SUMMARY
Myc activity is emerging as a key element in acquisition and maintenance of stem cell properties. We have previously shown that c-Myc deficiency results in accumulation of defective hematopoietic stem cells (HSCs) due to niche-dependent differentiation defects. Here we report that immature HSCs coexpress c-myc and N-myc mRNA at similar levels. Although conditional deletion of N-myc in the bone marrow does not affect hematopoiesis, combined deficiency of c-Myc and N-Myc (dKO) results in pancytopenia and rapid lethality. Interestingly, proliferation of HSCs depends on both myc genes during homeostasis, but is c-Myc/N-Myc independent during bone marrow repair after injury. Strikingly, while most dKO hematopoietic cells undergo apoptosis, only self-renewing HSCs accumulate the cytotoxic molecule GranzymeB, normally employed by the innate immune system, thereby revealing an unexpected mechanism of stem cell apoptosis. Collectively, Myc activity (c-Myc and N-Myc) controls crucial aspects of HSC function including proliferation, differentiation, and survival.
INTRODUCTION
Long-term hematopoietic stem cells (HSCs) are defined by their unique ability to self-renew while concomitantly differentiating to generate all mature blood cell types. HSCs possess the clonal capacity to provide life-long reconstitution of all hematopoietic lineages upon transplantation into lethally irradiated mice (Purton and Scadden, 2007; Shizuru et al., 2005; Till and Mc Culloch, 1961). To therapeutically take advantage of the exclusive regenerative properties of LT-HSCs, it is fundamental to elucidate the mechanisms by which these cells maintain the balance between self-renewal and differentiation. The advent of genetically engineered mice has facilitated identification of several molecules that play a role in stem cell maintenance and function. For example, mice lacking signaling components such as TPO-cMPL (Qian et al., 2007; Yoshihara et al., 2007), Ang1-Tie2 (Arai et al., 2004), or SCF-cKit (Thoren et al., 2008), or nuclear regulators such as FOXO proteins (Tothova et al., 2007) or Bmi-1 (Park et al., 2003), have impaired HSC function. Most of these molecules participate in cell-cycle control, regulation of apoptosis, and response to oxidative stress, or interact with the surrounding niche environment (Orford and Scadden, 2008; Wilson and Trumpp, 2006). In HSCs, these processes are tightly regulated, most likely through distinct mechanisms during homeostasis or under stress conditions. For instance, myeloablative chemotherapy transiently induces cell-cycle and cell-surface marker changes in HSCs, allowing them to enter an activated state in order to re-establish normal hematopoiesis (Randall and Weissman, 1997; Venezia et al., 2004).
The Myc family members, c-Myc, N-Myc, and L-Myc (DePinho et al., 1987), encode basic helix-loop-helix leucine zipper transcription factors that are potent oncogenes. Myc proteins have been implicated in many biological processes, such as proliferation, cellular growth, angiogenesis, apoptosis, differentiation, and regulation of chromatin structure (Eisenman, 2001; Knoepfler, 2007; Murphy et al., 2005; Nilsson and Cleveland, 2003). Moreover, c-Myc (or N-Myc) has been identified as an important element in the induced reprogramming of adult fibroblasts into embryonic stem cells like iPS cells (Knoepfler, 2008; Lewitzky and Yamanaka, 2007). While many studies have shed light on the mechanisms by which overexpression of Myc promotes tumorigenesis (Pelengaris et al., 2002), its physiological role still remains elusive in many tissues in vivo. While L-Myc appears dispensable during development (Hatton et al., 1996), deletion of c-myc or N-myc leads to embryonic lethality (Charron et al., 1992; Dubois et al., 2008; Trumpp et al., 2001). We have previously reported that deleting c-myc in the adult bone marrow (BM) via the inducible MxCre-loxP system unexpectedly results in an accumulation of functionally defective HSCs (Wilson et al., 2004). In the absence of c-Myc, differentiation of these cells into more committed progenitors is inhibited as they upregulate a number of adhesion molecules that anchor them in the niche, thus preventing their differentiation. Surprisingly, and in contrast to differentiated progenitors, c-Myc-deficient HSCs can still divide, and their proliferation capacity is not affected. Since N-myc is expressed in normal and c-Myc-deficient HSCs (Ivanova et al., 2002; Wilson et al., 2004), we have genetically addressed the individual role of N-Myc, as well as that of c-Myc and N-Myc together, for HSC self-renewal, survival, and differentiation.
RESULTS
Expression of c-myc, N-myc, and L-myc in Hematopoietic Lineages
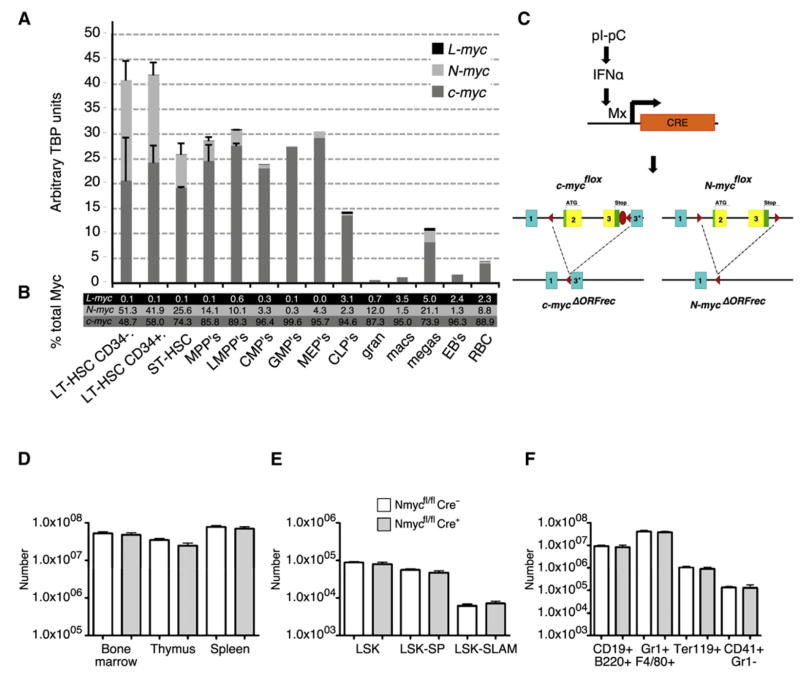
To address whether, in addition to c-Myc, other Myc family members are involved in controlling HSC and progenitor function, the expression levels of c-myc, N-myc, and L-myc were determined by qRT-PCR in various stem and progenitor populations isolated by FACS (Figure 1A). Identical amplification efficiencies for all three genes were established to not only quantitatively determine the expression of each gene, but also to compare the relative amounts of each transcript expressed in each individual cell type. Total myc mRNA levels (c- + N- + L-myc) were found to be highest in the most immature HSCs and progressively decreased during differentiation (Figure 1A). While c-myc and N-myc are detected in most progenitor subsets, L-myc only modestly contributes to the general Myc activity in CLPs, megakaryocytes, and macrophages (3%–5% of total Myc) and is not expressed in any stem/progenitor cells. The highest expression levels of total myc transcripts are found in the most immature HSCs (CD34− and CD34+LT-HSCs), where c-myc and N-myc contribute approximately equal amounts. During the initial differentiation step into ST-HSCs, the total myc level drops by approximately 30% but then remains constant in more committed progenitor stages (MPPs and LMPPs). This is mostly due to the progressive decrease of N-Myc transcripts while c-Myc mRNA expression remains stable. Surprisingly, despite c-Myc being essential for proliferation of most differentiated hematopoietic cell types, it is only expressed at modest levels in mature cell types (Figures 1A and 1B). These data show that expression of N-myc is essentially restricted to HSCs, thus raising the possibility that in addition to c-Myc, N-Myc may also play an important role in HSC function.
Figure 1. N-myc Is Highly Expressed in HSCs, but Its Conditional Deletion Does Not Impair Steady-State Hematopoiesis.
(A and B) qRT-PCR assessment of the expression levels of c-myc, N-myc, and L-myc in selected bone marrow populations. LT-HSCs (LSK CD150+CD48−CD34− and LSK CD150+CD48−CD34+). Cell surface marker definition of the other populations is indicated in Table S1. (A) Relative expression of each gene after TBP normalization. (B) Percent contribution of c-myc, N-myc, and L-myc to the total Myc pool in each population.
(C) Conditional deletion of c-myc and N-myc by pIpC treatment of MxCre;c-mycflox/flox;N-mycflox/flox mice. pIpC triggers IFNα production that, in turn, leads to activation of the Mx promoter, which drives the Cre recombinase.
(D–F) Analysis of N-myc-deficient hematopoietic cells at homeostasis 5 weeks after pIpC treatment. (D) Total numbers of BM, thymus, or spleen cells in N-Myc-deficient animals (Nmycfl/flCre+) compared to normal littermates (Nmyc fl/flCre−). (E) Total numbers of BM stem and progenitor cell populations Lin−Sca-1+c-kit+ (LSK), LSK-SP, and LSK-CD150+CD48− (LSK-SLAM) and (F) of major BM differentiated cell types B cells (CD19+B220+), Myeloid cells (GR1+ and/or F480+), Erythroid (Ter119+), and Megakaryocytes (CD41+GR1−). Results are mean ± SD.
