Abstract
Amphipols are a new class of surfactants that make it possible to handle membrane proteins in detergent-free aqueous solution as though they were soluble proteins. The strongly hydrophilic backbone of these polymers is grafted with hydrophobic chains, making them amphiphilic. Amphipols are able to stabilize in aqueous solution under their native state four well-characterized integral membrane proteins: (i) bacteriorhodopsin, (ii) a bacterial photosynthetic reaction center, (iii) cytochrome b6f, and (iv) matrix porin.
Keywords: detergent, bacteriorhodopsin, reaction center, porin, cytochrome b6f
Integral membrane proteins usually are extracted from membranes and are kept soluble in aqueous solutions using detergents (1, 2). Detergent molecules equilibrate between a monolayer covering the transmembrane surface of the protein (3, 4), free monomers, and protein-free micelles. The presence of free micelles is a source of difficulty in membrane protein biochemistry and biophysics. It can induce, for instance, protein inactivation caused by the dissociation of subunits, lipids, or hydrophobic cofactors, phase separation during crystallization attempts, and an increased viscosity of the solutions in NMR experiments. We have endeavored to develop a new class of polymers, “amphipols,” that are designed to keep membrane proteins soluble in water in the absence of free surfactant. High molecular weight (MW) congeners of amphipols are known to exhibit a high affinity for hydrophobic particles (5–9). We show here that low MW amphipols can keep soluble under their native state four integral membrane proteins: (i) bacteriorhodopsin (BR), (ii) the photosynthetic reaction center from Rhodobacter sphaeroides R-26 (RC), (iii) the cytochrome b6f complex from Chlamydomonas reinhardtii, and (iv) the matrix porin (OmpF) from Escherichia coli.
MATERIALS AND METHODS
Synthesis of Amphipols.
Amphipols were synthesized by forming amide bonds between octylamine and, in some cases, isopropylamine, and the carboxylic groups of low MW polyacrylic acid precursors (10, 11). The end-products are co- or terpolymers in which the grafts are randomly distributed along the chain (Fig. 1). A grafting ratio of 25% octyl units confers to the polymers a high amphiphilicity without making them insoluble in water. The four amphipols tested in this study will be denoted by the letter A (for “anionic”) followed by two numbers, refering to the average apparent MW (≈8 or ≈34 kDa) and to the fraction of free carboxylic groups (35 or 75%; cf. Fig. 1).
Figure 1.
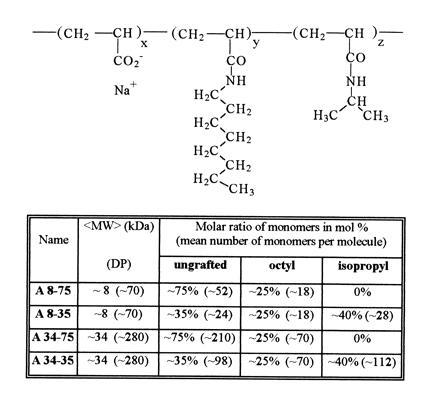
Molecular structure of amphipols. The weight average apparent molecular weight (〈MW〉) was deduced from the 〈MW〉 of the polyacrylate precursors as estimated by gel permeation chromatography using narrow MW polyoxyethylene calibration standards (which may entail systematic errors). DP (〈MW〉/MW of monomer) is the corresponding number of units per chain. The number of monomers per chain in the most abundant molecules (representing ≈80% of the mass) actually ranges over one decade. x, y, and z are the molar percentages of each type of unit, randomly distributed along the chain. The average number of each type of unit per amphipol molecule is given between parentheses.
Protein Purification.
