Abstract
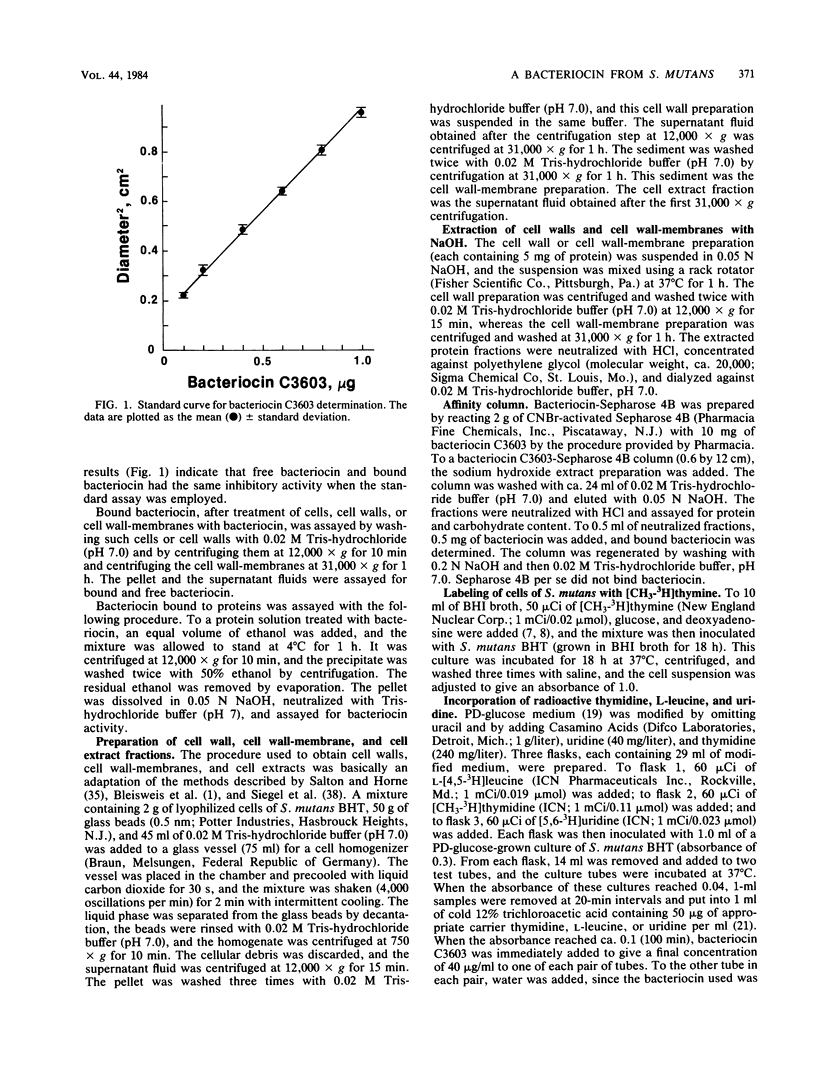
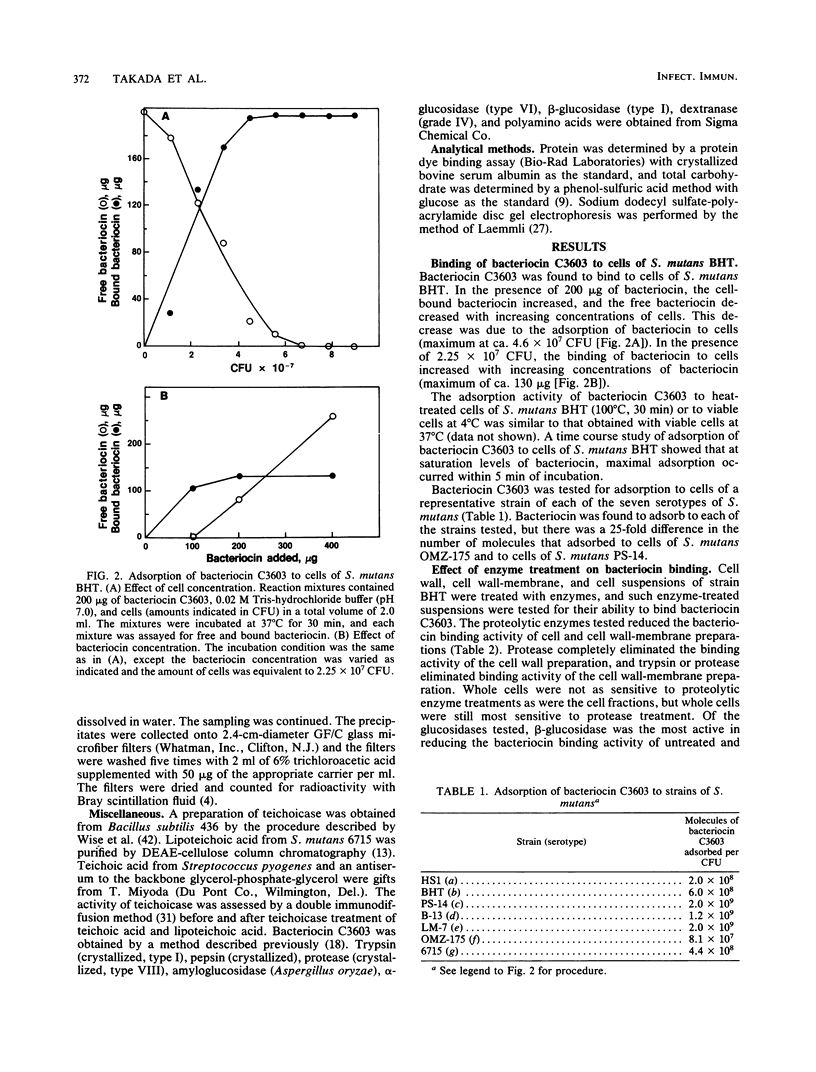
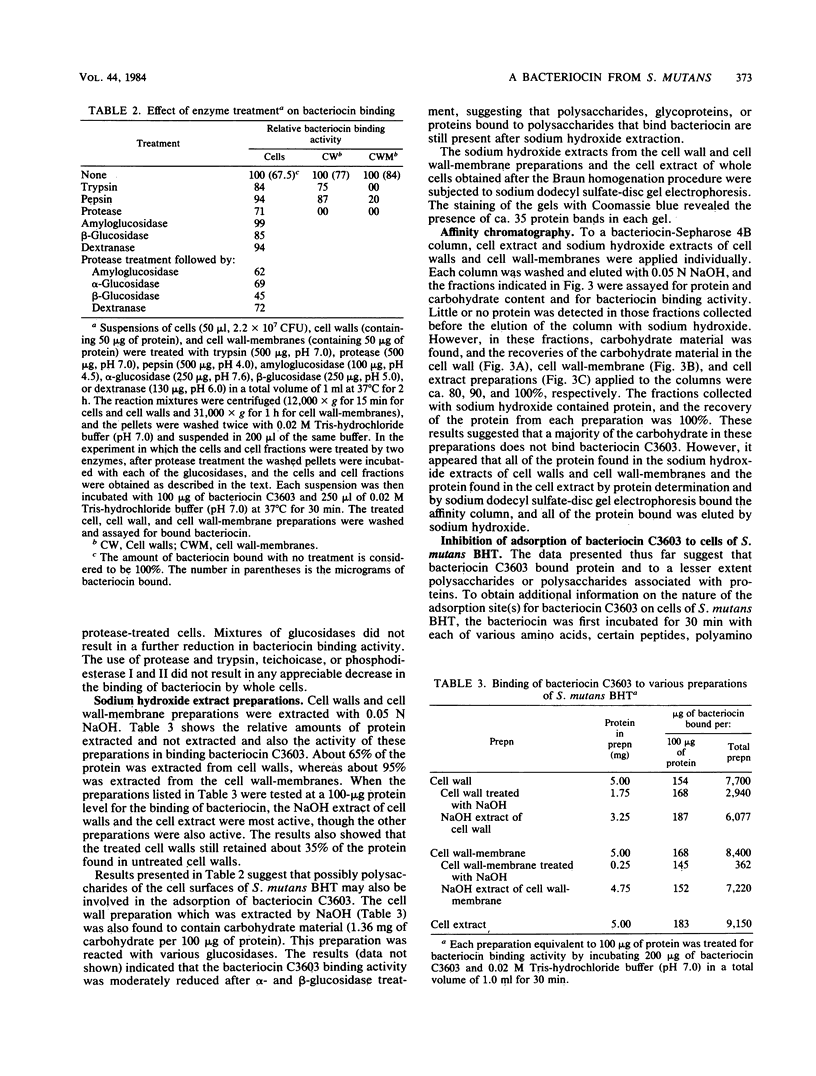
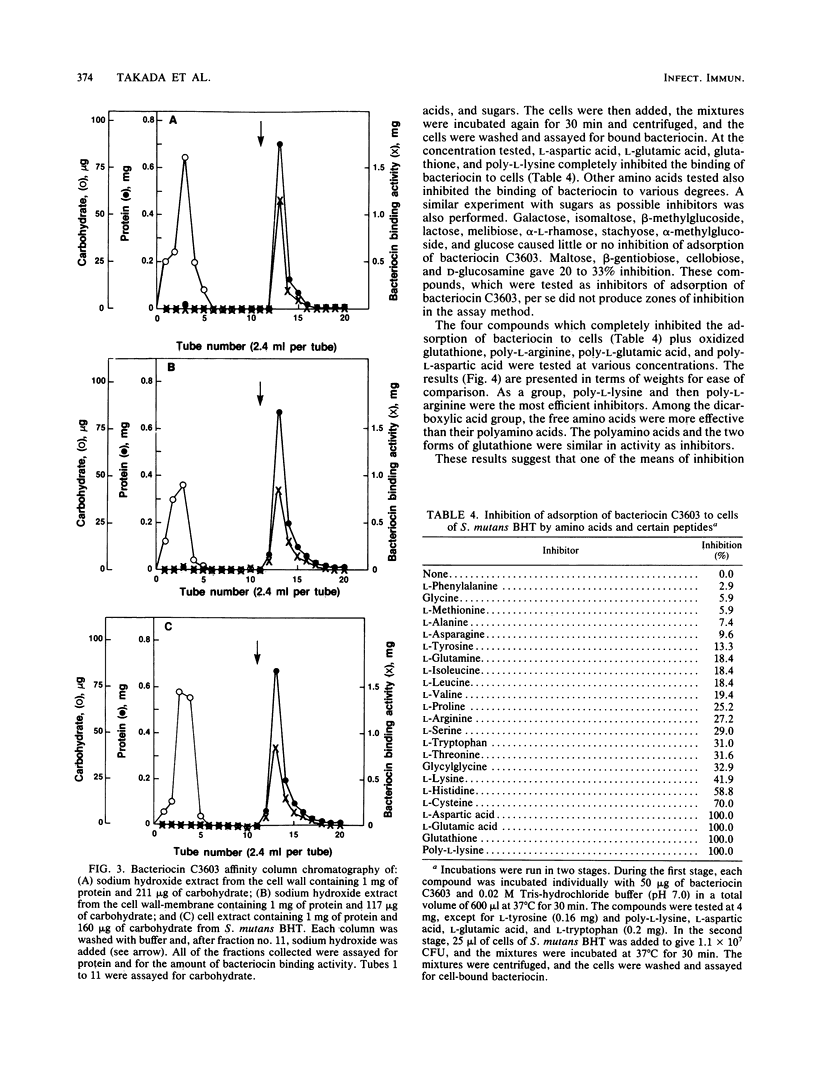
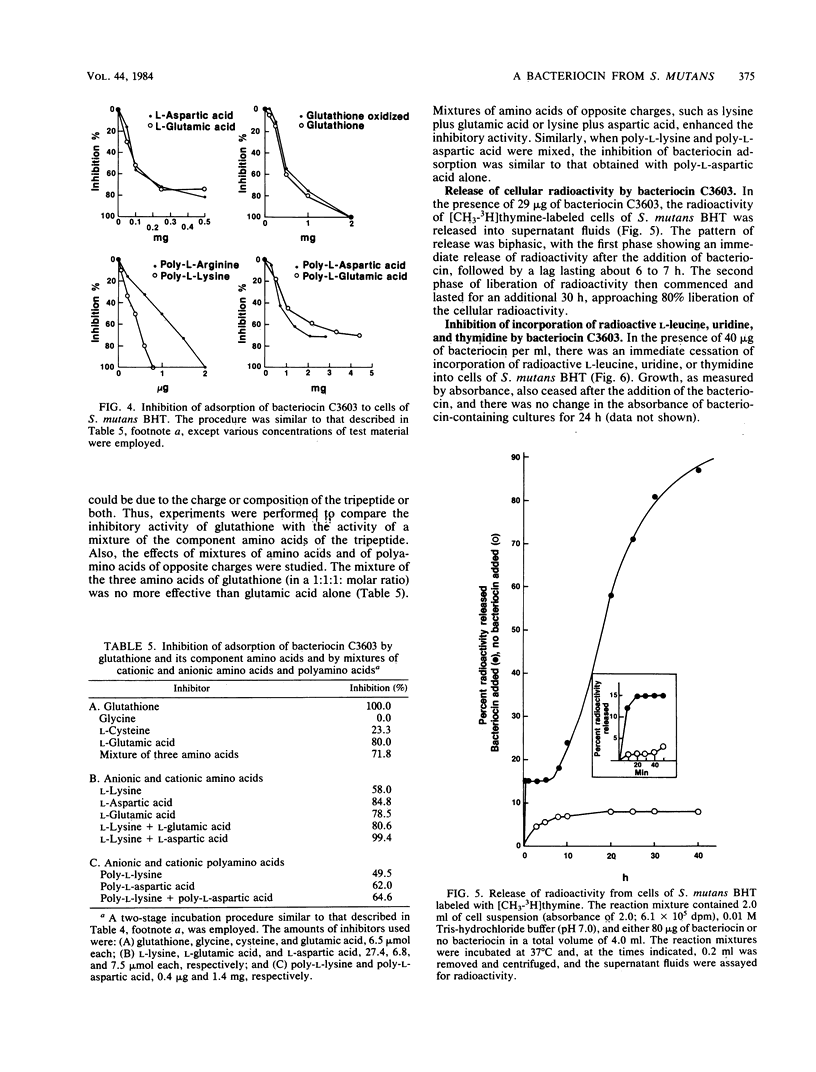
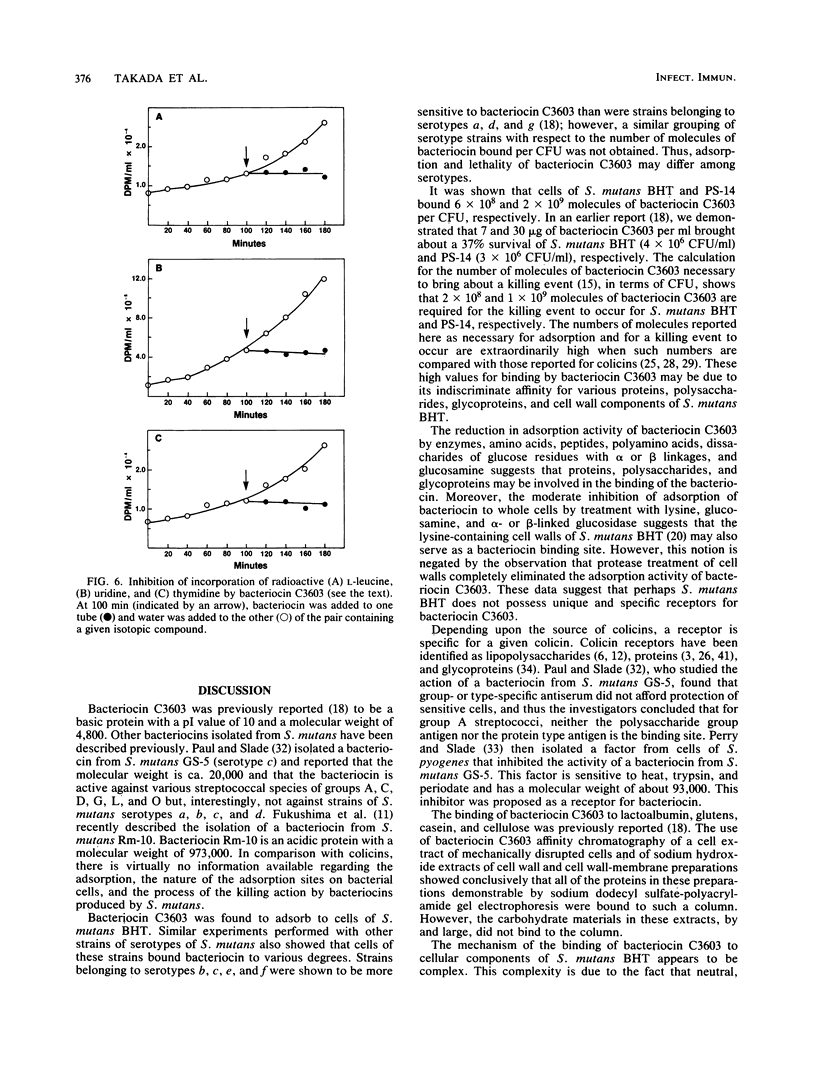
The basis for the lethal activity of a bacteriocin produced by Streptococcus mutans C3603 (serotype c) was studied. Bacteriocin C3603 was found to adsorb to cells of representative strains of the seven serotypes of S. mutans. S. mutans BHT (serotype b) was used to study the adsorption and the lethal properties of bacteriocin C3603. The adsorption of bacteriocin to cells of S. mutans BHT was inhibited by treatment of cells with protease and beta-glucosidase and by such ligands as poly-L-lysine, poly-L-arginine, L-aspartic acid, L-glutamic acid, glutathione, oxidized glutathione, poly-L-aspartic acid, and poly-L-glutamic acid. The adsorption to cells was also inhibited by oligosaccharides and glucosamine. Mixtures of anionic and cationic amino acids or polyamino acids did not greatly enhance or antagonize the inhibition of adsorption of bacteriocin C3603 to cells. Sodium hydroxide extracts of cell walls and cell wall-membranes contained carbohydrates and proteins; however, only proteins were found to bind to bacteriocin or to a bacteriocin affinity column. The sodium hydroxide extracts contained about 35 protein bands as determined by sodium dodecyl sulfate-polyacrylamide disc gel electrophoresis. Bacteriocin C3603 was found to immediately inhibit the synthesis of proteins, DNA, and RNA of cells and to slowly release DNA from cells of S. mutans BHT.