N-Myc Is Not Essential for Steady-State Hematopoiesis
To investigate the role of N-Myc during hematopoiesis, adult MxCre;N-mycflox/flox mice were generated and treated with pIpC to conditionally eliminate this gene in all hematopoietic cell types, including HSCs (Figure 1C) (Knoepfler et al., 2002; Kühn et al., 1995). Five weeks postdeletion of N-myc, total numbers of cells in the BM, thymus, or spleen of mutant mice were indistinguishable from those of control littermates (Figure 1D). In addition, similar proportions of all major BM populations (Figure 1F), including stem/progenitor cell types such as LSK, LSK-SP (Side Population), or LSK-SLAM (LSK CD150+CD48−) populations (Figure 1E) were observed in control and mutant mice. While deletion of N-myc was complete (Figure S1A available online), no significant compensatory upregulation of either c-myc or L-myc was observed (data not shown). To address whether N-Myc-deficient HSCs are functional, limiting dilution assays in a competitive chimera setting were performed. N-Myc-deficient BM cells were indistinguishable from control BM cells in their capacity to engraft (>2% donor contribution after 21 weeks) (Table S2). In summary, these data suggest that deletion of N-myc alone has no major impact on HSC numbers and does not affect the capacity to maintain adult hematopoiesis.
Simultaneous Elimination of Both c-Myc and N-Myc Leads to Rapid Hematopoietic Failure, Including HSC Loss
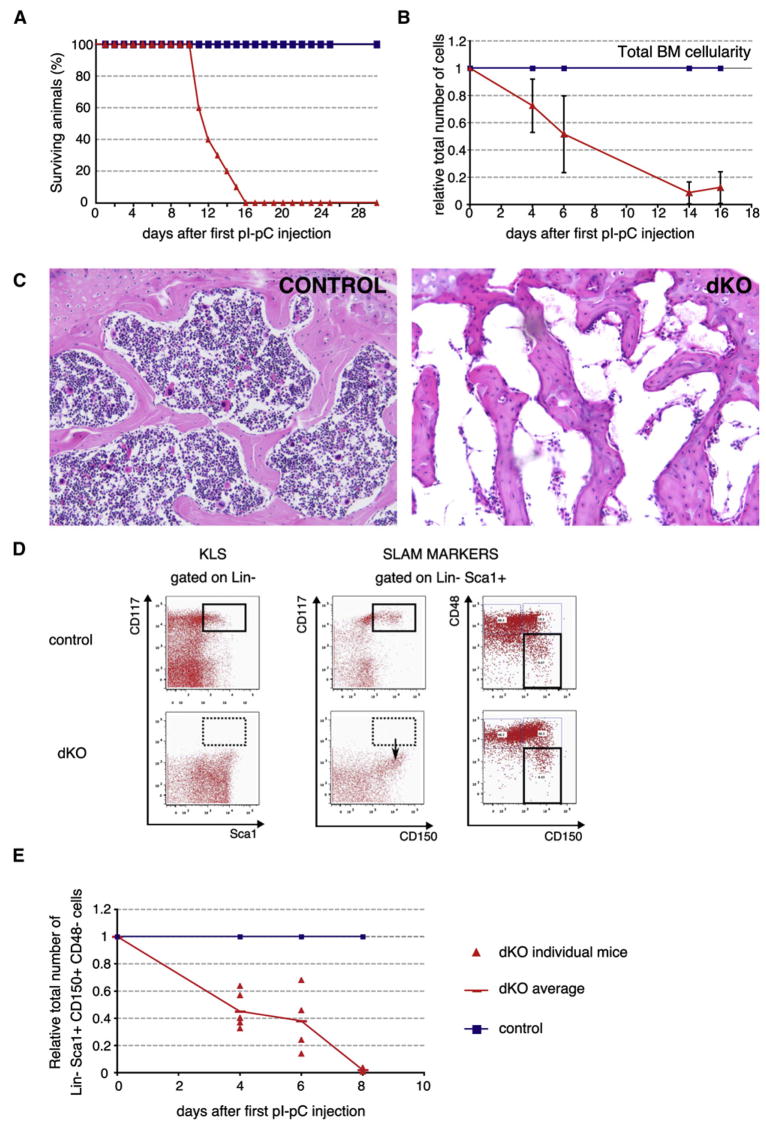
We have previously shown that c-Myc-deficient HSCs have a severe niche-dependent differentiation defect, indicating that c-Myc activity is required for proper HSC function. Interestingly, c-Myc-deficient HSCs proliferate normally, suggesting that the remaining N-Myc expression may be sufficient to maintain self-renewing divisions in c-Myc null HSCs. Moreover, the fact that both c-myc and N-myc transcripts are equally expressed in HSCs raises the possibility that both genes control HSC self-renewal and possibly other HSC functions in a cooperative or additive manner. To genetically address this hypothesis, MxCre;c-mycflox/flox;N-mycflox/flox double knockout (dKO) mice were generated (Trumpp et al., 2001; Knoepfler et al., 2002). Treatment of these mice with pIpC resulted in the loss of both c-myc and N-myc transcripts from day 4 onward. Although deletion of both c-myc and N-myc was very efficient (90% and 98%, respectively) as measured by qRT-PCR, no significant (p = 0.28) compensatory upregulation of L-Myc was observed in dKO BM subsets (Figure S1B). Simultaneous deletion of c-myc and N-myc rapidly induced severe pancytopenia (much faster than after deletion of c-Myc alone; Wilson et al. [2004]), as dKO mice died as early as 12 days after Cre induction (Figure 2A). Just prior to death, dKO mice were severely anemic, and their bone cavities were almost completely devoid of hematopoietic cells, further confirming complete BM failure upon loss of both genes (Figure 2C). Total BM cellularity rapidly decreased (>90%) 2 weeks postdeletion (Figure 2B). All major BM cell types, including myeloid, lymphoid, and erythroid cells, were progressively lost, albeit with distinct kinetics (Figure 2B and Figure S2A). Cell counts in peripheral hematopoietic organs, such as spleen and peripheral blood, were also strongly reduced in dKO animals (Figures S2B and S2C). Collectively, these results show that simultaneous elimination of c-Myc and N-Myc in the adult BM results in rapid hematopoietic failure and subsequent lethality 2 weeks postdeletion.
Figure 2. Simultaneous Deletion of c-myc and N-myc Results in Rapid BM Failure and Depletion of the HSC Pool.
(A) Kaplan-Meyer survival curve of MxCre;c-mycflox/flox;N-mycflox/flox (dKO) mice after pIpC-induced deletion.
(B) Kinetic analysis of dKO BM cells relative to control mice from 4 to 16 days after the first pIpC injection. Days 4 and 6, n > 8; and days 14 and 16, n = 3. Data are from at least two separate experiments per time point. Data represent mean ± SD.
(C) Hematoxylin-eosin staining of transversal sections of the femur trabecular zone 14 days after the first pIpC injection. 10× magnification. Control on the left; dKO on the right.
(D) Representative FACS profiles of early hematopoietic stem/progenitor cells isolated from control and dKO mice 6 days after pIpC treatment (bottom). BM cells were stained with Lineage, cKit (CD117), Sca1, CD150, and CD48 antibodies and gated as indicated. cKit+ Sca1+ labeling alone would erroneously predict a complete loss of stem cells and early precursors (left panel). The use of the SLAM markers CD150 and CD48 shows that the dKO Lin−Sca1+ compartment downregulates cKit cell-surface expression (middle panel) but still contains a subset of HSCs (Lin−Sca1+CD150+CD48−, right panel).
(E) Quantification of the number of SLAM-HSCs post pIpC induction. Data are from at least two experiments per time point, 2 < n < 4 per genotype and time point.
At the stem/progenitor level, the LSK compartment was no longer recognizable in dKO mice as early as day 4 postdeletion (Figure 2D, left panels). Instead, Lin−Sca1+cKit− cells accumulated, suggesting that c-Kit expression is lost in mutant LSKs. Downregulation of c-Kit cell surface expression on functional HSCs has also been reported in response to 5-FU-induced myelosuppression. Such severe loss of myeloid cells is thought to activate dormant HSCs, inducing them to cycle and generate progenitors and new mature cells to quickly reachieve homeostasis (Randall and Weissman, 1997; Wilson et al., 2008). Since simultaneous loss of c-Myc and N-Myc results in a similar reduction of BM cells to that observed after treatment with 5-FU, HSCs could still be present in dKO mice, but they would have also downregulated surface expression of the cKit receptor by an analogous mechanism. dKO BM was thus reanalyzed using the SLAM receptors CD150 and CD48 (Kiel et al., 2005). As all Lin−Sca1+CD150+ cells in control mice are also cKit+, HSCs can be phenotypically identified without cKit by analyzing the Lin−Sca1+CD150+CD48− population (hereafter called SLAM-HSC). Around 40% to 60% of SLAM-HSCs are still present in dKO mice shortly after deletion. However, their number steadily declines over time, and almost all SLAM-HSCs are lost by day 8 postdeletion (Figure 2E). Relative and absolute numbers of SLAM-HSCs in the spleen also decreased significantly (Figure S2D and data not shown), indicating that there was no significant mobilization of dKO HSCs from the BM to this organ and no subsequent extramedullary hematopoiesis. In summary, these results demonstrate that simultaneous deletion of both c-myc and N-myc results not only in rapid hematopoietic failure, but also in the complete loss of phenotypic HSCs.