Bacteriorhodopsin. Purple membrane from Halobacterium salinarium (a gift from M. Weik and G. Zaccaï, Institut de Biologie Structurale, Grenoble, France) was solubilized in 100 mM octylthioglucoside (OTG; cf. ref. 12) in 100 mM ammonium phosphate (AP) (pH 8.0), and BR was purified by centrifugation on a 5–20% (wt/wt) sucrose gradient in the same buffer containing 10 mM OTG [10 h at 54,000 rpm (250,000 × g) in the TLS55 rotor of a TL100 ultracentrifuge (Beckman)]; the final concentration was ≈0.1 g/liter in 100 mM AP (pH 8.0), ≈10% sucrose, and 10 mM OTG.
Cytochrome b6f complex.
Cytochrome b6f complex from C. reinhardtii was purified in the presence of Hecameg [6-O-(N-heptylcarbamoyl)-methyl-α-d-glucopyranoside (HG); a neutral detergent; cf. ref. 13] and egg phosphatidylcholine (EPC) as described (14); the final solution contained ≈5 μM b6f complex in 20 mM HG, 0.1 g/liter EPC, and 400 mM NaOH/AP buffer, pH 8.0. Photosynthetic reaction center from R. sphaeroides R-26 (15) (≈3 g/liter in 20 mM HG/20 mM NaOH·Tricine buffer, pH 8.0) was a gift of B. Schoepp (Centre National des Arts et Métiers, Paris). OmpF porin from E. coli (16) [≈4 g/liter in 0.2% (wt/wt) octylpolyoxyethylene (octyl-POE) in the same buffer] was a gift from J. P. Rosenbusch (Biozentrum, Basel).
Analytical Techniques.
Electrophoretical, spectroscopic, and other analytical techniques were carried out as described previously (14).
RESULTS AND DISCUSSION
The ability of amphipols to keep integral membrane proteins soluble was tested on purified preparations of (i) BR in OTG (12), (ii) b6f complex in HG/EPC mixed micelles (14), (iii) RC (15) in pure HG, and (iv) OmpF in octyl-POE (16). The solutions were or were not supplemented with amphipols, diluted below the critical micellar concentration (cmc) of the detergent, and centrifuged to remove aggregated proteins. Dilution below the cmc in the absence of polymers or in the presence of ungrafted polyacrylate resulted in the nearly complete precipitation of all four proteins (Fig. 2). In the presence of amphipols, on the contrary, most of each protein remained soluble (Fig. 2). When observed, partial precipitation resulted from too low an amphipol/protein ratio and could be avoided either by increasing the amount of amphipol (Fig. 2A) or by decreasing the protein concentration (data not shown). Optimal ratios varied from one protein to another. As an example, the amount of A8-75 required to keep in solution 80% of the RC under our experimental conditions was ≈0.3 g A8-75 per g protein.
Figure 2.
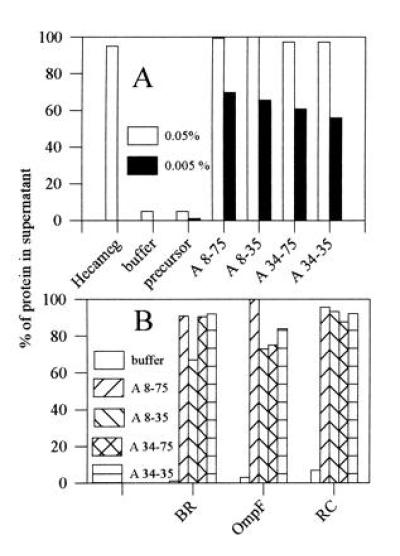
Solubility of membrane protein/amphipol complexes in aqueous solution. Aliquots from stock solutions of amphipols (5 g/liter in water) were added to purified membrane proteins in detergent solution and the mixtures diluted 10× with detergent-free buffer or with water. After 15 min incubation at 4°C, the solutions were centrifuged for 30 min at 4°C in the A-110 rotor of an Airfuge (Beckman) at 20 psi (1 psi = 6.89 kPa; ≈210,000 × g). The concentration of protein in the supernatant was determined from the absorbance at 564 nm (redox difference spectrum of b6), 546 nm (BR), 278 nm (OmpF), or 802 nm (RC). (A) Cytochrome b6f complex. Stock solution was ≈5 μM b6f complex in 20 mM HG (cmc ≈19.5 mM), 0.1 g/liter EPC, 400 mM NaOH/AP buffer (pH 8.0). Final amphipol concentrations following 10× dilution with water were 0.5 g/liter (open bars) or 0.05 g/liter (solid bars). Control experiments included dilution with a 20 mM HG solution (Hecameg) or with water (buffer) in the absence of amphipols, and dilution with water in the presence of nonderivatized low MW polyacrylate (precursor). (B) Other proteins. Stock solutions: bacteriorhodopsin, ≈0.1 g/liter in 100 mM AP (pH 8.0), ≈10% sucrose, 10 mM OTG (cmc ≈9 mM); OmpF porin, ≈4 g/liter in 0.2% (wt/wt) octyl-POE (≈9.2 mM; cmc ≈7 mM) in the same buffer; reaction center, ≈3 g/liter in 20 mM HG in 20 mM NaOH·Tricine buffer (pH 8.0). Tenfold dilution with 100 mM AP (pH 8.0) to a final amphipol concentration of 0.5 g/liter.