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLEIWEIS A. S., KARAKAWA W. W., KRAUSE R. M. IMPROVED TECHNIQUE FOR THE PREPARATION OF STREPTOCOCCAL CELL WALLS. J Bacteriol. 1964 Oct;88:1198–1200. doi: 10.1128/jb.88.4.1198-1200.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGER W. C., STAHMANN M. A. The agglutination and growth inhibition of bacteria by lysine polypeptides. Arch Biochem Biophys. 1952 Jul;39(1):27–36. doi: 10.1016/0003-9861(52)90257-9. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Chang Y. Y., Hager L. P. Inhibition of colicin e2 activity by bacterial lipopolysaccharide. J Bacteriol. 1970 Dec;104(3):1106–1109. doi: 10.1128/jb.104.3.1106-1109.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Giuffrida A. Method for the lysis of Gram-positive, asporogenous bacteria with lysozyme. Appl Environ Microbiol. 1980 Jan;39(1):153–158. doi: 10.1128/aem.39.1.153-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on cellular metabolism. J Bacteriol. 1969 Jan;97(1):64–77. doi: 10.1128/jb.97.1.64-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H., Fukushima S., Umemoto T., Fukuhara H., Sagawa H. Purification and chemical analysis of a bacteriocin from the oral bacterium streptococcus mutans rm-10. Arch Oral Biol. 1982;27(9):721–727. doi: 10.1016/0003-9969(82)90020-6. [DOI] [PubMed] [Google Scholar]