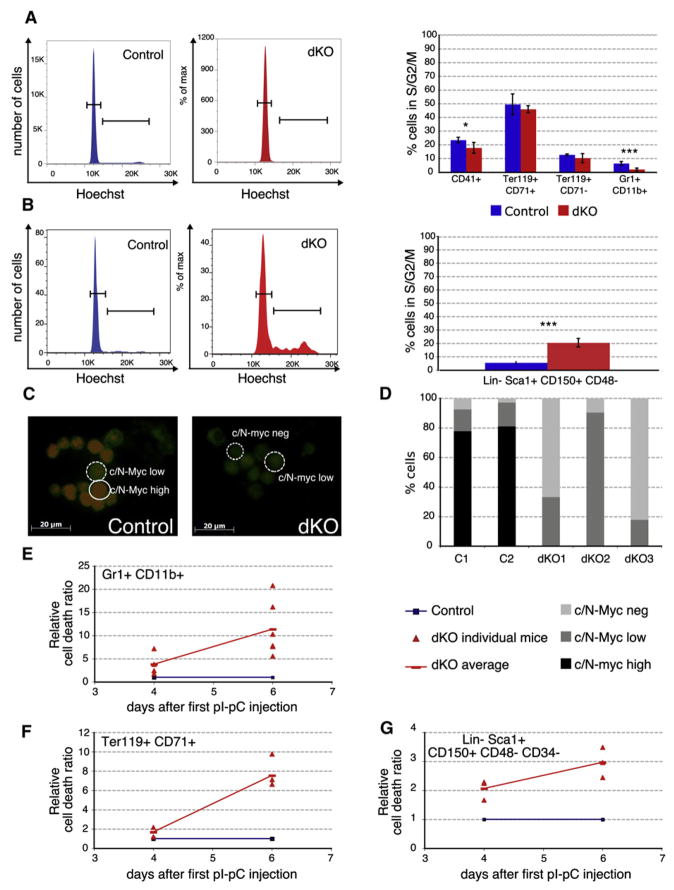
c-Myc;N-Myc Double-Deficient HSCs and Differentiated Cells Display Aberrant Proliferation and Enter an Apoptosis Program
To determine the effects of total loss of Myc activity on cellular proliferation, the cell-cycle status of all major hematopoietic populations, including HSCs, was determined in dKO animals 6 days after pIpC injection. As expected, a highly significant reduction of cells in the S/G2/M phases of the cell cycle was observed in the dKO myelomonocytic population (~3-fold; p < 0.01; Figure 3A). In contrast, dKO SLAM-HSCs cycled significantly more than control HSCs (~4-fold; p < 0.001; Figure 3B). To ensure that these unexpectedly proliferating HSCs had not simply escaped deletion of either c-myc or N-myc, the proliferative BrdU+ populations of HSCs (Lin−Sca1+CD150+BrdU+) from control and dKO mice were isolated by FACS and analyzed by immunofluorescence for the expression of c-Myc and N-Myc proteins. While abundant expression of both c-Myc and N-Myc was observed in more than 80% of control cells, HSCs isolated from dKO mice showed only very low or undetectable levels of c-Myc or N-Myc protein, confirming that MxCre-mediated deletion of both genes was highly efficient. These data demonstrate that, surprisingly, dKO SLAM-HSCs are able to proliferate even in the absence of both c-Myc and N-Myc (Figures 3C and 3D).
Figure 3. Simultaneous Deletion of c-myc and N-myc Affects Proliferation and Survival of Hematopoietic Cells in a Context-Dependent Manner.
(A and B) Cell-cycle status (DNA content; Hoechst 33342 staining) of control and dKO cells 6 days after pIpC treatment. Representative examples of granulocytic (Gr1+CD11b+) cells (A) and of SLAM-HSCs (Lin−Sca1+CD150+CD48−) (B) are shown in the left panels. Mean percent of cells in S/G2/M phase (±SD) is shown in the corresponding right panel. n = 3.
(C and D) c-Myc/N-Myc expression levels were determined by immunofluorescence on cytospins from FACS sorted BrdU+Lin−Sca1+CD150+ cells. BrdU was administered to the mice via the drinking water 15 hr prior to analysis. Representative pictures are shown in (C), with BrdU in green and c-Myc/N-Myc in red. Scale bar, 20 μm. (D) c-Myc/N-Myc expression levels were blindly scored on two control and three dKO mice. A minimum of 25 cells per genotype was analyzed, and each cell was assigned an intensity of Myc expression as exemplified in (C). Genetic controls for the antibodies used are presented in Figure S6.
(E–G) Quantitative analysis (FACS) of apoptosis (TUNEL) in the granulocyte (E), erythroblast (F), and SLAM-HSC (G) populations. Data represent mean ± SD, n > 3 per time point.
We also analyzed whether an increase in the relative amount of apoptosis occurred upon deletion of c-myc and N-myc by performing TUNEL and AnnexinV analyses ex vivo and after in vitro culture. dKO granulocytes, erythroblasts, and all B cell stages except the most mature BM B cells (IgM+) showed a substantial increase in apoptosis from day 4 postdeletion onward, with a further increase by day 6 (Figures 3E and 3F and data not shown). Moreover, increased cell death was also observed in the dKO LT-HSC (Lin−Sca1+CD150+CD48−CD34−) population as early as 4 days after the first pIpC injection (Figure 3G). Apoptosis was also induced in cultured mutant progenitors upon MxCre induction by IFNα, indicating that cell death induced by the loss of c-Myc and N-Myc is likely to be cell-autonomous and is not a result of putative alterations of the stem cell environment (Figure S3A). In addition to TUNEL assays, AnnexinV/7AAD analysis independently confirmed these results (data not shown). In summary, most of the major hematopoietic lineages, including HSCs, undergo apoptosis within a few days of the simultaneous loss of c-myc and N-myc. This not only provides a plausible explanation for the rapid general BM failure, but also reveals that while the presence of either N-Myc or c-Myc alone ensures survival, hematopoietic cells lacking both proteins are quickly lost due to cell death.
Bone Marrow Failure upon Loss of c-Myc and N-Myc Is Hematopoietic Cell Autonomous
The MxCre transgene induces significant deletion also outside the hematopoietic system, including the BM stromal compartment and the liver (Kühn et al., 1995). Nevertheless, c-myc and N-myc deletion in nonhematopoietic cell types does not contribute to the severe phenotype observed, as all the defects described above for dKO mice could be qualitatively and quantitatively recapitulated in noncompetitive BM transplantation experiments in which the c-myc and N-myc genes were deleted after reconstitution (Figure S4A).
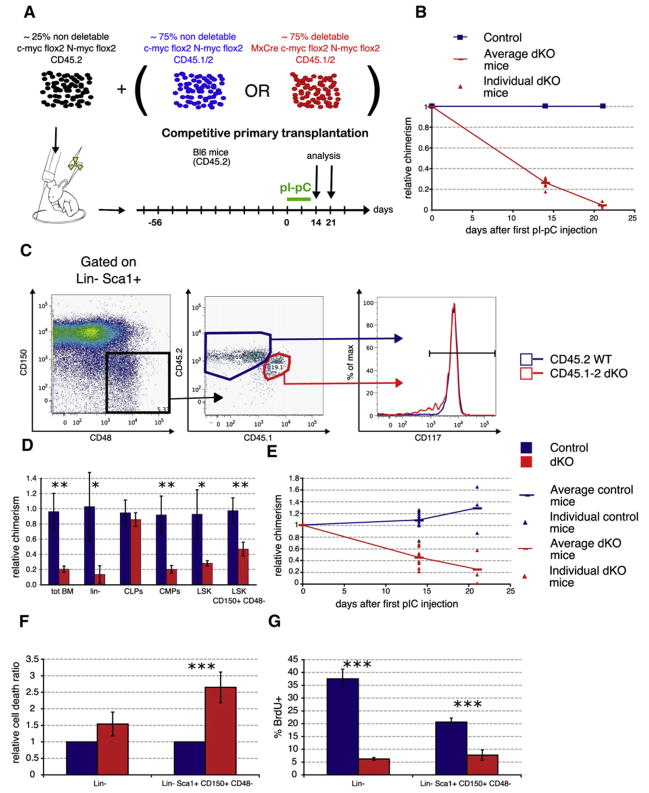
To determine the functional capacity of dKO HSCs in a competitive situation, competitive BM transplantation experiments were performed (Figure 4A). CD45.2+ recipient mice were lethally irradiated and reconstituted with either a 1:1 or a 3:1 mixture of experimental, but not yet deleted, BM cells (MxCre; c-mycflox/flox;N-mycflox/flox;CD45.1+/2+) together with nondeletable control (c-mycflox/flox;N-mycflox/flox;CD45.2+) cells (mixed chimeras). After stable reconstitution was established, deletion of both myc genes was induced by pIpC and the BM analyzed 14 or 21 days later (Figure 4A). In this set-up, the nondeletable control (“wild-type”) cells maintain hematopoiesis even if the dKO cells are lost after pIpC treatment and, therefore, prevent the development of a severe BM phenotype. This strategy conveniently permits differentiation between cell autonomous effects due to loss of c-Myc and N-Myc, and any effects secondary to the severe BM cytopenia that occurs in dKO mice and non-mixed chimeras. As expected, these competitive chimeric mice did not appear anemic upon deletion and had normal total BM cell numbers (data not shown). However, dKO donor cells were specifically lost as their relative chimerism decreased by 70% on day 14 and 90% 21 days after the first pIpC injection (Figure 4B). Furthermore, dKO donor cells proliferated significantly less compared to control cells and displayed increased apoptosis (Figure S5), in agreement with the results observed in dKO mice and nonmixed chimeras. In summary, these data show that dKO BM cells are lost even in a wild-type microenvironment (competitive and noncompetitive chimeras) and are unable to compete with wild-type hematopoietic cells in a steady-state (mixed BM chimera) or cytopenic (dKO and non-competitive transplantation) situation.
