Abstract
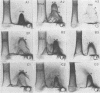
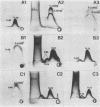
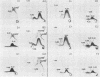
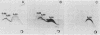
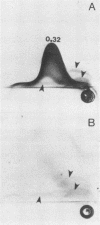
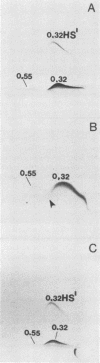
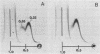
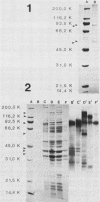
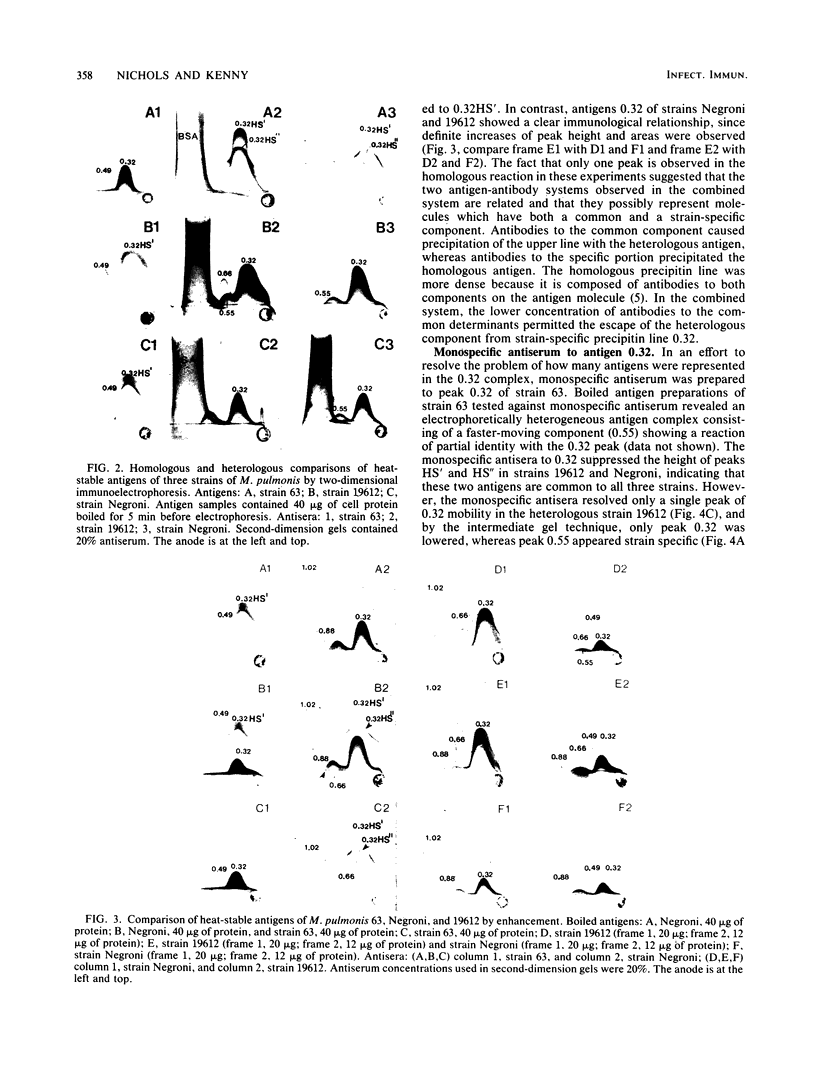
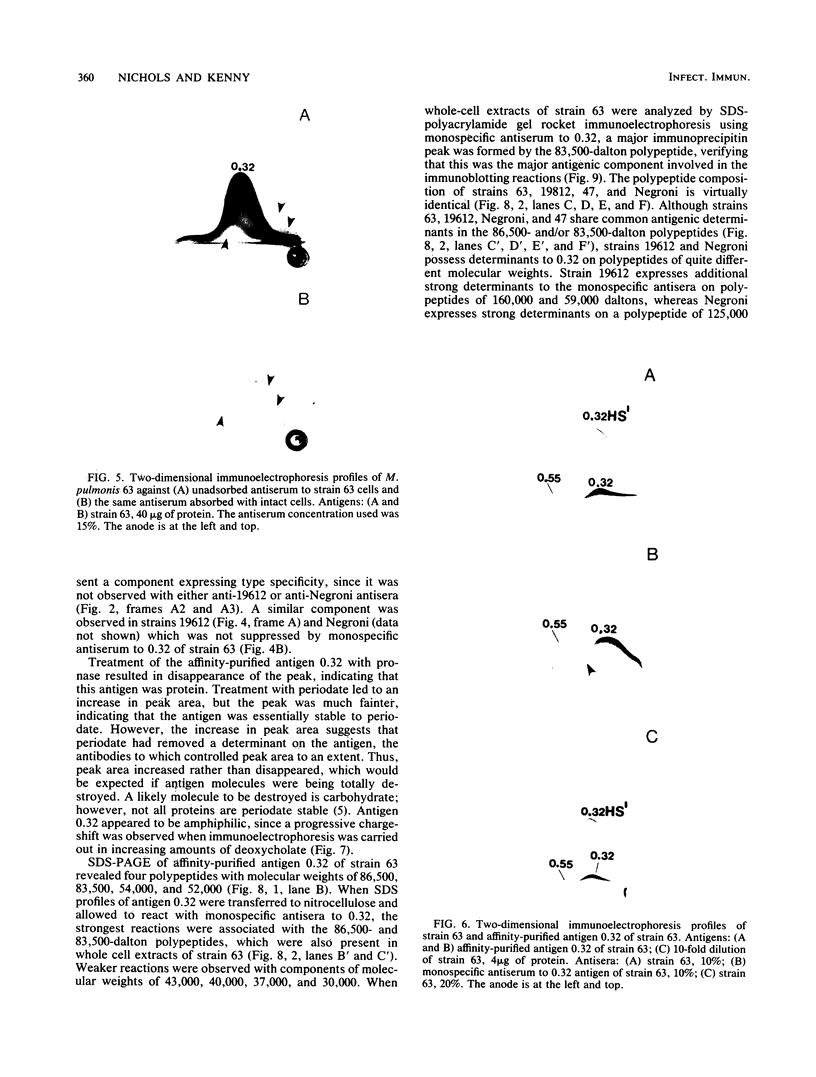
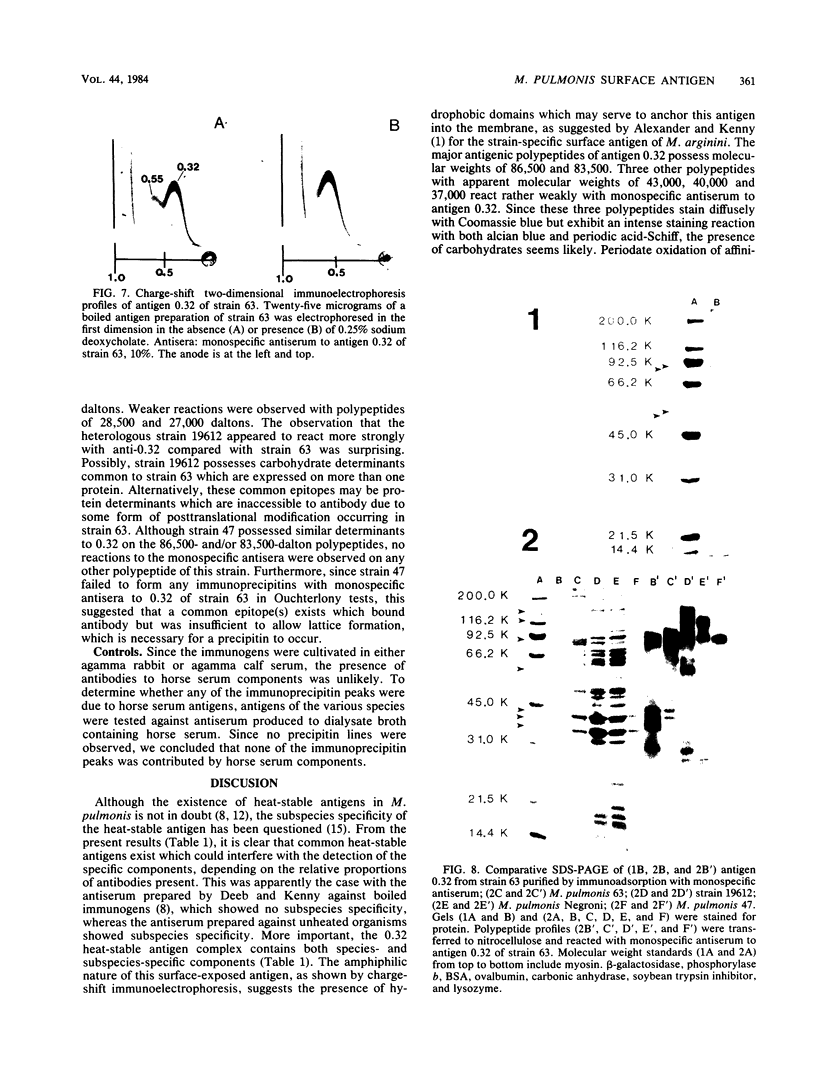
We sought to characterize the strain-specific antigens of four strains of Mycoplasma pulmonis (47, 63, Negroni, and 19612) by crossed immunoelectrophoresis. Although the strains possessed a number of common antigens, type-specific antigens of 0.32 mobility (bovine albumin was assigned a value of 1) were detected in strains 47 and 63. Strains 19612 and Negroni cross-reacted and represented a third group. Each strain possessed a major heat-stable antigen complex of 0.32 mobility characterized by a faster-moving component of 0.55 mobility. Monospecific antiserum to heat-stable antigen 0.32 of strain 63 demonstrated that this antigen complex consisted of at least three antigen-antibody precipitating systems characterized by type-specific and group-specific determinants. Adsorption of antiserum with whole cells revealed that the 0.32 antigen complex was surface exposed. The antigen complex is pronase sensitive and only partially sensitive to periodate. Purification of antigen 0.32 from detergent-extracted cells by affinity chromatography using monospecific antiserum revealed two major polypeptides of 86,500 and 83,500 dalton which reacted strongly with monospecific antiserum by immunoblotting. These reactive polypeptides were present in all strains