Upon separation from detergent and unbound amphipol by rate zonal centrifugation on surfactant-free sucrose gradients, protein/amphipol complexes migrated as small particles, apparently monodisperse, containing no large aggregates (Fig. 3). Detergent depletion under these conditions appears to be extensive; in experiments in which the detergent distribution was followed using [14C]laurylmaltoside (LM) as a tracer, the amount of detergent present in the b6f/amphipol complex fractions was less than ≈12 molecules per b6f dimer, most of which were probably contaminants (data not shown). As a comparison, the same complex in 0.2 mM LM solution binds 260 ± 20 molecules of detergent (17). No significant protein pellet was observed, even though the gradients contained neither detergent nor amphipol. This suggests that in the absence of a competing surfactant, either the association between the polymers and the proteins is irreversible on the time scale of these experiments (5–10 h), or the binding constant is so high that aqueous solutions can be saturated by the release of a negligible fraction of adsorbed polymer. In keeping with these observations, cytochrome b6f/amphipol complexes could be dialyzed for at least 2 days against surfactant-free buffer without precipitating, even though the free polymers readily cross dialysis membranes (data not shown). The quasi-irreversibility of the association was confirmed by the observation that protein-bound [14C]amphipols did not dissociate upon centrifugation in a sucrose gradient unless the gradient contained detergent (data not shown). The amount of protein-bound amphipol measured in these experiments varied from 0.2 to 0.5 g per g protein depending on the protein studied (data not shown).
Figure 3.
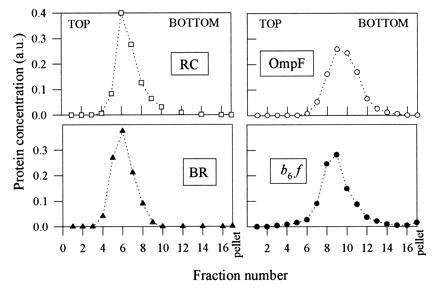
Sedimentation velocity analysis of protein/amphipol complexes on detergent-free sucrose gradients. Aliquots (100 μl) of membrane proteins in detergent solution (cf. legend to Fig. 2) were supplemented with 1 g/liter A8-75 (plus, in the case of b6f, 0.33 g/liter EPC), diluted 2-fold with 100 mM AP (pH 8.0), layered on top of 2-ml 5–20% (wt/wt) sucrose gradients in the same buffer and centrifuged at 54,000 rpm in the TLS55 rotor of a TL100 ultracentrifuge. After 5.25 h (RC), 5 h (b6f), 6.5 h (OmpF), or 10 h (BR), gradients were collected by 120-μl fractions. Protein concentrations in the 16 fractions and in the resuspended pellet were determined spectrophotometrically (cf. legend to Fig. 2).