- Guterman S. K., Luria S. E. Escherichia coli: strains that excrete an inhibitor of colicin B. Science. 1969 Jun 20;164(3886):1414–1414. doi: 10.1126/science.164.3886.1414. [DOI] [PubMed] [Google Scholar]
- HOLLAND I. B. A BACTERIOCIN SPECIFICALLY AFFECTING DNA SYNTHESIS IN BACILLUS MEGATERIUM. J Mol Biol. 1965 Jun;12:429–438. doi: 10.1016/s0022-2836(65)80265-0. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Purification and immunochemical characterization of type e polysaccharide antigen of Streptococcus mutans. Infect Immun. 1976 Jul;14(1):68–76. doi: 10.1128/iai.14.1.68-76.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges A. J. An examination of single-hit and multi-hit hypotheses in relation to the possible kinetics of colicin adsorption. J Theor Biol. 1966 Aug;11(3):383–410. doi: 10.1016/0022-5193(66)90100-7. [DOI] [PubMed] [Google Scholar]
- Holland E. M., Holland I. B. Induction of DNA breakdown and inhibition of cell division by colicin E2. Nature of some early steps in the process and properties of the E-2-specific nuclease system. J Gen Microbiol. 1970 Dec;64(2):223–239. doi: 10.1099/00221287-64-2-223. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Iwanami T., Hirasawa M., Watanabe C., McGhee J. R., Shiota T. Purification and certain properties of a bacteriocin from Streptococcus mutans. Infect Immun. 1982 Mar;35(3):861–868. doi: 10.1128/iai.35.3.861-868.