Abstract
Recombinant Listeria monocytogenes expressing a type-common herpes simplex virus (HSV) gB-peptide was shown previously to protect against footpad inoculation with HSV-1. We tested this construct for protection against vaginal challenge with HSV-2. Primed mice demonstrated strong recall responses, had modest reductions in HSV-2 DNA in vaginal mucosa, but were not protected from disease.
Viruses of the family Herpesviridae, genus simplexvirus, species human herpesvirus 2 (HHV-2, also known as herpes simplex virus 2 or HSV-2) are the most common cause of genital ulcer disease worldwide [4]. Despite extensive efforts, there are currently no licensed vaccines to prevent HSV acquisition in humans [24]. CD8+ T-cells are critical to immune control of HSV infection [13]. In humans, HSV-specific CD8+ T-cells reside in latently infected ganglia [27], persist in skin for weeks after resolution of recurrent HSV [30], and infiltrate recurrent lesions in association with viral clearance [11]. In mice, HSV-specific CD8+ T-cells in latently infected ganglia control viral reactivation in an interferon-γ (IFN-γ) dependent manner [10]. Together, these studies suggest that strategies that prime HSV-specific CD8+ T-cells may confer immunity to primary and reactivation disease.
Experimental HSV infection in mice has identified a dominant H-2Kb restricted CD8+ T-cell epitope in the gB glycoprotein which accounts for >70% of the cellular HSV-specific immune response [26, 29]. Several investigators have shown that adaptive immunity against this single MHC class I epitope common to HSV-1 and HSV-2 protects C57BL/6 mice against subsequent challenge by various routes [1, 8, 17, 18]. However, none of these studies has evaluated protection against intravaginal HSV-2 challenge.
Live attenuated strains of recombinant Listeria monocytogenes (Lm) are a promising class of vaccine vectors for priming antigen-specific CD8+ T-cell responses to heterologous antigen. In a recent study [18], we demonstrated that inoculation with recombinant Lm expressing the dominant HSV-gB antigen triggers a robust gB-specific CD8+ T-cell response. We were able to show that these CD8+ T-cells produce IFN-γ, and that they can protect against lethal HSV-1 challenge in the footpad infection model [18]. In the current study, we examine whether prior inoculation with this recombinant Lm strain can protect against lethal intravaginal challenge with HSV-2.
The attenuated recombinant Lm strains used in this study were derived from LmΔactA strain DPL1942, and were transformed by penicillin treatment as described [19]. These Lm mutants contain a targeted deletion in the virulence determinant actA [15], and are thus unable to spread from cell to cell. Transformed strains contain an expression construct containing the Lm hly promoter and signal sequence ligated with DNA sequences expressing the heterologous gene of interest tagged with hemagglutinin [18]. These strains were confirmed to secrete recombinant proteins containing either the type-common HSV gB epitope SSIEFARL [2] (Lm-gB496–503; gB amino acid numbering is based on HSV-2 strain HG52 [5]), or a control (irrelevant peptide) sequence derived from Mycobacterium tuberculosis (Lm-ESAT62–20) [18]. Lm strains were grown and subcultured in brain-heart infusion medium containing chloramphenicol (20 µg/ml) to early log-phase (OD600 0.1). Bacteria were washed and diluted in saline to deliver 1 × 106 CFU in 200 µL intravenously into female C57BL/6 mice (purchased at 6–8 weeks of age from The Jackson Laboratory, and inoculated prior to 10 weeks of age).
HSV-2 strain 186 was grown and titered on mycoplasma-free Vero cells. We sequenced the region of the HSV-2 186 gene containing SSIEFARL, and confirmed the presence of this sequence (data not shown). Virus was thawed and diluted in normal saline immediately prior to infection, and delivered intravaginally via micropipette in 10 µL. To confer susceptibility, mice were given 2 mg medroxyprogesterone acetate (Pharmacia, Kalamazoo, MI) subcutaneously in 50 µL saline six days prior to intravaginal infection as described [21]. After infection, animals were assessed daily for 14–28 days using a clinical scoring system ranging from 0 to 5: 0-no apparent infection, 1-mild redness/swelling of external vagina, 2-moderate redness/swelling of external vagina and surrounding tissue, 3-severe redness/swelling of external vagina and surrounding tissue, 4-genital ulceration with severe redness, and 5-severe genital ulceration extending to surrounding tissue [7]. Mice with severe clinical disease (limb paralysis, urinary retention, severe worsening lesions) or severe pathology (clinical scores of 5) were sacrificed by CO2 overdose, as these animals will progress to death [16, 20].