Amphipols appear to preserve the native state of membrane proteins. Cytochrome b6f is a particularly fragile complex. Upon exposure to an excess of HG or LM micelles, it monomerizes, loses one of its subunits, and becomes enzymatically inactive (14, 17, 18). The b6f complex stabilized by amphipols retained all of its subunits (data not shown) and showed no or very little tendency to monomerize (Fig. 3). However, as also observed with detergent solutions (14, 17), stability was greater when the complex had been trapped by amphipols in the presence of small amounts of lipids, some of which comigrated with the b6f complex upon sucrose gradient fractionation (data not shown). Following dilution into 0.25 mM LM solution, amphipol-stabilized b6f catalyzed electron transfer from decylplastoquinol to plastocyanin (data not shown), albeit with a lower turnover number than preparations diluted from HG solution (14). The rate of association between plastocyanin and cytochrome f, which is the limiting step in this reaction (14), is primarily controlled by electrostatic interactions (see ref. 19). The observation that inhibition by amphipols was lower in the presence of the less highly charged amphipol, A8-35, than in the presence of A8-75 (a decrease of the turnover number by factor of ≈2× versus ≈10×, respectively) suggests that this effect may be due to electrostatic repulsion between amphipols and the negatively charged plastocyanin. Upon storage for 2 weeks, the activity of cytochrome b6f complexed by amphipols decayed slightly more rapidly than that of a control preparation kept in HG/EPC (Fig. 4B). The sensitivity of the b6f complex to amphipols therefore appears comparable to or slightly higher than that to classical detergents.
Figure 4.
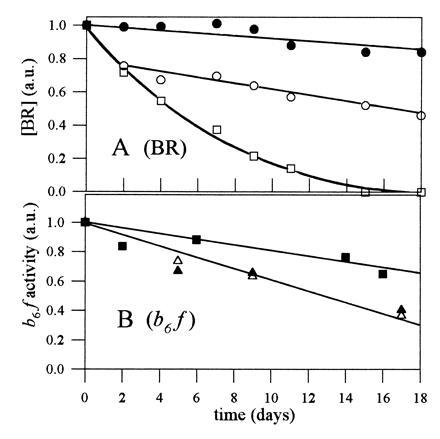
Stability of BR and cytochrome b6f complexed by amphipols. (A) BR was either kept in 10 mM OTG in 100 mM AP, pH 8.0 (□; final concentration of OTG was 10 mM), or complexed by A8-75 (○) or A8-35 (•) as described in the legend of Fig. 2B (final concentration of amphipol was 0.5 g/liter). The three samples were stored at 4°C in the dark. Every second or third day, each sample was centrifuged in the Airfuge (cf. legend to Fig. 2) before measuring the absorbance at 546 nm. (B) Cytochrome b6f was either kept in 20 mM HG, 0.1 g/liter EPC, 400 mM AP (pH 8.0) (▪), or complexed with A8-75 (▵) or A8-35 (▴) and isolated by sucrose gradient centrifugation as described in the legend of Fig. 3. The samples were stored at 4°C in the dark. Enzymatic activity was determined by diluting an aliquot 50- to 200-fold in 0.25 mM LM, 20 mM Tricine·NaOH buffer (pH 8.0), and measuring the rate of stigmatellin-sensitive electron transfer from decylplastoquinol to oxidized plastocyanin (14).
BR, which is not very stable at pH 8 in detergent solution (20), tolerated association with amphipols extremely well. Its dark-adapted absorption spectrum exhibited a “detergent-induced” blue shift (21) to 546 nm and remained invariant upon storage for at least 2 weeks (not shown). In A8-35, practically no aggregation of the protein occurred over time under these experimental conditions, whereas in A8-75 about 25% of BR aggregated during the first 2 days of storage. Under the same conditions, BR kept in OTG denatured rapidly (Fig. 4A). In regard to the other two test proteins, the spectrum of the bacterial RC was not affected by any of the amphipols (not shown), and—unsurprisingly given its high stability—matrix porin remained a trimer, as probed by electrophoresis on polyacrylamide gels in the presence of sodium dodecylsulfate (not shown). These observations show that amphipols are able to keep integral membrane proteins soluble under a native-like form in detergent-free aqueous solutions.