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Shiota T., McGhee J. R., Otake S., Michalek S. M., Ochiai K., Hirasawa M., Sugimoto K. Virulence of Streptococcus mutans: comparison of the effects of a coupling sugar and sucrose on certain metabolic activities and cariogenicity. Infect Immun. 1978 Feb;19(2):477–480. doi: 10.1128/iai.19.2.477-480.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Hamada S., Kotani S., Kato K. Enzymic lysis and structure of the cell walls of the oral Streptococcus mutans BHT. Arch Oral Biol. 1979;24(7):529–537. doi: 10.1016/0003-9969(79)90132-8. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Characteristics of the killing effect of a Staphylococcus epidermidis bacteriocin. Antonie Van Leeuwenhoek. 1974;40(1):177–183. doi: 10.1007/BF00394565. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Effects of colicin A and staphylococcin 1580 on amino acid uptake into membrane vesicles of Escherichia coli and staphylococcus aureus. Biochim Biophys Acta. 1973 Jul 18;311(4):483–495. doi: 10.1016/0005-2736(73)90124-7. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Mode of action of a Staphylococcus epidermidis bacteriocin. Antimicrob Agents Chemother. 1972 Dec;2(6):456–463. doi: 10.1128/aac.2.6.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATCHALSKI E., BICHOWSKI-SLOMNITZKI L., VOLCANI B. E. The action of some water-soluble poly-alpha-amino acids on bacteria. Biochem J. 1953 Nov;55(4):671–680. doi: 10.1042/bj0550671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J., Cowell B. S. Interaction of colicin Ia with bacterial cells. Direct measurement of Ia-receptor interaction. J Biol Chem. 1972 Oct 25;247(20):6524–6529. [PubMed] [Google Scholar]