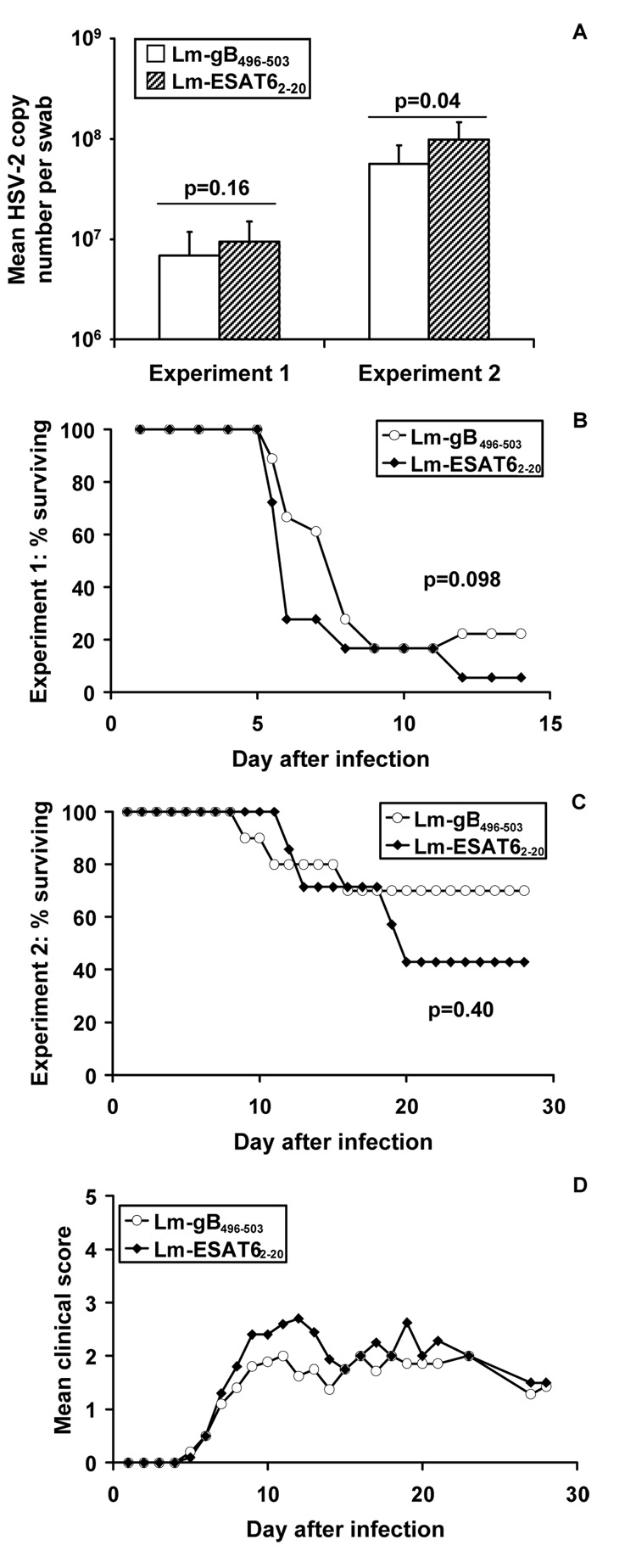
Peripheral blood was collected by orbital bleed from four mice in each group prior to intravaginal challenge with 5 × 105 PFU of HSV-2 (approximately 10 × LD50). HSV gB epitope-specific CD8+ T-cells were analyzed using H-2Kb DimerX loaded with HSV-2 gB496–503 peptide according to the manufacturer’s instructions (BD Biosciences). By 100 days after inoculation with Lm-gB496–503, circulating gB-specific CD8+ T-cells had undergone significant contraction, since they were at background levels comparable to control mice (Fig 1A). However three days after intravaginal HSV-2 challenge, mice primed with Lm-gB496–503 but not Lm-ESAT62–20 had a pronounced recall response, evidenced by expansion of peptide-specific T-cells among total circulating CD8+ T-cells (Fig.1B). Control mice had a significantly smaller expansion of circulating peptide-specific CD8+ T-cells. To investigate the effect of priming with Lm-gB496–503 on local viral replication, we quantified HSV-2 DNA from sterile polyester swabs (Copan, Corona, CA) used to collect vaginal secretions from mice prior to and three days after challenge. The swabs were immediately placed in digestion buffer (100 mM KCl, 10 mM Tris [pH 8.0], 25 mM EDTA, 0.5% Nonidet P-40) [22] and stored at −20°C. DNA extraction, real-time, quantitative PCR, and inhibition controls for HSV DNA were performed as described [28]. Three days after challenge, no significant differences in HSV DNA copy number in the vaginal mucosa between the two Lm infection groups could be detected (Fig 2A, Experiment 1, means compared by unpaired t-test). Similarly, no significant differences were noted between groups of mice in mortality (Fig 2B). Although there was no difference in mortality between the two groups, a slightly reduced clinical score was noted in the Lm-gB496–503 group over the first 6–7 days after infection (data not shown).
Figure 1.
HSV gB-specific T-cell response in blood of mice day 100 after priming with Lm-gB496–503 or Lm-ESAT62–20 (A), and three days after intravaginal challenge with 5 × 105 PFU of HSV-2 strain 186 (B). Means ± standard deviation from 4–5 mice per group are shown; p-values compare mean values between groups by unpaired t-test.