Figure 4. Bone Marrow Failure in dKO Mice Is Hematopoietic Autonomous, and dKO HSCs Show Decreased Proliferation and Survival.
(A) Competitive bone marrow chimeras. Mice were reconstituted with equal amounts of c-mycflox/flox;N-mycflox/flox cells and undeleted MxCre;c-mycflox/flox; N-mycflox/flox BM cells. After stable engraftment (8 weeks), pIpC was administered to the mice to induce deletion. The chimeras were analyzed 14 or 21 days after the first pIpC injection.
(B) Kinetics of loss of donor dKO cells in mixed chimeras. Results are mean ± SD. D14, n = 8; D21, n = 3.
(C) dKO HSCs present in mixed chimeras do not downregulate cKit (CD117) cell-surface expression. BM from competitive chimeras 14 days after pIpC treatment was analyzed and gated on Lin−Sca1+CD150+CD48−. The expression levels of cKit in the CD45.2+ (WT) rescue and in the CD45.1+/2+ (dKO) population were compared. Representative FACS plots from a total of six mice are shown.
(D) Degree of relative chimerism on progenitor and stem cell populations. CLPs (Lin−cKitintSca1int CD127+), CMPs (Lin−cKit+Sca1−); n > 3.
(E) Degree of relative chimerism of SLAM-HSCs. Triangles, individual mice; horizontal bar, the mean value for each genotype. D14, n = 8; D21, n = 3.
(F) Chimeras 14 days after the first pIpC treatment: BM was stained with stem cell markers, and apoptosis was assessed by TUNEL using FACS. Mean ± SD is shown; n = 3.
(G) Chimeras 14 days after the first pIpC treatment were administered BrdU for 15 hr before percent BrdU incorporation was assessed. Mean ± SD is shown; n = 3.
Role of Myc Proteins in HSC Proliferation and Survival during Homeostasis and Stress Response
In contrast to dKO mice and nonmixed chimeras, no downregulation of cKit cell surface expression was observed in dKO SLAM-HSCs in mixed chimeras (Figure 4C). Since these animals are not anemic, these data confirm that loss of cKit expression in dKO mutants is a secondary consequence of the BM hypoplasia similar to what is observed after 5-FU-induced BM ablation (Randall and Weissman, 1997) and is not a direct effect of Myc activity on c-Kit expression or cell-surface localization. Importantly, significant numbers of dKO SLAM-HSCs were still present in competitive chimeras. In comparison to their initial numbers before deletion, the total number of dKO SLAM-HSCs (LSKCD150+CD48−) had only halved by day 14 after deletion and only further decreased to 25% by day 21 (Figure 4E), a rather modest reduction compared to dKO mutants where all HSCs are lost within 8 days (Figure 2E). Analysis of the proportion of apoptotic cells in mixed chimeras revealed that in contrast to Lin− progenitors in which apoptotic rates are normal, dKO HSCs displayed a 2.5-fold increase in cell death compared to control cells (Figure 4F), highly similar to the situation in dKO mutant mice (Figure 3G). However, in striking contrast to HSCs in dKO mice, which show increased proliferation, the percentage of actively cycling dKO SLAM-HSCs (%S/G2/M) in mixed BM chimeras was strongly reduced compared to controls (~3-fold; p < 0.001; Figure 4G). These results suggest that c-Myc and N-Myc are indeed critical for the proliferation of HSCs, but only during homeostasis (mixed BM chimeras) and not in situations of severe BM stress (dKO animals). This result supports the notion that Myc-dependent and Myc-independent HSC proliferation programs can be engaged during homeostasis and repair.
Proliferating Cells with HSC Phenotype Are More Dependent on Myc Activity Than Quiescent HSCs
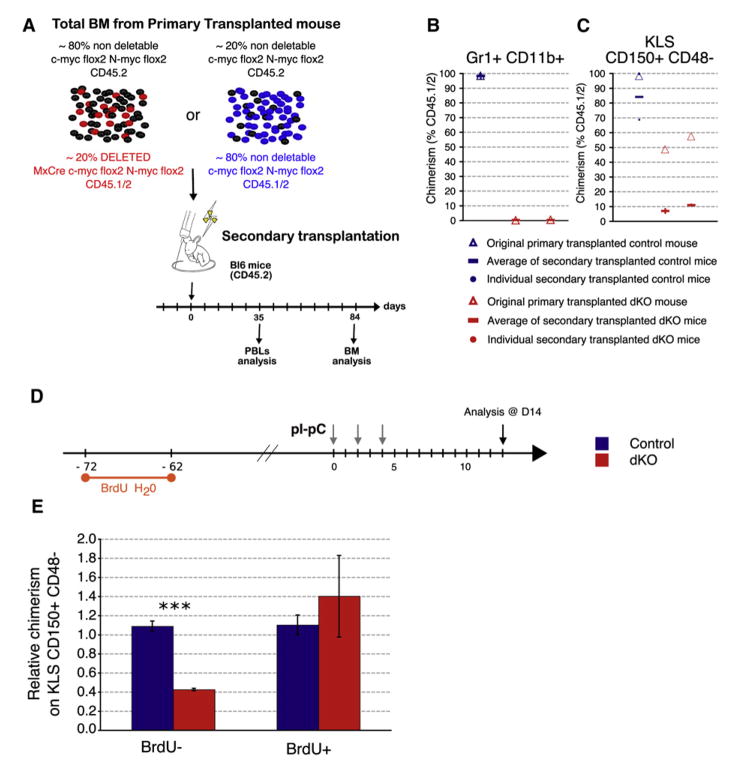
To investigate whether c-Myc- and N-Myc-deficient HSCs retain any long-term repopulation ability, serial transplantations were performed. BM from the primary competitive chimeras described above was harvested 14 days after pIpC treatment and transferred into lethally irradiated (CD45.2+) recipients and analyzed 12 weeks later (Figures 5A and 5B). As expected, no contribution from dKO-deficient cells to either the myeloid or erythroid compartments was observed (Figure 5B and data not shown), showing that no stem cell activity is retained in dKO SLAM-HSCs. Nevertheless, dKO SLAM-HSCs were still present in secondary recipients although their number had decreased about 6-fold compared to that at the time of transfer (Figure 5C). These results demonstrate that although HSCs lacking c-myc and N-myc can be serially transplanted, they do not give rise to differentiated progeny and, thus, have lost one of the critical features of stem cells.
Figure 5. Proliferating HSCs, but Not Quiescent HSCs, Are Preferentially Lost in dKO Mice.
(A) Secondary transplantation. BM from primary transplanted mice was harvested 14 days after deletion (see Figure 4A) and transferred into lethally irradiated CD45.2+ hosts. Percent CD45.1+/2+ myeloid cells (B) or SLAM-HSCs (C) in BM of secondary transplanted recipients 84 days after transplantation. (D) Label-Retaining Cell (LRC) assay. Undeleted mixed chimeric mice were administered BrdU in their drinking water for 10 days, followed by 70 days of chase (no BrdU). During the chase period, rapidly dividing cells dilute out the BrdU label, while long-lived quiescent cells (among which are dormant HSCs) retain this marker. Deletion was induced so that the day of analysis corresponded to both 70 days of chase and 14 days after the first pIpC injection. (E) BM from LRC mice was isolated and stained for stem cell markers. Relative chimerism (mean ± SD) of BrdU− or BrdU+ SLAM-HSCs is shown. n = 3.
The persistence of dKO dormant/quiescent HSCs indicates that these cells are less susceptible to the loss of both myc genes compared to actively proliferating HSCs. To test this hypothesis, a “label-retaining cell” experiment (LRC; Arai et al., 2004; Wilson et al., 2008) that permits distinction between quiescent and self-renewing stem cells, was performed on mixed chimeras to monitor the outcome of c-Myc/N-Myc deletion on long term dormant HSCs while avoiding putative cytopenia-induced effects (Figure 5D). After elimination of both myc genes, about 60% of the self-renewing SLAM-HSCs (BrdU−), but none of the dKO LRC SLAM-HSCs, were lost 14 days after the first pIpC injection (Figure 5E). These results indicate that during homeostasis, deletion of both c-myc and N-myc rather modestly, if at all, impairs maintenance of the quiescent, and therefore most immature, HSCs but strongly affects self-renewal and survival capacities of activated HSCs. Thus, Myc activity becomes critical during the activation process promoting quiescent HSCs into a self-renewal mode.