- Konisky J., Liu C. T. Solubilization and partial characterization of the colicin I receptor of Escherichia coli. J Biol Chem. 1974 Feb 10;249(3):835–840. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maeda A., Nomura M. Interaction of colicins with bacterial cells. I. Studies with radioactive colicins. J Bacteriol. 1966 Feb;91(2):685–694. doi: 10.1128/jb.91.2.685-694.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui E., Mizuno D. Stabilization of colicin E2 by bovine serum albumin. J Bacteriol. 1969 Nov;100(2):1136–1137. doi: 10.1128/jb.100.2.1136-1137.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Paul D., Slade H. D. Production and properties of an extracellular bacteriocin from Streptococcus mutans bacteriocidal for group A and other streptococci. Infect Immun. 1975 Dec;12(6):1375–1385. doi: 10.1128/iai.12.6.1375-1385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Slade H. D. Isolation and characterization of a Streptococcus mutans bacteriocin inhibitor from Streptococcus pyogenes. Infect Immun. 1978 May;20(2):578–580. doi: 10.1128/iai.20.2.578-580.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. J., HORNE R. W. Studies of the bacterial cell wall. II. Methods of preparation and some properties of cell walls. Biochim Biophys Acta. 1951 Jul;7(2):177–197. doi: 10.1016/0006-3002(51)90017-0. [DOI] [PubMed] [Google Scholar]