Figure 2.
Mean mucosal HSV-2 copy numbers day 3 post-challenge (A), mortality rate (B and C), and average clinical disease score among surviving mice in Experiment 2 (D), after challenge with 5 × 105 PFU HSV-2 in mice inoculated with Lm-gB496–503 or Lm-ESAT62–20 100 days prior to challenge (Experiment 1) or with 5×104 PFU HSV-2 in mice inoculated with Lm-gB496–503 or Lm-ESAT62–20 30 days prior to challenge (Experiment 2). Data for Experiment 1 are from two independent challenges with 18 mice per group. Experiment 2 data are from 10 mice in the Lm-gB496–503 group and 7 mice from the Lm-ESAT62–20 group; 3 Lm-ESAT62–20 survivor mice did not initiate productive infection, as there were no clinical signs of infection, no recovery of DNA from vaginal swabs collected on day 3 after infection, and no anti-HSV-2 antibodies by ELISA (data not shown). DNA copy numbers were below the detection limit in all mice prior to infection (data not shown). Time to death in (B and C) is compared by log-rank test.
This apparent lack of protection could have been due to challenge with a dose of HSV-2 which overwhelmed the memory response, or alternatively could have reflected waning ability of gB-specific T-cells to protect in the late memory phase (challenge 100 days after priming with recombinant Lm). Waning protection has been previously observed for several different HSV vaccine strategies using the immunodominant gB epitope in C57BL/6 mice [8, 17]. Accordingly, a second experiment was conducted using a lower challenge dose (5×104 PFU, or approximately the LD50), in mice primed with the same Lm strains 30 days prior to challenge. We have previously shown measurable levels of circulating epitope-specific T-cells at this time point after priming [18]. Following challenge at this dose, there was a modest but significant reduction in the amount of HSV-2 DNA recovered from mice primed with Lm-gB496–503 compared with Lm-ESAT62–20 (Fig 2A, Experiment 2). Surprisingly, this apparent reduction in viral load in mice primed with Lm-gB496–503 compared with Lm-ESAT62–20 was not associated with significantly increased rates of survival (Figure 2C) or reductions in disease score (Fig 2D).