Myc Depletion Results in Upregulation of Granzyme B Expression in HSCs
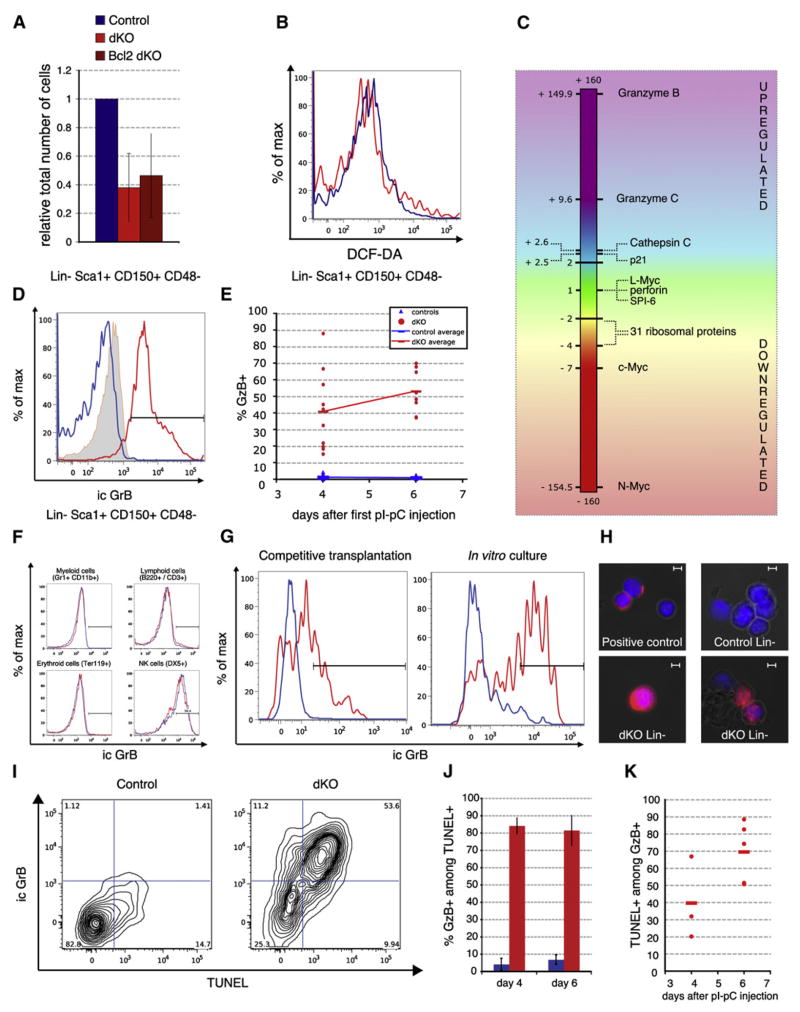
HSC survival can be increased by expression of the antiapoptotic factor BCL-2 (Domen and Weissman, 2000) and can be strongly inhibited by an increase in Reactive Oxygen Species (ROS) (Ito et al., 2006; Tothova et al., 2007). While overexpression of BCL-2 (via the H2K-BCL-2 transgene) rescued apoptosis in dKO B lymphocytes, it did not diminish the apoptosis rate of SLAM-HSCs, nor did it rescue their rapid decline in numbers (Figure 6A and data not shown). To test whether the apoptosis induced upon loss of c-Myc and N-Myc in HSCs is mediated by upregulation of ROS, the intracellular concentration of ROS in dKO SLAM-HSCs was determined by DCF-DA staining. However, no significant differences could be detected between control and dKO SLAM-HSCs (Figure 6B), indicating that apoptotic cell death of dKO HSCs is not mediated by an increase in ROS.
Figure 6. c-Myc and N-Myc Promote Survival of HSCs and Prevent GrB Accumulation.
(A) Absolute numbers, relative to control mice, of SLAM-HSCs in dKO and H2K::BCL2 dKO mice, 6 days after pIpC treatment. Mean ± SD is shown; n ≥ 3.
(B) SLAM-HSCs from dKO and control animals were stained with DCF-DA to measure the intracellular concentration of ROS. A representative FACS plot is shown; n = 3.
(C) Differential expression levels of selected genes as analyzed by microarray analysis 4 days after the first pIpC injection.
(D) Intracellular FACS staining to determine expression of Granzyme B (GrB) protein (blue line, control; red line, dKO; tinted, isotype control; n ≥3 for each condition).
(E) Quantification of GrB expression at day 4 and day 6. Percent GrB+ in gated Lin−Sca1+CD150+CD48− cells. The Lin cocktail includes DX5, NK1.1, CD3, CD4, and CD8 antibodies in order to eliminate any possible contamination from canonical GrB-producing cells, such as CTLs and NK cells. Blue triangles, individual control mice; red circles, dKO mice. Horizontal bars indicate averages of at least 6 mice per genotype.
(F) Intracellular GrB staining on indicated cell types 4 days after pIpC treatment.
(G) Representative GrB expression profiles gated on LSKCD150+CD48− in mixed chimeras (left panel) and in cultured SLAM HSCs 48 hr after IFNα addition.
(H) Representative ictures of GrB by immunofluorescence. Lin− cells or activated NK cells (DX5+, positive control) were cytospun onto slides and stained for GrB protein and DAPI (blue). Scale bar, 5 μm.
(I–K) GrB/TUNEL double staining on gated Lin−Sca1+CD150+CD48− cells at 4 and 6 days after c-myc and N-myc deletion. (I) Representative FACS plots at day 6. (J and K) Quantification of percentage of GrB+ cells among TUNEL+ SLAM HSCs ([J], mean ± SD) and of TUNEL+ cells among GrB+ SLAM HSCs (K). Day 4, n = 3; day 6, n = 2 for controls and n = 5 for dKO.
To elucidate the mechanisms underlying apoptosis of dKO HSCs, the global gene expression profiles of FACS-purified SLAM-HSCs and committed progenitors isolated from control and dKO mice were established using Affymetrix arrays (Figure 6C). As expected, c-myc and N-myc transcripts were strongly downregulated while L-myc expression was not significantly changed, in agreement with the data obtained by qRT-PCR (Figure S1B). In addition, dKO HSCs showed higher mRNA levels of the gene encoding the CDK inhibitor p21CIP, which is known to be repressed by c-Myc (Oskarsson et al., 2006; Wu et al., 2003). An HSC signature was generated by comparing the global gene expression profiles of wild-type LT-HSCs and progenitors. The expression of genes present in this wild-type HSC signature was only marginally changed in dKO-SLAM-HSCs, indicating that no significant loss of stem cell identity occurs upon loss of Myc activity (data not shown). This is consistent with our finding that the most quiescent dKO HSCs are maintained. Interestingly, the expression of 31 genes encoding ribosomal proteins was significantly downregulated (Figure S3), suggesting that ribosome biogenesis is strongly inhibited as a result of c-myc and N-myc gene loss. Intriguingly, the most upregulated transcript upon loss of both Myc family members was the gene encoding for Granzyme B (GrB, 150-fold and 130-fold as determined by microarray and qRT-PCR analysis, respectively; Figure 6C and Figure S7A), a highly potent cytotoxic molecule. The serine-protease Granzyme B plays a key role in the granule-exocytosis pathway, which is used by activated cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells to eliminate cells infected with intracellular pathogens or tumor cells (Russell and Ley, 2002). Normal SLAM-HSCs do not express GrB as determined by intracellular FACS staining (Figures 6D and 6E; Revell et al., 2005). In contrast, most dKO SLAM-HSCs show highly elevated levels of intracellular GrB protein (Figures 6D and 6E). Strikingly, GrB upregulation upon c-myc and N-myc deletion is specific to HSCs since no increased GrB could be detected in any other dKO hematopoietic cell type (Figure 6F). Importantly, GrB is also upregulated in dKO HSCs present in mixed chimeras (Figure 6G, left panel), and deletion of both Myc genes in cultured progenitors in vitro also resulted in the upregulation of intracellular GrB protein (Figure 6G, right panel), indicating a cell-autonomous effect of Myc deficiency on GrB production.
Processing of an N-terminal propeptide by the protease cathepsin C (or DPPI) is required for the proteolytical activity of GrB (Caputo et al., 1993; Pham and Ley, 1999). Cathepsin C transcripts are significantly upregulated (2.5-fold) in dKO SLAM-HSCs, while the expression level of the principal GrB inhibitor, Serine Protease Inhibitor 6 (Spi-6), is unchanged (Figure 6C and Figure S7A). Several groups have recently reported GrB-leakage-induced cell death in NK cells and CTLs, in which functionally active GrB escapes into the cytoplasm, thus provoking apoptosis of the GrB producing cell itself. The major determinant of such endogenous GrB-mediated killing seems to be an excess of free active GrB over its cytoplasmic inhibitor Spi-6 (Ida et al., 2003; Laforge et al., 2006; Phillips et al., 2004; Zhang et al., 2006), which correlates with diffuse cytoplasmic localization of the GrB protein. In agreement with this notion, in the vast majority of dKO progenitor cells (70.3% ± 0.4), GrB protein is located throughout the cytoplasm (diffuse pattern, Figure 6H, bottom panels, Figure S7B), in sharp contrast to the specifically localized and punctuate pattern observed in most (85.1% ± 2.0) activated NK cells (Figure 6H, top right panel; Figure S7B). All conditions for cell-autonomous killing by GrB are thus met in dKO HSCs. To assess whether GrB+ dKO HSCs are indeed dying as a consequence of GrB upregulation, HSCs were simultaneously assayed for TUNEL and GrB expression (Figures 6I–6K). Around 80% of dying dKO SLAM-HSCs were also GrB+ (Figure 6J). Moreover, the percentage of TUNEL+ cells among GrB+ dKO SLAM-HSCs increased over time, from about 40% at day 4 to around 70% at day 6 post-pIpC injection (Figure 6K), indicating that GrB upregulation precedes HSC apoptosis. Taken together, our results suggest that Myc depletion results in the massive increase of catalytically active GrB in SLAM-HSCs. As GrB is one of the most toxic molecules known, we conclude that Myc activity is required to ensure stem cell survival via repression of Granzyme B, thus identifying an unexpected mechanism of HSC apoptosis.