- SPITNIK P., LIPSHITZ R., CHARGAFF E. Studies on nucleoproteins. III. Deoxyribonucleic acid complexes with basic polyelectrolytes and their fractional extraction. J Biol Chem. 1955 Aug;215(2):765–775. [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Schlegel R., Slade H. D. Alteration of macromolecular synthesis and membrane permeability by a Streptococcus sanguis bacteriocin. J Gen Microbiol. 1974 Mar;81(1):275–277. doi: 10.1099/00221287-81-1-275. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Cashion P. J., Suzuki S., Joseph J. P., Demarco P., Cohen M. B. The action of pancreas deoxyribonuclease I (deoxyribonucleate oligonucleotidohydrolase, EC-number 3.1.4.5.) on calf thymus nucleohistone. Arch Biochem Biophys. 1972 Apr;149(2):513–527. doi: 10.1016/0003-9861(72)90351-7. [DOI] [PubMed] [Google Scholar]
- Siegel J. L., Hurst S. F., Liberman E. S., Coleman S. E., Bleiweis A. S. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981 Feb;31(2):808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzien H. U., Jesaitis M. A. The nature of the cilicin K receptor of Escherichia coli Cullen. J Exp Med. 1971 Mar 1;133(3):534–553. doi: 10.1084/jem.133.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Glickman R. S., Teimer E. Teichoic acid hydrolase activity in soil bacteria (Bacillus subtilis-sporulation-phosphodiesterase-polyamines-concanavalin A). Proc Natl Acad Sci U S A. 1972 Jan;69(1):233–237. doi: 10.1073/pnas.69.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]


