Abstract
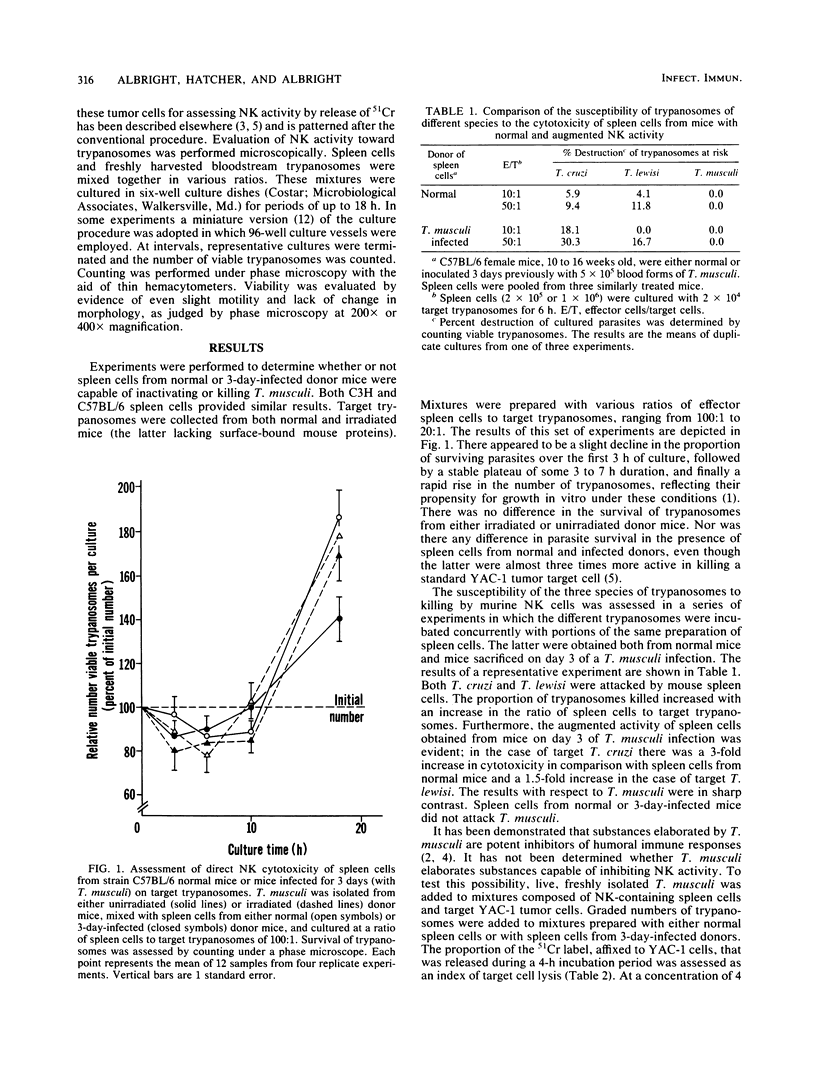
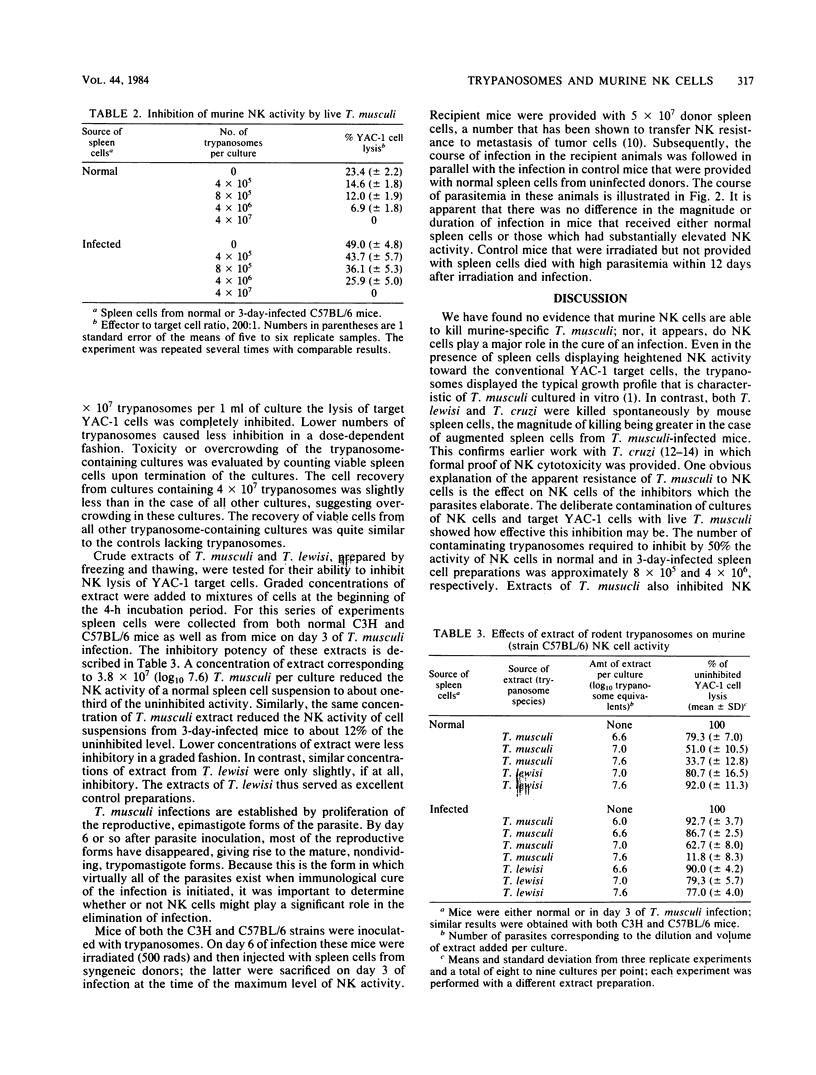
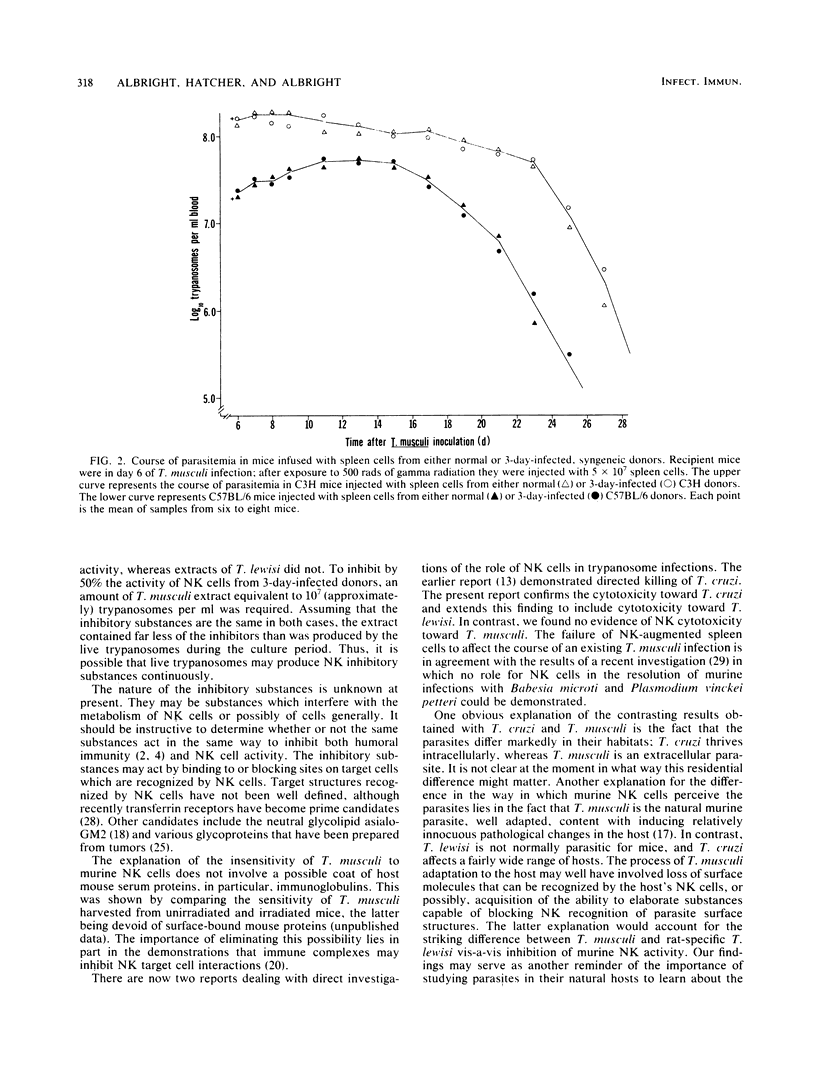
The involvement of natural killer (NK) cells in the immunological resistance of mice to murine-specific Trypanosoma musculi was evaluated. Murine NK cells were found to be unable to kill or inhibit T. musculi or to protect recipients from infection. In addition, the ability of spleen cells from normal mice and from mice on day 3 of T. musculi infection, at the time of maximum NK augmentation, to kill Trypanosoma cruzi and Trypanosoma lewisi was evaluated. Spleen cells from normal mice displayed significant killing of both T. cruzi and T. lewisi. Furthermore, augmented spleen cells from T. musculi-infected mice were considerably more effective than normal spleen cells in killing both T. cruzi and T. lewisi. The activity of NK cells toward YAC-1 tumor target cells was inhibited in a dose-dependent fashion by either live T. musculi or extracts of T. musculi, but not by extracts of rat-specific T. lewisi. The results suggest that well-adapted protozoan parasites may be nonsusceptible to the natural cell-mediated resistance mechanisms of their hosts. Their nonsusceptibility could result from the ability to elaborate substances that either inactivate NK cells or block NK cell interaction with complementary sites on the parasite surface.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Albright J. F. Age-associated impairment of murine natural killer activity. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6371–6375. doi: 10.1073/pnas.80.20.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F., Dusanic D. G. Mechanisms of trypanosome-mediated suppression of humoral immunity in mice. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3923–3927. doi: 10.1073/pnas.75.8.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. Growth of Trypanosoma musculi in cultures of murine spleen cells and analysis of the requirement for supportive spleen cells. Infect Immun. 1978 Nov;22(2):343–349. doi: 10.1128/iai.22.2.343-349.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. Trypanosome-mediated suppression of murine humoral immunity independent of typical suppressor cells. J Immunol. 1980 May;124(5):2481–2484. [PubMed] [Google Scholar]