DISCUSSION
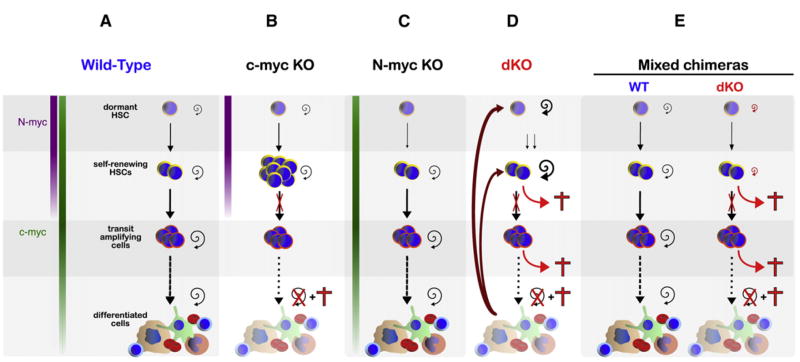
In this paper, we show that c-myc and N-myc are coexpressed in the most immature hematopoietic stem-progenitor populations and provide genetic evidence that total Myc activity (c-Myc, N-Myc, and L-Myc) controls HSC function at various levels (Figure 7). We demonstrate that c-Myc and N-Myc are required for HSC proliferation, metabolic growth, differentiation, long-term self-renewal activity, and survival of stem cells by inhibiting GrB-mediated apoptosis.
Figure 7. A Model for the Consequ ences of Loss of Myc in the Hematopoietic System.
(A) Schematic representation of steady-state hematopoiesis in a normal mouse. The HSC compartment comprises both self-renewing HSCs that contribute to day-to-day hematopoiesis and dormant HSCs that are only activated upon injury. N-myc expression levels contribute significantly to the Myc pool only in the most primitive HSCs. All other hematopoietic cell types are predominantly controlled by the c-Myc homolog.
(B) Upon loss of c-myc alone (Wilson et al., 2004), HSCs are unable to differentiate into progenitors. However, the presence of N-Myc allows them to maintain their self-renewal capacity, resulting in HSC accumulation in the bone marrow niche. The majority of early and late progenitors stop proliferating.
(C) In the presence of c-Myc, elimination of N-myc does not impact steady-state hematopoiesis.
(D and E) Deletion of both c-myc and N-myc reveals that c-Myc and N-Myc are crucial to promote survival throughout the hematopoietic system, as Myc-deficient self-renewing HSCs accumulate GrB and rapidly die. When no wild-type cells are present (“straight” dKO situation) (D), the rapid cell death of most differentiated cell types induces pancytopenia. As a consequence, a positive-feedback loop is generated, leading to activation of dormant HSCs. In the absence of c-Myc and N-Myc, self-renewing HSCs enter apoptosis and are therefore rapidly exhausted. In mixed chimeras (E), pancytopenia does not occur due to the presence of wild-type cells. Thus, no feedback loop is generated and dKO-dormant HSCs are maintained.
c-myc and N-myc Are Coexpressed in HSCs, and Both Contribute to Stem Cell Function
Our analysis of the precise expression pattern of the Myc family members throughout hematopoiesis has led to a number of important observations. First, no hematopoietic cell type expresses only N-myc or L-myc without c-myc. Consistent with the genetic data discussed below, this suggests that c-Myc provides the baseline Myc function in hematopoietic cells. The additional expression of either or both of the other two myc genes through their distinct regulatory regions is likely to fine-tune total Myc activity in these cell types. Second, the striking coexpression of c-myc and N-myc in immature HSCs combined with the rapid decrease of N-myc (but not c-myc) transcripts at the onset of HSC differentiation suggests that N-Myc plays a critical role in HSC function. However, when ablated in the presence of c-Myc, N-myc is dispensable for stem cell maintenance during steady-state conditions or following stress accompanied by transplantation (Figure 7C and Table S2). In contrast, when N-myc is deleted in the absence of c-Myc (dKO situation), HSC proliferation, survival, and differentiation are severely impaired (Figures 7D and 7E). As deletion of c-myc alone (Figure 7B) does not alter HSC proliferation and survival rates (Wilson et al., 2004), our dKO data reveal a role for N-myc expression in sustaining the survival and proliferation of c-myc null HSCs. Moreover, no compensatory upregulation of one family member is observed upon deletion of the other, implying that when N-myc is deleted, the unchanged c-myc transcript levels are sufficient for maintaining all aspects of HSC maintenance. In addition, when c-myc is deleted, the normally equivalent levels of N-myc transcripts can provide enough Myc activity to ensure HSC survival and proliferation but are not sufficient to permit exit from the niche and differentiation past the ST-HSC stage (Wilson et al., 2004). How can we explain such a discrepancy? Gene-replacement studies in which the coding sequences and intervening intron of c-myc were replaced by those of N-myc (NCR allele) exclude the possibility that the N-Myc protein functionally differs from c-Myc, since mice homozygous for this NCR allele are viable and produce normal proportions of all differentiated cell types (Malynn et al., 2000). Our own analysis of NCR BM confirms these data as no obvious differentiation defect in their HSC and progenitor compartments was detectable (data not shown). Thus, N-Myc, if expressed in the exact pattern as endogenous c-Myc, can provide all c-Myc functions during steady-state hematopoiesis, including exit of HSCs from the niche. Nevertheless, it remains possible that c-Myc and N-Myc activities are quantitatively different in stem cells as a number of studies have reported that N-Myc transactivation properties are weaker than those of c-Myc (Cole and McMahon, 1999; Malynn et al., 2000). Alternatively, differential posttranscriptional regulation of c-Myc and N-Myc degradation may result in a predominance of c-Myc over N-Myc at the protein level in HSCs (Zhao et al., 2008). Further experiments will be required to address these appealing aspects.
A third interesting observation is that total myc expression peaks in the most immature HSCs and decreases in progenitors and more mature cell types. Thus, absolute myc transcript levels positively correlate with multipotency and self-renewal activity. These results are consistent with reports suggesting Myc genes as stem cell markers (Bettess et al., 2005; Cartwright et al., 2005; Ivanova et al., 2002; Knoepfler et al., 2006; Ramalho-Santos et al., 2002). Moreover, although not absolutely required, c-Myc or N-Myc have been shown to participate in reprogramming tail-tip fibroblasts into iPS cells, multipotent cells highly similar to ESCs (Lewitzky and Yamanaka, 2007). The mechanism by which Myc activity promotes this reprogramming remains hypothetical, but its positive effects on cell-cycle and metabolic growth, as well as its recently uncovered effect on chromatin structure, have been suggested to be causally involved (Knoepfler, 2008). Our study confirms that Myc activity is critical for stem cell maintenance as total loss of Myc strongly affects both self-renewal division and survival of activated HSCs, suggesting that Myc expression is tightly regulated in order to balance scheduled differentiation with maintenance of a rarely self-renewing stem cell pool.
Interestingly, deletion of c-myc and N-myc in more committed progenitors (Lin−) severely diminishes their proliferation capacity but does not compromise their survival, indicating that in highly cycling progenitors (in contrast to HSCs), Myc principally controls proliferation rates. Similarly, most lineage committed cells (Lin+) are exquisitely sensitive to c-Myc levels as they undergo G0 arrest after elimination of c-Myc (Trumpp et al., 2001; Wilson et al., 2004). Collectively, our data show that proliferation of most hematopoietic cell types, including homeostatic HSCs, is Myc dependent. Nonetheless, we also provide evidence that dKO HSCs retain their ability to rapidly cycle in response to injury signals. Indeed, simultaneous deletion of c-myc and N-myc in a noncompetitive setting causes severe pancytopenia due to cell-cycle exit and apoptosis of lineage committed cell types. As a consequence, dKO stem cells become “activated” in order to repair the injured BM (like 5-FU treated HSCs) (Randall and Weissman, 1997; Venezia et al., 2004). This is accompanied by downregulation of c-Kit cell-surface expression and enhanced proliferation (Figure 7D). Since this proliferative burst occurs without upregulation of the barely detectable L-myc transcripts, our data strongly suggest that situations exist where hematopoietic cell cycling can occur in a Myc-independent manner. In agreement with recent gene-profiling studies (Venezia et al., 2004), this observation implies that different proliferation/self-renewal programs control HSC cycling during homeostasis or in an injury response situation. Importantly, the injury-activated and proliferating dKO HSCs all undergo apoptosis, leading to the complete loss of all HSCs in the “straight” mutants. In contrast, in competitive chimeras where no cytopenia (injury) develops, quiescent dKO HSCs are maintained, suggesting that the loss of the Myc-mediated apoptotic response is linked to an activated self-renewal program (Figure 7). In conclusion, myc genes appear to be both critical and pleiotropic control elements regulating not only homeostatic HSC cycling, but also other key stem cell functions such as differentiation and survival.