examined. Additional polypeptides of different molecular weights in strains 19612 and Negroni produced strong reactions with monospecific antiserum, although sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles of the two strains were strikingly similar. Common heat-stable antigens were observed also. This study demonstrates that M. pulmonis strains possess an antigenically variable heat-stable surface antigen which is unique in that it contains not only strain-specific determinants but also species-specific determinants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander A. G., Kenny G. E. Application of charge shift electrophoresis to antigenic analysis of mycoplasmic membranes by two-dimensional (crossed) immunoelectrophoresis. Infect Immun. 1978 Jun;20(3):861–863. doi: 10.1128/iai.20.3.861-863.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A. G., Kenny G. E. Characterization of membrane and cytoplasmic antigens of Mycoplasma arginini by two-dimensional (crossed) immunoelectrophoresis. Infect Immun. 1977 Jan;15(1):313–321. doi: 10.1128/iai.15.1.313-321.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A. G., Kenny G. E. Characterization of the strain-specific and common surface antigens of Mycoplasma arginini. Infect Immun. 1980 Aug;29(2):442–451. doi: 10.1128/iai.29.2.442-451.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOBBITT J. M. Periodate oxidation of carbohydrates. Adv Carbohydr Chem. 1956;48(11):1–41. doi: 10.1016/s0096-5332(08)60115-0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Deeb B. J., Kenny G. E. Characterization of Mycoplasma pulmonis variants isolated from rabbits. I. Identification and properties of isolates. J Bacteriol. 