Abstract
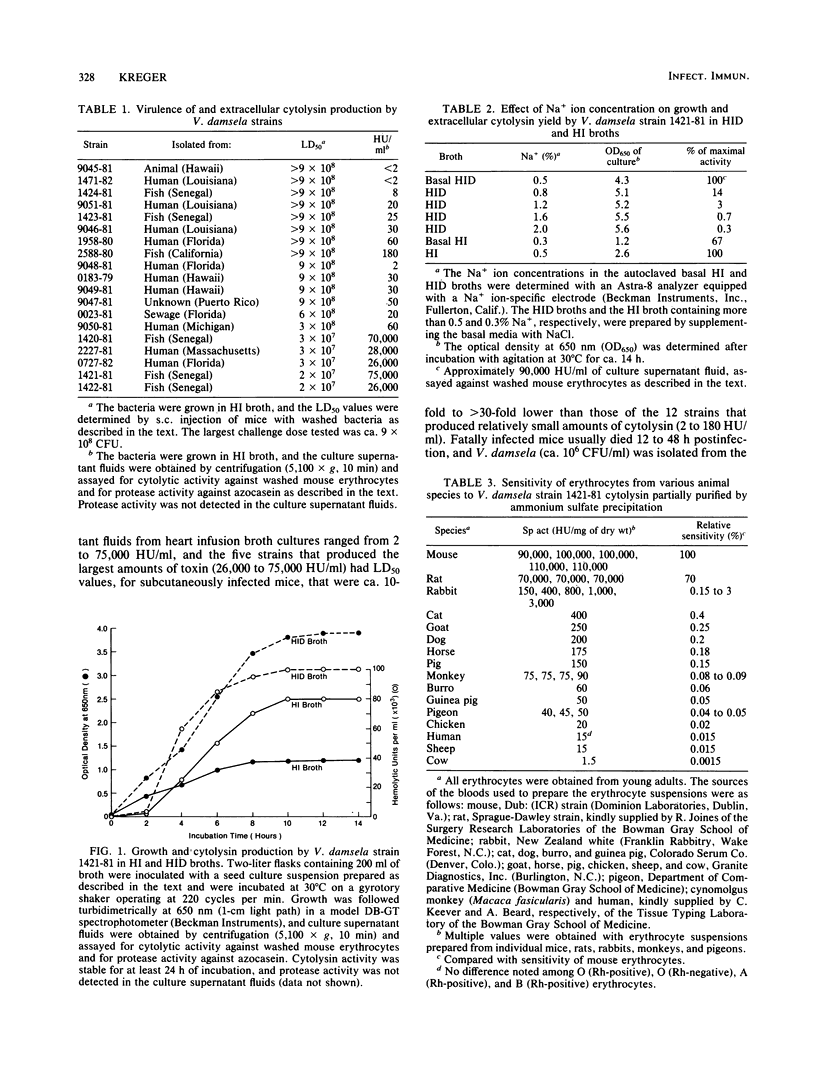
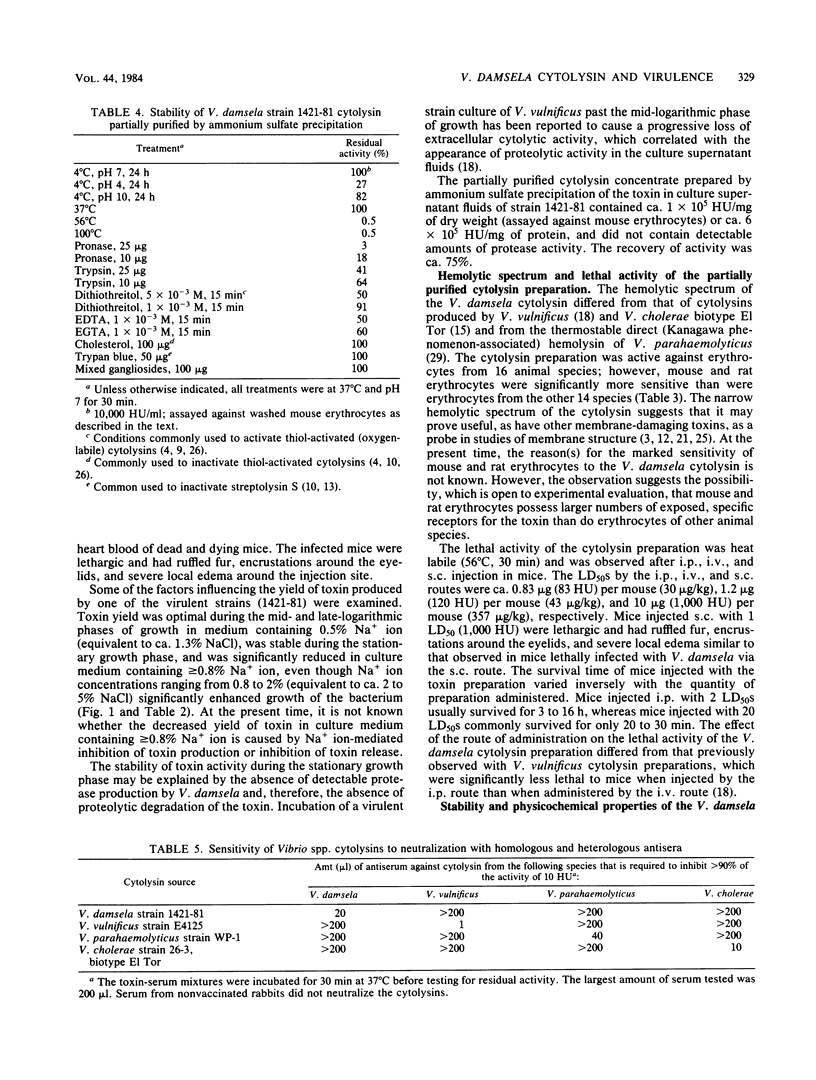
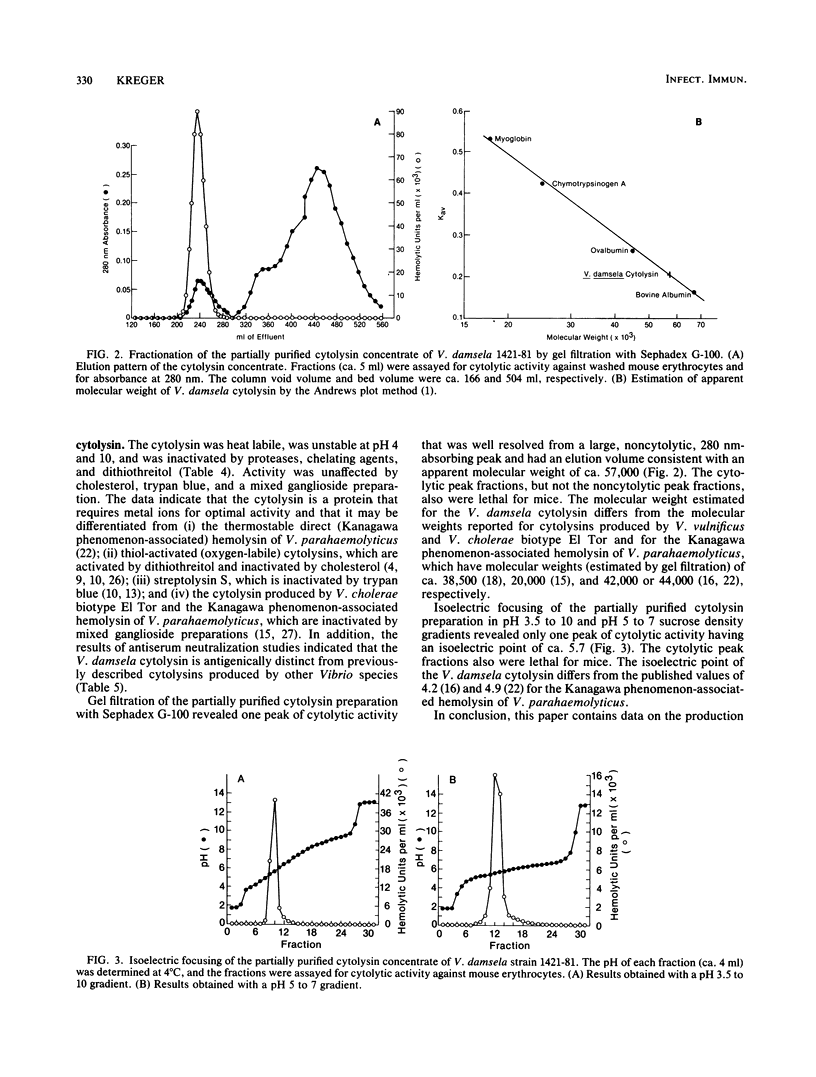
A correlation was observed between the ability of 19 isolates of Vibrio damsela, a halophilic bacterium recently recognized as a human pathogen, to cause disease in mice and to produce large amounts of a cytolytic toxin in vitro. The yield of toxin in the culture supernatant fluids was optimal during the mid- and late-logarithmic phases of growth in medium containing 0.5% Na+ ion, was stable during the stationary growth phase, and was significantly reduced in culture medium containing greater than or equal to 0.8% Na+ ion, even though Na+ ion concentrations ranging from 0.8% to 2% significantly enhanced growth of the bacterium. The activity in toxin preparations partially purified by ammonium sulfate precipitation was deleteriously affected by heat, low and high pH, proteases, dithiothreitol, and chelating agents, but was unaffected by cholesterol, trypan blue, and mixed gangliosides. The toxin had a molecular weight (estimated by gel filtration) of ca. 57,000 and an isoelectric point of ca. 5.7 and was antigenically distinct from previously described cytolysins produced by Vibrio vulnificus, Vibrio parahaemolyticus, and the El Tor biotype of Vibrio cholerae. Bacteriologically sterile, partially purified toxin preparations were lethal for mice after intraperitoneal, intravenous, and subcutaneous administration, and subcutaneous injection elicited grossly observable changes similar to those observed during the lethal experimental infection caused by subcutaneous injection of V. damsela.