- Albright J. W., Huang K. Y., Albright J. F. Natural killer activity in mice infected with Trypanosoma musculi. Infect Immun. 1983 Jun;40(3):869–875. doi: 10.1128/iai.40.3.869-875.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G., Hochman P. S. Do natural killer cells engage in regulated reactions against self to ensure homeostasis? Immunol Rev. 1979;44:13–41. doi: 10.1111/j.1600-065x.1979.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasite Immunol. 1980 Winter;2(4):277–292. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Giannini S. H., D'Alesandro P. A. Trypanostatic activity of rat IgG purified from the surface coat of Trypanosoma lewisi. J Parasitol. 1982 Oct;68(5):765–773. [PubMed] [Google Scholar]
- Hanna N., Burton R. C. Definitive evidence that natural killer (NK) cells inhibit experimental tumor metastases in vivo. J Immunol. 1981 Nov;127(5):1754–1758. [PubMed] [Google Scholar]
- Hansson M., Kärre K., Kiessling R., Roder J., Andersson B., Häyry P. Natural NK-cell targets in the mouse thymus: characteristics of the sensitive cell population. J Immunol. 1979 Aug;123(2):765–771. [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E., Cerrone M. C., Burton R. C. Increased natural killer cell activity in experimental American trypanosomiasis. J Immunol. 1981 Sep;127(3):1126–1130. [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Destruction of Trypanosoma cruzi by Natural killer cells. Science. 1982 Oct 15;218(4569):295–296. doi: 10.1126/science.6812218. [DOI] [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Spontaneous lytic activity against allogeneic tumor cells and depression of specific cytotoxic responses in mice infected with Trypanosoma cruzi. J Immunol. 1981 Jun;126(6):2436–2442. [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Natural killer cells induced by acute and chronic toxoplasma infection. Cell Immunol. 1982 May 15;69(2):330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Hirokawa K., Eishi Y., Albright J. W., Albright J. F. Histopathological and immunocytochemical studies of Trypanosoma musculi infection of mice. Infect Immun. 1981 Dec;34(3):1008–1017. doi: 10.1128/iai.34.3.1008-1017.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. Surveillance of primitive cells by natural killer cells. Curr Top Microbiol Immunol. 1981;92:107–123. doi: 10.1007/978-3-642-68069-4_7. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. Responses to infection with metazoan and protozoan parasites in mice. Adv Immunol. 1979;28:451–511. doi: 10.1016/s0065-2776(08)60803-2. [DOI] [PubMed] [Google Scholar]
