Abstract
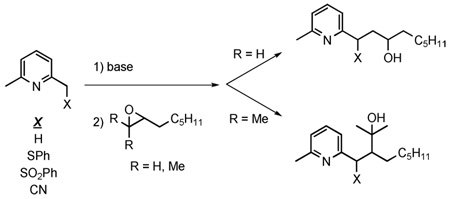
Substituted (6-methyl-2-pyridyl)methyllithium species were reacted with 1,2-epoxyoctane and 2-methyl-2,3-epoxynonane. The monosubstituted epoxide reacted efficiently with lutidyllithium and a number of 2-substituted-(6-methyl-2-pyridyl)methyllithium derivatives. The trisubstituted epoxide gave low yields of adducts with all (2-pyridyl)methyllithium species studied. These results are discussed in the context of a proposed synthesis of cananodine.
Cananondine (1) is a guaipyridine alkaloid isolated from the fruits of Cananga odorata shown to inhibit the growth of two hepatocellular carcinoma cell lines.1,2 The guaipyridines are a small class of natural products whose syntheses have not been extensively studied.3 As part of our ongoing efforts toward the synthesis of cananodine, we planned an intramolecular epoxide opening to form the seven-membered carbocycle of the natural product. A color change consistent with the formation of an α-pyridyl anion was observed when epoxy sulfone 2 was treated with either n-BuLi or LDA in THF, but we were unable to isolate any of the cyclized product 3. The majority of starting 2 was recovered in every case.4 Since the planned 7-exo-tet cyclization does not run afoul of Baldwin’s rules for ring closure,5 we concluded that the sulfone-stabilized anion is not nucleophilic enough and/or the trisubstituted epoxide is too hindered to be a competent electrophile.
Lateral lithiation of heterocyclic systems for subsequent functionalization is a powerful synthetic tool.6 The literature offers limited insight as to the solution of the present problem, however, as there are only a scattering of examples of α-pyridyl anions reacting with ethylene oxide, simple monosubstituted,7 or disubstituted epoxides.8 We have found no examples of a reaction of an α-pyridyl anion with a trisubstituted epoxide, nor have we found any examples of reactions with epoxides where the α-pyridyl carbon bears an acidifying group. Thus, we engaged in a directed study to address the questions raised above in the context of intermolecular reactions of α–substituted lutidyllithium species with epoxides.9
Amide bases have been used to generate picolyllithium species,7a,b but more often n-BuLi or PhLi is used for this purpose.7c–k The pKa value of 2-picoline has been determined to be 34 (Figure 1).10 This makes n-BuLi or PhLi (pKa butane ~ 50; pKa benzene = 43) the obvious choice over LDA (pKa of diisopropylamine = 36) for quantitative deprotonation of 2-picoline. Substitution at the α-position of the picoline increases acidity dramatically, however, as seen for 2-benzylpyridine and 2-(methylphenylsulfonyl)pyridine (Figure 1).11 Indeed, the conjugate base of 2-(methylphenylsulfonyl)pyridine has been reported to be unreactive toward alkyl halides and Michael acceptors.12 Given that our ultimate goal is an intramolecular epoxide opening, we were mindful of the compatibility of the chosen base with epoxide functionality. Thus, we explored the use of both LDA and n-BuLi as bases and the use of various acidifying groups at the α-position.
Figure 1.
Relative acidities of 2-picoline derivatives.
Addition of 2,6-lutidine (4a) to a solution of LDA in THF at −78 °C resulted in a deep reddish solution, characteristic of the picolyl-type lithium species. Addition of 1,2-epoxyoctane provided a good yield of the adduct 5a7g after workup and purification (Table 1, entry 1). Use of boron trifluoride as an additive did not dramatically improve the yield of 5a (entry 2), but did decrease the reaction time from 12–18 h to 15 min. HMPA as an additive offered no advantages (entry 3). Using n-BuLi instead of LDA resulted in a somewhat higher yield of 5a (entry 4), and formation of a ‘higher-order’ cuprate13 species from the lutidyllithium using 0.5 equiv of CuCN gave the highest yield of 5a (entry 5).
Table 1.
Reaction of lutidyllithium derivatives with 1,2-epoxyoctane.