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Hickman-Brenner F. W., Lee J. V., Steigerwalt A. G., Fanning G. R., Hollis D. G., Farmer J. J., 3rd, Weaver R. E., Joseph S. W., Seidler R. J. Vibrio furnissii (formerly aerogenic biogroup of Vibrio fluvialis), a new species isolated from human feces and the environment. J Clin Microbiol. 1983 Oct;18(4):816–824. doi: 10.1128/jcm.18.4.816-824.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell J. L., Grushoff-Kosyk P. S., Bernheimer A. W. Purification of cereolysin and the electrophoretic separation of the active (reduced) and inactive (oxidized) forms of the purified toxin. Infect Immun. 1976 Jul;14(1):144–154. doi: 10.1128/iai.14.1.144-154.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L. Streptococcal growth and toxin production in vivo. Infect Immun. 1983 May;40(2):501–505. doi: 10.1128/iai.40.2.501-505.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C. R., Pankey G. A. Tissue invasion by unnamed marine vibrios. JAMA. 1975 Sep 15;233(11):1173–1176. [PubMed] [Google Scholar]
- Hickman F. W., Farmer J. J., 3rd, Hollis D. G., Fanning G. R., Steigerwalt A. G., Weaver R. E., Brenner D. J. Identification of Vibrio hollisae sp. nov. from patients with diarrhea. J Clin Microbiol. 1982 Mar;15(3):395–401. doi: 10.1128/jcm.15.3.395-401.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Purification and characterization of a hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect Immun. 1979 Dec;26(3):1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Taga S., Takeda T., Hasibuan M. A., Takeda Y., Miwatani T. Identification of lethal toxin with the thermostable direct hemolysin produced by Vibrio parahaemolyticus, and some physicochemical properties of the purified toxin. Infect Immun. 1976 Jan;13(1):133–139. doi: 10.1128/iai.13.1.133-139.