Myc Activity Is Essential for HSC Survival by Repressing a GrB-Mediated Apoptosis Pathway
GrB, one of the most toxic proteases known, is, so far, only thought to be produced by NK cells and CTLs, major components of the innate immune system. NK cells possess finely tuned production, shuttling, and targeting pathways that permit delivery of GrB granules to target cells without any self-inflicted damage. Here we demonstrate that the vast majority (80%) of apoptotic dKO HSCs express significant levels of intracellular GrB protein. Although GrB expression has not yet been reported in murine stem cells, GrB expression has been observed in human CD34+ cells after challenge with a combination of chemotherapeutic drugs and G-CSF (Berthou et al., 1995). As no increase in Spi-6, which not only inactivates GrB proteolytic activity but also contributes to its compartmentalization into exocytic granules (Sun et al., 1997; Zhang et al., 2006), is detected upon loss of both myc genes, HSCs are unlikely to be equipped with the molecular machinery necessary to compartmentalize GrB and/or counteract its devastating proteolytic effects. As a consequence, aberrant production of GrB is most likely the main cause of cell death of Myc-deficient HSCs, thus revealing a previously unrecognized mechanism of stem cell apoptosis.
Importantly, GrB accumulation is restricted to the stem cell compartment. However a variety of other cell types undergo apoptosis in the absence of Myc, implying that distinct apoptotic pathways are elicited in different Myc-deficient cellular contexts. Indeed, our results suggest that BCL2 overexpression rescues apoptosis in lymphoid cells at different stages of maturation, but not in HSCs or in myeloid cells. Moreover, granulocytic cells, but not HSCs or lymphoid cells, show upregulation of ROS (data not shown). Although it is possible that Myc directly controls cell-type-specific antiapoptotic target genes, it seems more likely that the total absence of Myc activity generates a general cellular emergency situation that then secondarily elicits a cell death program inherent to each hematopoietic cell type. Our microarray profiling data have revealed the downregulation of 31 different ribosomal proteins in dKO HSCs, consistent with previous reports showing that Myc controls transcription mediated by all three RNA polymerases (Oskarsson and Trumpp, 2005). An interesting possibility is that the absence of all Myc activity results in the inability of dormant HSCs to increase protein biosynthesis upon activation/stress signals. This metabolic/growth impairment might in turn trigger GrB-mediated apoptosis. This is consistent with our finding that the most quiescent/dormant HSCs, in which protein metabolism is naturally low compared to actively self-renewing HSCs (Passegue et al., 2005; Wilson et al., 2008), are significantly less affected by simultaneous c-myc and N-myc deletion than activated HSCs. Future experiments will address whether induction of GrB accumulation in dKO HSCs is due to their inability to grow in the absence of Myc, combined with activating/stress signals caused by the resultant severe cytopenia or by pI-pC/IFNa treatment that also activates quiescent HSCs in vivo (M.A.G. Essers, S. Offner, W.E.B.-B., Z. Waibler, U. Kalinke, M.A. Duchosal, and A.T., unpublished data).
Collectively, our genetic data not only show that the absolute Myc levels resulting from the combined expression of the c-myc and N-myc genes are critical for several biological functions of HSCs, but also identify GrB as a novel pathway used to eliminate defective stem cells in vivo and may raise new possibilities to target malignant cells including leukemic stem cells.
EXPERIMENTAL PROCEDURES
Conditional Deletion of c-myc and N-myc
Six- to twelve-week-old N-mycflox/flox (±MxCre) or c-mycflox/flox N-mycflox/flox(±MxCre) mice were used throughout our study (Knoepfler et al., 2002; Trumpp et al., 2001). Deletion of the flox alleles was induced with pIpC treatment (Invitrogen). Control and experimental littermate mice were injected i.p. (up to five times) every 2 days with 10 μg/g mouse (or 1 μg/g mouse in competitive transplantation settings). For day 4 analyses, two injections on 2 consecutive days were performed at 5 μg/g mouse.
Flow Cytometry and Purification of HSCs
BM was taken from hindlegs, forelegs, and/or backbone. Single-cell suspensions were made by crushing bones after removal of muscle and connective tissue. For a complete list of antibodies used, refer to the Supplemental Data.
Transplantation Assays
In the noncompetitive setting, 5 × 106 Thy1 depleted CD45.2+ c-mycflox/flox N-mycflox/flox ± MxCre were injected i.v. into CD45.1+ lethally irradiated (900 rads in two doses of 450 rads 4 hr apart) recipient mice that had been treated with 100 μg anti-NK1.1 mAb 24 hr beforehand. Similarly, in the competitive setting, a mixture of 3 × 106 Thy1-depleted CD45.1/2+ c-mycflox/flox N-mycflox/flox ± MxCre and 3 × 106 Thy1 depleted competitive wild-type BM (c-myc flox/flox N-mycflox/flox; CD45.2+) was i.v. transferred into CD45.2+ lethally irradiated recipient animals. Mice were maintained on antibiotic (Bactrim, Roche, Basel, Switzerland)-containing water for 3 weeks and reconstitution of peripheral blood, BM, and spleen was analyzed at the indicated time points. Limiting dilution experiments are described in the Supplemental Data.
BrdU Uptake and Label-Retaining Cell Assays
In vivo 5-Bromodeoxy-uridine (BrdU, Sigma) labeling was performed by injecting control and experimental mice with 200 μl (1.8 mg BrdU/ml) i.p. then giving water containing 1 mg/ml BrdU and 5% glucose for 15 hr. For the LRC assays, control and experimental mice not yet treated with pIpC were maintained on 1 mg/ml BrdU and 5% glucose drinking water for 10–14 days, then on normal water for the entire period of chase (70 days). In both assays, BrdU staining was quantitated by FACS, combining staining of adequate cell surface markers with BrdU intracellular staining using the BrdU-APC staining kit according to the manufacturer’s instructions (BD Biosciences).
c-Myc/N-Myc Immunofluorescence
Experimental and control mice were labeled with BrdU for 15 hr as described above. Following BM isolation and depletion of Lin+ cells, cells were stained with CD150-APC and Sca1-biotin antibodies. Secondary staining was performed with a SAV-APCCy7 conjugate. BrdU intracellular staining was carried out using a BrdU-FITC staining kit (BD Biosciences). Lin−Sca1+CD150+BrdU+ cells were then sorted on a FACS Aria Flow Cytometer (BD) and finally spun on slides with a cytocentrifuge. c-Myc/N-Myc immunofluorescent staining was performed as described in Figure S6.
Cell-Cycle Analysis
Cell-cycle analysis was performed by surface staining to define BM subsets, followed by fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences). Intracellular staining with Hoechst 33342 (Molecular Probes, Invitrogen) was done in Permwash (BD Biosciences) for 30 min.
Apoptosis Assays
Following cell surface staining, cells were incubated with 7-AAD and AnnexinV-Cy5 (BD Biosciences) following the manufacturer’s instructions. TUNEL labeling by FACS was performed with the in situ cell death detection kit (Roche) according to the manufacturer’s protocol.
Determination of Intracellular Reactive Oxygen Species
BM samples prestained for the cell-surface markers of interest were loaded with 5 μM DCF-DA (Sigma), incubated on a shaker for 30 min at 37° C, and then immediately analyzed on a FACS Canto instrument (BD).
GrB Intracellular Staining
For FACS analyses, total BM or Lin− cells were stained for surface markers and intracellular GrB as described (Revell et al., 2005) with a directly conjugated GrB-PE antibody (CalTag, Invitrogen). Mouse IgG1-PE was used as the appropriate IgG control. For immunofluorescence assays, Lin− cells were fixed in 3.7% PFA, then cytospun onto slides. Following permeabilization in PBS + 0.2% Triton X-100 and blocking with PBS + 15% Goat Serum, the slides were stained overnight with anti-murine GrB biotinylated Ab (R&D Systems), then for 1 hr with SAV-Alexa 568. Nuclei were counterstained with DAPI.
Microarray Analysis and RT-PCR
Total RNA isolation was performed from the indicated populations with TRizol reagent (Invitrogen) according to the manufacturer’s instructions. Microarray analysis raw data are available for download from Gene Expression Omnibus (http://ncbi.nlm.nih.gov/geo; gene accession number: GSE12538). Microarray and real-time PCR protocols are described in the Supplemental Data.
Statistical Analysis
All analyses were performed using two-tailed t tests assuming equal variance. Statistical significance is indicated by *p < 0.1, **p < 0.05, and ***p < 0.01.
Supplementary Material
The Supplemental Data include Supplemental Experimental Procedures, Supplemental References, seven figures, and three tables and can be found with this article online at http://www.cellstemcell.com/supplemental/S1934-5909(08)00458-X.