1967 Apr;93(4):1416–1424. doi: 10.1128/jb.93.4.1416-1424.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb B. J., Kenny G. E. Characterizion of Mycoplasma pulmonis variants isolated from rabbits. II. Basis for differentiation of antigenic subtypes. J Bacteriol. 1967 Apr;93(4):1425–1429. doi: 10.1128/jb.93.4.1425-1429.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Forshaw K. A., Fallon R. J. Serological heterogeneity of Mycoplasma pulmonis. J Gen Microbiol. 1972 Oct;72(3):501–510. doi: 10.1099/00221287-72-3-501. [DOI] [PubMed] [Google Scholar]
- Geary S. J., Tourtellotte M. E., Cameron J. A. Inflammatory toxin from Mycoplasma bovis: isolation and characterization. Science. 1981 May 29;212(4498):1032–1033. doi: 10.1126/science.7233196. [DOI] [PubMed] [Google Scholar]
- Haller G. J., Boiarski K. W., Somerson N. L. Comparative serology of Mycoplasma pulmonis. J Infect Dis. 1973 Mar;127(Suppl):S38–S42. doi: 10.1093/infdis/127.supplement_1.s38. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Charge shift electrophoresis: simple method for distinguishing between amphiphilic and hydrophilic proteins in detergent solution. Proc Natl Acad Sci U S A. 1977 Feb;74(2):529–532. doi: 10.1073/pnas.74.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. Antigenic differences within the species Mycoplasma hominis. J Hyg (Lond) 1970 Sep;68(3):469–477. doi: 10.1017/s0022172400042376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. Membrane antigens of Mycoplasma hominis. J Hyg (Lond) 1972 Mar;70(1):85–98. doi: 10.1017/s0022172400022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Taylor G. Variation in the virulence of strains of Mycoplasma pulmonis related to susceptibility to killing by macrophages in vivo. J Gen Microbiol. 