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978 Feb;19(2):630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A., Lockwood D. Detection of extracellular toxin(s) produced by Vibrio vulnificus. Infect Immun. 1981 Aug;33(2):583–590. doi: 10.1128/iai.33.2.583-590.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M., Teebken-Fisher D., Hose J. E., Farmer J. J., 3rd, Hickman F. W., Fanning G. R. Vibrio damsela, a Marine Bacterium, Causes Skin Ulcers on the Damselfish Chromis punctipinnis. Science. 1981 Dec 4;214(4525):1139–1140. doi: 10.1126/science.214.4525.1139. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Obara Y., Nikkawa T., Yamai S., Kato T., Yamada Y., Ohashi M. Simplified purification and biophysicochemical characteristics of Kanagawa phenomenon-associated hemolysin of Vibrio parahaemolyticus. Infect Immun. 1980 May;28(2):567–576. doi: 10.1128/iai.28.2.567-576.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr, Miller H. G., Wilson R., Tacket C. O., Hollis D. G., Hickman F. W., Weaver R. E., Blake P. A. Illness caused by Vibrio damsela and Vibrio hollisae. Lancet. 1982 Jun 5;1(8284):1294–1297. doi: 10.1016/s0140-6736(82)92853-7. [DOI] [PubMed] [Google Scholar]
- Takeda R., Honda T., Sakural J., Otomo N. Inhibition of hemolytic activity of the thermostable direct hemolysin of Vibrio parahaemolyticus by ganglioside. Infect Immun. 1975 Oct;12(4):931–933. doi: 10.1128/iai.12.4.931-933.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen-Yoji H., Hitokoto H., Morozumi S., Le Clair R. A. Purification and characterization o;f a hemolysin produced by Vibrio parahaemolyticus. J Infect Dis. 1971 Jun;123(6):665–667. doi: 10.1093/infdis/123.6.665. [DOI] [PubMed] [Google Scholar]