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | base | additive | product | Yield (%)a | |
| 1 | 4a | H | LDA | none | 5a | 61 |
| 2 | 4a | H | LDA | BF3•OEt2 b | 5a | 66 |
| 3 | 4a | H | LDA | HMPAc | 5a | 56 |
| 4 | 4a | H | BuLi | none | 5a | 80 |
| 5 | 4a | H | BuLi | CuCNd | 5a | 89 |
| 6 | 4b | CN | LDA | none | 5b | 36e |
| 7 | 4c | SPh | LDA | none | 5c | 68e |
| 8 | 4c | SPh | BuLi | none | 5c | 70e |
| 9 | 4d | SO2Ph | LDA | none | 5d | 47e |
| 10 | 4d | SO2Ph | BuLi | none | 5d | 74e |
isolated yields of purified 5.
1 equiv.
2 equiv.
0.5 equiv.
mixture of diastereomers
Three acidifying groups on the α-position of lutidine were tried: cyano, thiophenyl, and sulfonylphenyl. These groups were chosen based on ease of substrate preparation and the options available for eventual removal of the acidifying group after reaction with the epoxide. Of these, the cyano substrate 4b14 was least effective in reacting with epoxyoctane (entry 6). The yield of 5b was diminished by formation of side products including lactone 6, which is formed by intramolecular attack of the intermediate alkoxide or hydroxyl group on the nitrile.15 Thiophenyl-substituted substrate 4c16 was successfully deprotonated with either n-BuLi or LDA to give similar yields of adduct 5c after reaction with 1,2-epoxyoctane (entries 7 and 8). The sulfonylphenyl substrate 4d gave modest yields of adduct 5d (entry 9) when LDA was used as base, but the yield of 5d was significantly improved using n-BuLi.
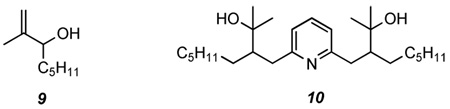
We next examined reactions of lutidyllithium with the trisubstituted epoxide 2-methyl-2,3-epoxynonane (7)17 (Table 2). Deprotonation of 4a with n-BuLi followed by addition of the epoxide gave a modest yield of adduct 8a (entry 1). Use of LDA to deprotonate 4a gave a much lower yield of 8a along with dialkylated product 10 (8%) and a mixture of unreacted 7 and allylic alcohol 918 (1:1 ratio, 50%) (entry 2). Use of boron trifluoride and HMPA as additives with LDA did not improve the yield of 8a, but did prevent the formation of 10. In both of these cases, however, a similar amount of unreacted 7 and allylic alcohol 9 was isolated as before (55 and 60%, respectively, entries 3 and 4). Use of a higher-order cuprate derived from 4a also gave a relatively poor yield of 8a. The thiophenyl substituted derivative 4c gave very low yields of adduct 8c, regardless of base used (entries 6 and 7), and the sulfonylphenyl-containing substrate 4d failed to produce 8d after deprotonation with n-BuLi and reaction with epoxide 7 (entry 8).
Table 2.
Reaction of lutidyllithium derivatives with 2-methyl-2,3-epoxynonane.
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | base | additive | product | Yield (%)a | |
| 1 | 4a | H | BuLi | none | 8a | 40 |
| 2 | 4a | H | LDA | none | 8a | 12b |
| 3 | 4a | H | LDA | BF3•OEt2 c | 8a | 8 |
| 4 | 4a | H | LDA | HMPAd | 8a | 29 |
| 5 | 4a | H | BuLi | CuCNe | 8a | 23 |
| 6 | 4c | SPh | BuLi | none | 8c | 9 f |
| 7 | 4c | SPh | LDA | none | 8c | 6 f |
| 8 | 4d | SO2Ph | BuLi | none | 8d | 0 |
isolated yields of purified 8.
dialkylated product 10 (8%) also isolated.
1 equiv.
2 equiv.
0.5 equiv.
mixture of diastereomers
Clearly the trisubstituted epoxide 7 is a much poorer electrophile than 1,2-epoxyoctane in the reaction with (6-methyl-2-pyridyl)methyllithium species. The dominant reaction path is epoxide elimination to produce 9. These results, within the context of our planned synthesis of cananodine, suggest a monosubstituted epoxide should be employed in the attempted cyclization.
As for the nature of the acidifying group in the α-position of the pyridine for the planned intramolecular reaction, our results indicate the sulfur-containing substituents will be more useful than the nitrile. Furthermore, the thiophenyl group is preferable to the sulfonylphenyl group if LDA is to be used in the intramolecular reaction (Table 1, compare entries 7 and 9).