Acknowledgments
The authors thank Sandra Offner and Christelle Dubey for animal husbandry, genetic screening, and technical help; Drs. Jos Domen for the H2K-BCL-2 mice; Fred Alt for the NCR mice; Mark Murphy for the initial breeding of the dKO mice; Keith Harshman, Otto Hagenbüchle, and other members of the Lausanne DNA Array Facility (DAFL) for their microarray service; and all members of the EPFL-MIM facility for outstanding help with microscopy and providing reagents. We would like to thank Steven Merlin and Joanna Roberts for FACS sorting, Tobias Vogt for TBP primers, and Rama for the mouse cartoon sketches. This work was supported in part by grants to A.T. from the Swiss National Science Foundation, the Swiss Cancer League, the EU- FP6 Program “INTACT,” and the EU-FP7 Program “EuroSyStem,” and by NIH grants PO1 HL084205 (to R.N.E. and I.D.B.) and RO1CA20525 (to R.N.E.).
References
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Berthou C, Marolleau JP, Lafaurie C, Soulie A, Dal Cortivo L, Bourge JF, Benbunan M, Sasportes M. Granzyme B and perforin lytic proteins are expressed in CD34+ peripheral blood progenitor cells mobilized by chemotherapy and granulocyte colony-stimulating factor. Blood. 1995;86:3500–3506. [PubMed] [Google Scholar]
- Bettess MD, Dubois N, Murphy MJ, Dubey C, Roger C, Robine S, Trumpp A. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol Cell Biol. 2005;25:7868–7878. doi: 10.1128/MCB.25.17.7868-7878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Garner RS, Winkler U, Hudig D, Bleackley RC. Activation of recombinant murine cytotoxic cell proteinase-1 requires deletion of an amino-terminal dipeptide. J Biol Chem. 1993;268:17672–17675. [PubMed] [Google Scholar]
- Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- Charron J, Malynn BA, Fisher P, Stewart V, Jeannotte L, Goff SP, Robertson EJ, Alt FW. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 1992;6:2248–2257. doi: 10.1101/gad.6.12a.2248. [DOI] [PubMed] [Google Scholar]
- Cole MD, McMahon SB. The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene. 1999;18:2916–2924. doi: 10.1038/sj.onc.1202748. [DOI] [PubMed] [Google Scholar]
- DePinho R, Mitsock L, Hatton K, Ferrier P, Zimmerman K, Legouy E, Tesfaye A, Collum R, Yancopoulos G, Nisen P, et al. Myc family of cellular oncogenes. J Cell Biochem. 1987;33:257–266. doi: 10.1002/jcb.240330404. [DOI] [PubMed] [Google Scholar]
- Domen J, Weissman IL. Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J Exp Med. 2000;192:1707–1718. doi: 10.1084/jem.192.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois NC, Adolphe C, Ehninger A, Wang RA, Robertson EJ, Trumpp A. Placental rescue reveals a sole requirement for c-Myc in embryonic erythroblast survival and hematopoietic stem cell function. Development. 2008;135:2455–2465. doi: 10.1242/dev.022707. [DOI] [PubMed] [Google Scholar]
- Eisenman RN. Deconstructing myc. Genes Dev. 2001;15:2023–2030. doi: 10.1101/gad928101. [DOI] [PubMed] [Google Scholar]
- Hatton KS, Mahon K, Chin L, Chiu FC, Lee HW, Peng D, Morgenbesser SD, Horner J, DePinho RA. Expression and activity of L-Myc in normal mouse development. Mol Cell Biol. 1996;16:1794–1804. doi: 10.1128/mcb.16.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida H, Nakashima T, Kedersha NL, Yamasaki S, Huang M, Izumi Y, Miyashita T, Origuchi T, Kawakami A, Migita K, et al. Granzyme B leakage-induced cell death: a new type of activation-induced natural killer cell death. Eur J Immunol. 2003;33:3284–3292. doi: 10.1002/eji.200324376. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS. Myc goes global: new tricks for an old oncogene. Cancer Res. 2007;67:5061–5063. doi: 10.1158/0008-5472.CAN-07-0426. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS. Why Myc? An Unexpected Ingredient in the Stem Cell Cocktail. Cell Stem Cell. 2008;2:18–21. doi: 10.1016/j.stem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16:2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS, Zhang XY, Cheng PF, Gafken PR, McMahon SB, Eisenman RN. Myc influences global chromatin structure. EMBO J. 2006;25:2723–2734. doi: 10.1038/sj.emboj.7601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Laforge M, Bidere N, Carmona S, Devocelle A, Charpentier B, Senik A. Apoptotic death concurrent with CD3 stimulation in primary human CD8+ T lymphocytes: a role for endogenous granzyme B. J Immunol. 2006;176:3966–3977. doi: 10.4049/jimmunol.176.7.3966. [DOI] [PubMed] [Google Scholar]
- Lewitzky M, Yamanaka S. Reprogramming somatic cells towards pluripotency by defined factors. Curr Opin Biotechnol. 2007;18:467–473. doi: 10.1016/j.copbio.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Malynn BA, de Alboran IM, O’Hagan RC, Bronson R, Davidson L, DePinho RA, Alt FW. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 2000;14:1390–1399. [PMC free article] [PubMed] [Google Scholar]
- Murphy MJ, Wilson A, Trumpp A. More than just proliferation: Myc function in stem cells. Trends Cell Biol. 2005;15:128–137. doi: 10.1016/j.tcb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Essers MA, Dubois N, Offner S, Dubey C, Roger C, Metzger D, Chambon P, Hummler E, Beard P, et al. Skin epidermis lacking the c-Myc gene is resistant to Ras-driven tumorigenesis but can reacquire sensitivity upon additional loss of the p21Cip1 gene. Genes Dev. 2006;20:2024–2029. doi: 10.1101/gad.381206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T, Trumpp A. The Myc trilogy: lord of RNA polymerases. Nat Cell Biol. 2005;7:215–217. doi: 10.1038/ncb0305-215. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci USA. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Opferman JT, Shah R, Liu N, Froelich CJ, Ashton-Rickardt PG. A role for the granzyme B inhibitor serine protease inhibitor 6 in CD8+ memory cell homeostasis. J Immunol. 2004;173:3801–3809. doi: 10.4049/jimmunol.173.6.3801. [DOI] [PubMed] [Google Scholar]
- Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SEW. Critical Role of Thrombopoietin in Maintaining Adult Quiescent Hematopoietic Stem Cells. Cell Stem Cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Randall TD, Weissman IL. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood. 1997;89:3596–3606. [PubMed] [Google Scholar]
- Revell PA, Grossman WJ, Thomas DA, Cao X, Behl R, Ratner JA, Lu ZH, Ley TJ. Granzyme B and the downstream granzymes C and/or F are important for cytotoxic lymphocyte functions. J Immunol. 2005;174:2124–2131. doi: 10.4049/jimmunol.174.4.2124. [DOI] [PubMed] [Google Scholar]
- Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- Sun J, Ooms L, Bird CH, Sutton VR, Trapani JA, Bird PI. A new family of 10 murine ovalbumin serpins includes two homologs of proteinase inhibitor 8 and two homologs of the granzyme B inhibitor (proteinase inhibitor 9) J Biol Chem. 1997;272:15434–15441. doi: 10.1074/jbc.272.24.15434. [DOI] [PubMed] [Google Scholar]
- Thoren LA, Liuba K, Bryder D, Nygren JM, Jensen CT, Qian H, Antonchuk J, Jacobsen SE. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008;180:2045–2053. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
- Till JE, Mc Culloch E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Trumpp A, Refaeli Y, Oskarsson T, Gasser S, Murphy M, Martin GR, Bishop JM. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature. 2001;414:768–773. doi: 10.1038/414768a. [DOI] [PubMed] [Google Scholar]
- Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA, Goodell MA. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2:e301. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, Pasche AC, Knabenhans C, Macdonald HR, Trumpp A. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser GM, van der Wath RC, Blanco-Bose WE, Dunant C, Bockamp E, Liò P, MacDonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008 doi: 10.1016/j.cell.2008.10.048. in press. [DOI] [PubMed] [Google Scholar]
- Wu S, Cetinkaya C, Munoz-Alonso MJ, von der Lehr N, Bahram F, Beuger V, Eilers M, Leon J, Larsson LG. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene. 2003;22:351–360. doi: 10.1038/sj.onc.1206145. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, et al. Thrombopoietin/MPL Signaling Regulates Hematopoietic Stem Cell Quiescence and Interaction with the Osteoblastic Niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Zhang M, Park SM, Wang Y, Shah R, Liu N, Murmann AE, Wang CR, Peter ME, Ashton-Rickardt PG. Serine protease inhibitor 6 protects cytotoxic T cells from self-inflicted injury by ensuring the integrity of cytotoxic granules. Immunity. 2006;24:451–461. doi: 10.1016/j.immuni.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Zhao X, Heng JI, Guardavaccaro D, Jiang R, Pagano M, Guillemot F, Iavarone A, Lasorella A. The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nat Cell Biol. 2008;10:643–653. doi: 10.1038/ncb1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Supplemental Data include Supplemental Experimental Procedures, Supplemental References, seven figures, and three tables and can be found with this article online at http://www.cellstemcell.com/supplemental/S1934-5909(08)00458-X.