1979 Oct;114(2):289–294. doi: 10.1099/00221287-114-2-289. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Immunogenicity of Mycoplasma pneumoniae. Infect Immun. 1971 Apr;3(4):510–515. doi: 10.1128/iai.3.4.510-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J. On the immunoelectrophoretical identification and quantitation of serum proteins. Scand J Clin Lab Invest. 1968;22(1):79–81. doi: 10.3109/00365516809160742. [DOI] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Svendsen J., Axelsen N. H. A modified antigen--antibody crossed electrophoresis characterizing the specificity and titre of human precipiting against Candida albicans. J Immunol Methods. 1972 Jan;1(2):169–176. doi: 10.1016/0022-1759(72)90044-0. [DOI] [PubMed] [Google Scholar]
- Thirkill C. E., Kenny G. E. Antigenic analysis of three strains of Mycoplasma arginini by two-dimensional immunoelectrophoresis. J Immunol. 1975 Mar;114(3):1107–1111. [PubMed] [Google Scholar]
- Thirkill C. E., Kenny G. E. Serological comparison of five arginine-utilizing Mycoplasma species by two-dimensional immunoelectrophoresis. Infect Immun. 1974 Sep;10(3):624–632. doi: 10.1128/iai.10.3.624-632.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardi A. H., Michos G. A. Alcian blue staining of glycoproteins in acrylamide disc electrophoresis. Anal Biochem. 1972 Oct;49(2):607–609. doi: 10.1016/0003-2697(72)90472-1. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bocchini V., Becker M., Givol D. A general method for the specific isolation of peptides containing modified residues, using insoluble antibody columns. Biochemistry. 1971 Jul 20;10(15):2828–2834. doi: 10.1021/bi00791a004. [DOI] [PubMed] [Google Scholar]