In order to gain further insight as to the suitability of the thiophenyl substituent for the cananodine synthesis, we explored methods to reductively cleave this group from adduct 5c. Samarium(II) iodide,19 Raney nickel,20 and lithium di-tert-butylbiphenylide (LDBB)21 all successfully converted 5b to 5a, but Raney nickel gave the highest yield and the cleanest product mixture (Figure 2).
Figure 2.
Reductive cleavage of the -SPh group from 5c.
To summarize, the reaction of lutidyllithium with 1,2-epoxyoctane provides good yields of adducts, with slightly higher yields obtained when using n-BuLi versus LDA to generate the alkyllithium species. (6-Methyl-2-pyridyl)methyllithium species substituted in the α-position with a thiophenyl or sulfonylphenyl group also react effectively with 1,2-epoxyoctane, while the cyano-substituted substrate was less effective. In contrast, a trisubstituted epoxide was a poor electrophile for all picolyl-type lithium species studied. These results suggest a monosubstituted epoxide is more likely to be successful in an intramolecular reaction required for our planned synthesis of cananodine.
Experimental Section22
1-(6-Methylpyridin-2-yl)nonan-3-ol (5a)
A 25-mL round-bottomed flask under argon was charged with LDA (3.0 mmol, prepared from diisopropylamine and n-BuLi in ~15 mL THF) and cooled in a dry ice / 2-propanol bath. 2,6-Lutidine (0.35 mL, 0.322 g, 3.0 mmol) was added dropwise to the flask. The solution turned red, and after 15 minutes the dry ice bath was removed and the flask allowed to warm to room temperature. The flask was then recooled in the dry ice slush bath, 1,2-epoxyoctane (0.46 mL, 0.38 g, 3.0 mmol) was added, and the solution was allowed to slowly warm to room temperature overnight. Saturated NH4Cl solution was then added and the mixture was extracted with ether (3×15 mL). The combined organic layers washed with saturated NH4Cl and brine before drying over Na2SO4. After solvent evaporation, the crude product was purified by radial chromatography (1:1 hexanes:ethyl acetate on a 2 mm silica rotor, 7.5 mL/min flow rate) to provide 5a as a pale yellow oil (0.429 g, 61%). When this procedure was repeated using n-BuLi in the place of LDA, 5a was isolated in 80% yield after chromatographic purification. IR (neat): 3344 (br), 1594, 1578, 1458, 1376 and 1061 cm−1. 1H NMR (500 MHz, CDCl3): δ 7.49 (t, J = 7.8, 1H), 6.97 (d, J = 7.8, 2H), 5.25 (br s, 1H, OH), 3.66 (m, 1H), 3.00, (ddd, J = 14.7, 8.8, 5.4, 1H), 2.92 (ddd, J = 14.7, 7.3, 5.4, 1H), 2.56 (s, 3H), 1.93 (dddd, J = 12.7, 8.3, 5.4, 2.9, 1H), 1.76 (dddd, J = 12.7, 8.8, 7.8, 5.4, 1H), 1.6 – 1.4 (m, 3H), 1.4 – 1.2 (m, 7H), 0.86 (t, J = 6.8, 3H). 13C NMR (125 MHz, CDCl3): δ 161.0, 157.3, 137.1, 120.6, 120.0, 71.6, 37.9, 36.0, 35.1, 31.9, 29.5, 25.9, 24.1, 22.6, and 14.1. HRMS (FAB) calcd for (C15H25NO+H)+ 236.2014, found 236.2033; Anal. Calcd for C15H25NO: C, 76.55; H, 10.71; N, 5.95. Found: C, 76.33; H, 10.94; N, 5.91.
