives began to emerge from CTSI's Novel Clinical and Translational Methodologies Core, which is designed to serve as an incubator for CTSI‐sponsored research that can broadly affect translational research and clinical practice. Each initiative addresses one of the two valleys of death. One involves the adaptation of innovative computer software for diagnostic purposes; the other lays the groundwork for broad public participation in research and clinical trials.
Addressing the first valley of death, the Diamond Program illustrates the value of multidisciplinary collaboration to apply emerging bioinformatics technologies to the improvement of clinical practice. Diamond is an open‐source software architecture jointly created by Intel and Carnegie Mellon University (both CTSI collaborators) for rapidly scanning large volumes of distributed data and filtering those data with domain‐specific software to find a few vaguely specified items in terabytes or petabytes of complex, loosely structured data like digital photographs and video streams. Clinical radiology images seemed to be an ideal proving ground for the software's medical capabilities. CTSI linked Diamond developers at Carnegie Mellon with clinicians and clinical scientists at the University of Pittsburgh; the ensuing collaboration resulted in a new approach for detecting breast cancer with Diamond's interactive search‐assisted diagnosis (ISAD) system to evaluate lesions depicted on mammograms and pathology images. ISAD retrieves relevant data from a large reference collection of region‐of‐interest images to enable radiologists to make better‐informed decisions about a given case by providing a selection of similar annotated cases. During its first year of CTSI support, this program developed an ISAD prototype using the Diamond search framework and assembled a mammogram reference database of 4,270 suspected breast mass regions, of which 1,792 depict histologically confirmed malignant masses, 717 depict known benign masses, and 1,761 depict false‐positive mass regions as identified by traditional computer‐assisted diagnosis (CAD). In addition to efficiently comparing mammograms with the reference database by distributing searching over multiple computer nodes, the program can intelligently structure the search and exploit cached results from similar previous queries. Evaluation of this algorithm suggests that Diamond outperforms other CAD methods and, therefore, has significant potential to improve the accuracy of breast cancer diagnosis using mammography. CTSI is now developing plans to translate this first known medical application of Diamond into clinical practice.
To address the second valley of death, CTSI is collaborating with its clinical partner, the UPMC, to implement a novel program to overcome the dearth of clinical research participants by developing an institutional clinical research participant registry. The registry will consist of a database of UPMC patients who consent to be contacted about clinical studies and a list of current studies being conducted through the University. Specific goals of the registry are: (1) to increase recruitment for clinical research by providing information about relevant studies to patients and their physicians, (2) to improve the efficiency of recruitment for research studies by electronically matching patients’ clinical characteristics and data from the UPMC electronic health records system (eRecord) with inclusion/exclusion criteria for specific studies, and (3) to develop research‐informed patients by providing registry participants with periodic educational materials about clinical research.
The CTSI registry will leverage UPMC's $500 million investment to build a fully interoperable eRecord system designed to improve healthcare quality and eliminate medical errors; at the same time, it gives clinical researchers potential access to patients who use UPMC's 538 physician offices and specialized outpatient locations as well as its 19 hospitals, which account for more than 3 million outpatient visits and more than 167,000 hospital admissions in 2006. The critical first step will be to introduce people to the registry through UPMC's electronic patient registration system when they check in for healthcare services. Patients will receive educational materials about clinical research and the potential benefits of becoming a research participant.
Using an Institutional Review Board‐approved consent form, they will be asked to provide written permission to participate in the registry; if they agree, their medical records will be matched electronically with inclusion criteria for nearly 7,000 active studies. In addition, participants will receive regular mailings about studies or medical issues that might be pertinent to them and be offered the chance to participate in specific studies. At the same time, safeguards will be established to protect the confidentiality of patient records and to prevent their use outside the registry's carefully prescribed parameters.
Although the primary purpose of the research registry is to recruit participants for clinical studies, CTSI's broader vision is to initiate a novel mechanism to facilitate clinical investigations of rare diseases throughout the United States. By exporting the registry infrastructure to other CTSA sites nationally, patients with rare diseases can be identified and matched with studies of those diseases being conducted by other CTSA institutions. Such an initiative would hasten the speed with which research on rare illnesses could be completed, potentially leading to treatment advances, as well as facilitate the formation of a national network of CTSA institutions that routinely collaborate on clinical and translational research.
These two initiatives exemplify the University of Pittsburgh Clinical and Translational Science Institute's emphasis on developing new research infrastructure and tools—particularly programs that attempt to cross one of the translational research valleys of death—to ultimately improve the prevention, diagnosis, and treatment of disease. In its second year, CTSI will expand this conceptual approach to develop specialized centers of excellence in cross‐cutting research areas that span the full translational research continuum. Such centers stand to gain the most from substantive transdisciplinary collaboration and will also provide foci for educational and training opportunities. Only when these two valleys have been effectively bridged will the nation's biomedical research enterprise truly have been transformed.
Acknowledgment
This publication was made possible by Grant Number 1UL1 RR024153‐01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
References
- 1. Tsugawa B, ed. Section 1: Research operations: bridging the valley of death. The Federal Meteorological Services and Supporting Research. Fiscal Year 2002; 2001, Section 1: 1–8.
- 2. Sung NS, Crowley WFJ, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K, Larson EL, Scheinberg D, Reece EA, Slavkin H, Dobs A, Grebb J, Martinez RA, Korn A, Rimoin D. Central challenges facing the national clinical research enterprise. JAMA. 2003;289: 1278–1287. [DOI] [PubMed] [Google Scholar]
- 3. Clinical Trials Networks Best Practices: NIH Roadmap. Available from: https://www.ctnbestpractices.org/networks/nih‐ctsa‐awardees/university‐of‐pittsburgh‐pittsburgh‐pa/view. Accessed November 14, 2007.
- 4. National Institutes of Health . NIH Roadmap for Medical Research Overview page. Available from: http://www.nihroadmap.nih.gov/overview.asp. Accessed November 14, 2007.
- 5. Pober JS, Neuhauser CS, Pober JM. Obstacles facing translational research in academic medical centers. FASEB J. 2001; 15: 2303–2313. [DOI] [PubMed] [Google Scholar]