The inability of parenteral administration of Lm-gB496–503 to provide protection against mucosal disease and mortality after intravaginal HSV-2 challenge may be due to several factors. We would not expect the lack of protection in these studies compared to the prior studies [18] to be related to the use of HSV-2 instead of HSV-1 for challenge. The gB epitope is present in both viruses, and previous studies have shown protection against HSV-2 challenge after immunization to promote cellular immunity against this peptide [1]. A second possibility is that epitope-specific T-cell immunity is unable to protect against vaginal challenge. This would not be expected, since prior studies have demonstrated that immunization of mice against the gB epitope protected against intravaginal challenge with HSV-1 [8]; however, the possibility that cellular immunity cannot specifically protect against intravaginal HSV-2 (as opposed to HSV-1) challenge cannot be excluded. It is also possible that the CD8+ T-cell response promoted by intravenous Lm-HSV infection may have generated responder cells unable to home to the mucosa quickly enough or in sufficient numbers to minimize viral replication or infection of supplying nerves. The factors promoting trafficking of T-cells to mucosal surfaces are not well understood, but may involve expression of the skin-homing molecules collectively known as E-selectin ligands by circulating memory T-cells [12, 14]. Importantly, previous studies have demonstrated a dependence on route of immunization for promotion of long-term, mucosally-directed CD8+ T-cell responses after immunization [6]. In fact, the prior studies showing protection against HSV-2 intranasal challenge immunized animals with a recombinant vaccinia contruct delivered intranasally [1]. Further studies are planned to modify the Lm-based immunization system, including tests of mucosal immunization prior to mucosal challenge, with the intent of promoting long-term, mucosally-directed immune responses.
There are advantages and limitations in considering the possible direct application of the described vaccine approaches to control of human disease. Attenuated Lm is safe, may allow delivery of numerous different heterologous antigens, and may lead to immune responses against these antigens [9, 15]. Unlike adenovirus vectors, which have been in development as mucosal vaccine vectors [23], use of Lm to deliver heterologous antigen may induce specific immunity, even in the presence of preexisting immunity to the bacterium [25]. The HSV gB glycoprotein is unlikely to be a useful single immunogen to promote protective responses in humans [3]; however, alternative targets of CD8+ T-cell-mediated immunity may allow further development of candidate Lm prophylactic or therapeutic HSV vaccines in humans [13].
Acknowledgments
This work was supported in part by the following grants to SSW: NIH-K08HD51584, a Wyeth Infectious Disease Society of America award, Puget Sound Partners for Global Health, and a March of Dimes Basil O'Conner starter research award. WJM was supported by NIH grant T32 AI007411 and by a Child Health Research Career Development Award through the Department of Pediatrics, Feinberg School of Medicine, and Children’s Memorial Research Center at Northwestern University. DMK is supported in part by NIH AI50132
We thank Dr. Gregg Milligan (University of Texas Medical Branch, Galveston, TX) for HSV-2 strain 186, which was grown and titered by Chris McClurkan.