2-Methyl-3-((6-methylpyridin-2-yl)methyl)nonan-2-ol (8a)
The procedure used for the preparation of 5a was followed using LDA (3.0 mmol), 2,6-lutidine (4a) (0.35 mL, 0.322 g, 3.0 mmol) and 2-methyl-2,3-epoxynonane (7) (0.469 g, 3.0 mmol). Extractive work-up and flash chromatography (gradient elution 15:1 hexanes:ethyl acetate to 1:2 hexanes:ethyl acetate) provided, in order of elution, unreacted 7 (0.114 g, 24%), allylic alcohol 918 (0.120 g, 26%), unreacted 4a (0.153 g, 48%), tertiary alcohol 8a (0.092 g, 12%), and dialkylated product 10 (0.098 g, 8%) as a mixture of diastereomers. When this procedure was repeated using n-BuLi in the place of LDA, 8a was isolated in 40% yield after chromatographic purification. Data for 8a: IR (neat): 3400 (br), 1594, 1578, and 1159 cm−1. 1H NMR (500 MHz, CDCl3): δ 7.49 (t, J = 7.8, 1H), 6.99 (d, J = 7.8, 1H), 6.96 (d, J = 7.3, 1H), 3.08 (dd, J = 15.6, 6.3, 1H), 2.86 (dd, J = 15.6, 2.9, 1H), 2.56 (s, 3H), 1.76 (m, 1H), 1.47 (m, 1H), 1.34 (s, 3H), 1.25 (m, 9H), 1.17 (s, 3H), 0.86 (t, J = 6.8, 3H). 13C NMR (125 MHz, CDCl3): δ 160.9, 157.3, 137.1, 120.7, 120.5, 71.9, 49.1, 37.4, 31.8, 30.7, 30.6, 29.4, 28.5, 25.9, 23.9, 22.6, and 14.1. CI-MS (MeOH): m/z 264 (M+H, 28), 247(18), and 246(100). HRMS (FAB) calcd for (C17H29NO+H)+ 264.2327, found 264.2331; Anal. Calcd for C17H29NO: C, 77.51; H, 11.10; N, 5.32. Found: C, 77.12; H, 11.39; N, 5.42.
Data for 10: 1H NMR (500 MHz, CDCl3): major diastereomer δ 7.49 (t, J = 7.8, 1H), 6.99 (d, J = 7.8, 2H), 3.16 (dd, J = 15.1, 5.4, 2H), 2.65 (dd, J = 15.1, 4.9, 2H), 1.86 (m, 2H), 1.45 (m, 2H), 1.30 (s, 6H), 1.3 – 1.1 (m, 20H), 1.15 (s, 6H), 0.83 (t, J = 7, 6H). 13C NMR (125 MHz, CDCl3): major diastereomer δ 161.2, 137.1, 120.7, 71.9, 50.0, 38.1, 31.7, 31.6, 29.7, 29.4, 28.7, 25.4, 22.6, and 14.1. CIMS (MeOH): m/z 420 (M+H, 48), 402(52), 385(56), and 59(100).
Supplementary Material
Additional experimental procedures, compound characterization data, and copies of 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
This work was supported by the National Cancer Institute, National Institutes of Health (R15 CA 122084).
References
- 1.Hsieh T-J, Chang F-R, Chia Y-C, Chen C-Y, Chiu H-F, Wu Y-C. J. Nat. Prod. 2001;64:616–619. doi: 10.1021/np0005208. [DOI] [PubMed] [Google Scholar]
- 2.Total synthesis of (+)-cananodine:Craig D, Henry GD. Eur. J. Org. Chem. 2006:3558–3561.
- 3.(a) Lião LM. Alkaloids. 2003;60:287–343. doi: 10.1016/s0099-9598(03)60005-2. [DOI] [PubMed] [Google Scholar]; (b) Büchi G, Goldman IM, Mayo DW. J. Am. Chem. Soc. 1966;88:3109–3133. [Google Scholar]; (c) Cren MC, Defaye G, Fetizon M. Bull. Soc. Chim. Fr. 1970:3020–3022. [Google Scholar]; (d) Van der Gen A, Van der Linde LM, Witteveen JG. Recueil Trav. Chim. Pays-Bas. 1972;91:1433–1440. [Google Scholar]; (e) Okatani T, Koyama J, Tagahara K, Suzuta Y. Heterocycles. 1987;26:595–597. [Google Scholar]; (f) Koyama J, Okatani T, Tagahara K, Suzuta Y, Irie H. Heterocycles. 1987;26:925–927. [Google Scholar]; (g) Koyama J, Okatani T, Ogura T, Tagahara K, Irie H. Chem. Pharm. Bull. 1991;39:481–482. [Google Scholar]
- 4.Meyer JA. M.S. Thesis. Bellingham, WA: Western Washington University; 2006. [Google Scholar]
- 5.(a) Baldwin JE. J. Chem. Soc., Chem. Commun. 1976:734–736. [Google Scholar]; (b) Baldwin JE, Kruse LI. J. Chem. Soc., Chem. Commun. 1977:233–235. [Google Scholar]; (c) Baldwin JE, Lusch MJ. Tetrahedron. 1982;38:2939–2947. [Google Scholar]
- 6.Review: Clark RD, Jahangir A. Org. React. 1995;47:1–314. Selected examples: Nelson JK, Burns CT, Smith MP, Twamley B, Natale NR. Tetrahedron Lett. 2008;49:3078–3082. doi: 10.1016/j.tetlet.2008.03.059.Smith TE, Mourad MS, Velander AJ. Heterocycles. 2002;57:1211–1217.Smith TE, Balskus EP. Heterocycles. 2002;57:1219–1225.