- Nunn M. E., Herberman R. B., Holden H. T. Natural cell-mediated cytotoxicity in mice against non-lymphoid tumor cells and some normal cells. Int J Cancer. 1977 Sep 15;20(3):381–387. doi: 10.1002/ijc.2910200309. [DOI] [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Salimonu L. S., Williams A. I., Akinwolere O. A., Shabo R., Alm G. V., Wigzell H. Positive correlation between degree of parasitemia, interferon titers, and natural killer cell activity in Plasmodium falciparum-infected children. J Immunol. 1981 Dec;127(6):2296–2300. [PubMed] [Google Scholar]
- Riccardi C., Santoni A., Barlozzari T., Herberman R. B. In vivo reactivity of mouse natural killer (NK) cells against normal bone marrow cells. Cell Immunol. 1981 May 1;60(1):136–143. doi: 10.1016/0008-8749(81)90254-9. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Ahrlund-Richter L., Jondal M. Target-effector interaction in the human and murine natural killer system: specificity and xenogeneic reactivity of the solubilized natural killer-target structure complex and its loss in a somatic cell hybrid. J Exp Med. 1979 Sep 19;150(3):471–481. doi: 10.1084/jem.150.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Human natural cell-mediated cytotoxicity against fetal fibroblasts. I. General characteristics of the cytotoxic activity. Cell Immunol. 1977 Oct;33(2):340–352. doi: 10.1016/0008-8749(77)90163-0. [DOI] [PubMed] [Google Scholar]
- Vodinelich L., Sutherland R., Schneider C., Newman R., Greaves M. Receptor for transferrin may be a "target" structure for natural killer cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):835–839. doi: 10.1073/pnas.80.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. R., Clark I. A. Apparent irrelevance of NK cells to resolution of infections with Babesia microti and Plasmodium vinckei petteri in mice. Parasite Immunol. 1982 Sep;4(5):319–327. doi: 10.1111/j.1365-3024.1982.tb00443.x. [DOI] [PubMed] [Google Scholar]