Footnotes
The original publication is available at www.springerlink.com
References
- 1.Blaney JJ, Nobusawa E, Brehm M, Bonneau R, Mylin L, Fu T, Kawaoka Y, Tevethia S. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol. 1998;72:9567–9574. doi: 10.1128/jvi.72.12.9567-9574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonneau R, Salvucci L, Johnson D, Tevethia S. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 3.Corey L, Langenberg A, Ashley R, Sekulovich R, Izu A, Douglas JJ, Handsfield H, Warren T, Marr L, Tyring S, DiCarlo R, Adimora A, Leone P, Dekker C, Burke R, Leong W, Straus S. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA. 1999;282:331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 4.Corey L. Herpes Simplex Virus. In: Mandell G, Bennett J, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia, Pennsylvania: Churchill Livingstone; 2005. [Google Scholar]
- 5.Dolan A, Jamieson F, Cunningham C, Barnett B, McGeoch D. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallichan W, Rosenthal K. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallichan W, Rosenthal K. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology. 1996;224:487–497. doi: 10.1006/viro.1996.0555. [DOI] [PubMed] [Google Scholar]
- 8.Gierynska M, Kumaraguru U, Eo S-K, Lee S, Krieg A, Rouse B. Induction of CD8 T-cell-specific systemic and mucosal immunity against herpes simplex virus with CpG-peptide complexes. J Virol. 2002;76:6568–6576. doi: 10.1128/JVI.76.13.6568-6576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton S, Badovinac V, Khanolkar A, Harty J. Listeriolysin O-deficient Listeria monocytogenes as a vaccine delivery vehicle: antigen-specific CD8 T cell priming and protective immunity. J Immunol. 2006;177:4012–4020. doi: 10.4049/jimmunol.177.6.4012. [DOI] [PubMed] [Google Scholar]
- 10.Khanna K, Bonneau R, Kinchington P, Hendricks R. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koelle D, Posavad C, Barnum G, Johnson M, Frank J, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koelle D, Liu Z, McClurkan C, Topp M, Riddell S, Pamer E, Johnson A, Wald A, Corey L. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koelle D, Corey L. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin Microbiol Rev. 2003;16:96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koelle D, Gonzalez J, Johnson A. Homing in on the cellular immune response to HSV-2 in humans. Am J Reprod Immunol. 2005;53:172–181. doi: 10.1111/j.1600-0897.2005.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollmann T, Reikie B, Blimkie D, Way S, Hajjar A, Arispe K, Shaulov A, Wilson C. Induction of protective immunity to Listeria monocytogenes in neonates. J Immunol. 2007;178:3695–3701. doi: 10.4049/jimmunol.178.6.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuklin N, Daheshia M, Karem K, Manickan E, Rouse B. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J Virol. 1997;71:3138–3145. doi: 10.1128/jvi.71.4.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumaraguru U, Gierynska M, Norman S, Bruce B, Rouse B. Immunization with chaperone-peptide complex induces low-avidity cytotoxic T lymphocytes providing transient protection against herpes simplex virus infection. J Virol. 2002;76:136–141. doi: 10.1128/JVI.76.1.136-141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orr M, Orgun N, Wilson C, Way S. Recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J Immunol. 2007;178:4731–4735. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SF, Stewart GS. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene. 1990;94:129–132. doi: 10.1016/0378-1119(90)90479-b. [DOI] [PubMed] [Google Scholar]
- 20.Parr E, Parr M. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol. 1997;71:8109–8115. doi: 10.1128/jvi.71.11.8109-8115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parr M, Kepple L, McDermott M, Drew M, Bozzola J, Parr E. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- 22.Ryncarz A, Goddard J, Wald A, Huang M, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol. 1999;37:1941–1947. doi: 10.1128/jcm.37.6.1941-1947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiver J, Emini E. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 24.Stanberry L. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes. 2004;11:161A–169A. [PubMed] [Google Scholar]
- 25.Starks H, Bruhn K, Shen H, Barry R, Dubensky T, Brockstedt D, Hinrichs D, Higgins D, Miller J, Giedlin M, Bouwer H. Listeria monocytogenes as a vaccine vector: virulence attenuation or existing antivector immunity does not diminish therapeutic efficacy. J Immunol. 2004;173:420–427. doi: 10.4049/jimmunol.173.1.420. [DOI] [PubMed] [Google Scholar]
- 26.Stock A, Jones C, Heath W, Carbone F. CTL response compensation for the loss of an immunodominant class I-restricted HSV-1 determinant. Immunol Cell Biol. 2006;84:543–550. doi: 10.1111/j.1440-1711.2006.01469.x. [DOI] [PubMed] [Google Scholar]
- 27.Verjans G, Hintzen R, van Dun J, Poot A, Milikan J, Laman J, Langerak A, Kinchington P, Osterhaus A. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wald A, Huang M, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345–1351. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 29.Wallace M, Keating R, Heath W, Carbone F. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol. 1999;73:7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Koelle D, Cao J, Vazquez J, Huang M, Hladik F, Wald A, Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]