- 7.(a) Pasquinet E, Rocca P, Marsais F, Godard A, Quéguiner G. Tetrahedron. 1998;54:8771–8782. [Google Scholar]; (b) Diana GD. J. Med. Chem. 1995;38:1355–1371. doi: 10.1021/jm00008a014. [DOI] [PubMed] [Google Scholar]; (c) Crabb TA, Fallah A. Magn. Res. Chem. 1990;28:431–436. [Google Scholar]; (d) Leighton P, Sanders JKM. J. Chem. Soc., Perkin Trans 1. 1987:2385–2393. [Google Scholar]; (e) Banting L, Crabb TA, Trethewey AN. Magn. Res. Chem. 1987;25:352–355. [Google Scholar]; (f) Crabb TA, Jupp PA. J. Chem. Soc., Perkin Trans 1. 1985:913–918. [Google Scholar]; (g) Jones TH, Highet RJ, Blum MS, Fales HM. J. Chem. Ecol. 1984;10:1233–1249. doi: 10.1007/BF00988551. [DOI] [PubMed] [Google Scholar]; (h) Sonnet PE, Oliver JE. J. Heterocycl. Chem. 1975;12:289–294. [Google Scholar]; (i) Oliver JE, Sonnet PE. J. Org. Chem. 1974;39:2662–2663. [Google Scholar]; (j) Reinecke MG, Kray LR. J. Org. Chem. 1964;29:1736–1739. [Google Scholar]; (k) Boekelheide V, Windgassen RJ., Jr J. Am. Chem. Soc. 1959;81:1456–1459. [Google Scholar]
- 8.(a) Crabb TA, Fallah A. J. Chem. Soc., Perkin Trans 2. 1992:1335–1342. [Google Scholar]; (b) Begley MJ, Crabb TA, Roch OG. Magn. Res. Chem. 1986;24:292–296. [Google Scholar]
- 9.Presented in part at 235th ACS National Meeting; April 6–10; New Orleans, LA. ORGN-356. [Google Scholar]
- 10.Fraser RR, Mansour TS, Savard S. J. Org. Chem. 1985;50:3232–3234. [Google Scholar]
- 11.Bordwell FG. Acc. Chem. Res. 1988;21:456–463.(b) Personal communication from Professor Hans J. Reich of unpublished data obtained from Professor Frederick G. Bordwell and coworkers of Northwestern University.
- 12.Ghera E, David YB, Rapoport H. J. Org. Chem. 1981;46:2059–2065. [Google Scholar]
- 13.Lipshutz BH, Kozlowski J, Wilhelm RS. J. Am. Chem. Soc. 1982;104:2305–2307. [Google Scholar]
- 14.Baker W, Buggle KM, McOmie JFW, Watkins DAM. J. Chem. Soc. 1958:3594–3603. [Google Scholar]
- 15.(a) Taylor SK, DeYoung D, Simons LJ, Vyvyan JR, Wemple MA, Wood NK. Synth. Commun. 1998;28:1691–1701. [Google Scholar]; (b) Larchevêque M, Debal A. Synth. Commun. 1980;10:49. [Google Scholar]
- 16.Canovese L, Visentin F, Uguagliati P, Chessa G, Pesce A. J. Organomet. Chem. 1998;566:61–71. [Google Scholar]
- 17.Steinreiber A, Mayer SF, Saf R, Faber K. Tetrahedron: Asymm. 2001;12:1519–1528. [Google Scholar]
- 18.Katzenellenbogen JA, Christy KJ. J. Org. Chem. 1974;39:3315–3318. [Google Scholar]
- 19.Kuenzer H, Stahnke M, Sauer G, Wiechert R. Tetrahedron Lett. 1991;32:1949–1952. [Google Scholar]
- 20.Bonner WA. J. Am. Chem. Soc. 1952;74:1034–1039. [Google Scholar]
- 21.Hossain MT, Timberlake JW. J. Org. Chem. 2001;66:6282–6285. doi: 10.1021/jo010212u. [DOI] [PubMed] [Google Scholar]
- 22.For general experimental details, see Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional experimental procedures, compound characterization data, and copies of 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.