To our knowledge, a single attempt at keeping membrane proteins soluble using polymers has been reported previously. Synthetic peptides designed to form amphipathic α-helices (“peptitergents”) were shown to stabilize BR and rhodopsin (but not PhoE porin) in detergent-free aqueous solution (22). Advantages of amphipols over peptitergents are their more general efficiency, their structural flexibility, and their low cost of production. On the other hand, the size heterogeneity and high charge of current amphipols (as well as, possibly, their ability to interact with nontransmembrane hydrophobic patches on proteins) have to be kept in mind when designing experiments. A sketch of our current view of the structure of amphipol/membrane protein complexes is depicted in Fig. 5.
Figure 5.
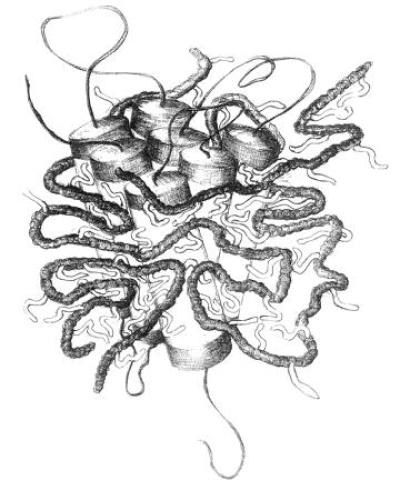
Sketch of an integral membrane protein complexed by amphipols. Protein and amphipols are drawn to scale (α-helix diameter, ≈1 nm; spacing of acrylic units along the polymer, ≈0.3 nm; length of octyl chains, ≈1 nm; expected mass of protein-bound amphipol, ≈20 kDa per mole of protein). The persistence length of the polymer, which determines the tightness of the loops, has been taken equal to ≈3 nm. It actually depends on such parameters as the density of charges along the chain and the ionic strength of the solution. The fractions of alkyl chains and polymer not in contact with the protein’s surface are largely speculative.
Altogether, the above observations suggest that amphipols can be useful tools for handling membrane proteins in aqueous solutions. Obvious applications include stabilizing proteins or protein complexes that are dissociated or inactivated by detergents, substituting for costly detergents in large-scale preparations, developing novel separation methods, and facilitating solution (e.g., NMR) studies. The vast resources of polymer chemistry should make it readily possible to tailor the physicochemical properties of amphipols toward specific uses in biochemistry and biophysics.
Acknowledgments
Particular thanks are due to J. P. Rosenbusch, B. Schoepp, and M. Weik and G. Zaccaï for gifts of OmpF, reaction center, and purple membrane, respectively; to C. Breyton, D. M. Engelman, J. Kyte, D. Lévy, and Y. Pierre for advice, discussions, and comments on the manuscript; and to J. Lionne-Scheiber for drawing Fig. 5. This project was initiated thanks to the Centre National de la Recherche Scientifique (CNRS) GDR 1082 interdisciplinary network “Colloïdes mixtes” and was mainly supported by the CNRS, the Ministère de la Recherche et de la Technologie (Mission Scientifique et Technique), and the European Economic Community Grant BIO2-CT93-0076 (J.L.P.).
Footnotes
Abbreviations: AP, ammonium phosphate; BR, bacteriorhodopsin; cmc, critical micellar concentration; EPC, egg phosphatidylcholine; HG, Hecameg [6-O-(N-heptylcarbamoyl)-methyl-α-d-glucopyranoside]; LM, laurylmaltoside; MW, molecular weight; octyl-POE, octylpolyoxyethylene; OTG, octylthioglucoside; RC, reaction center.
References
- 1.Helenius A, Simons K. Biochim Biophys Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- 2.Tanford C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes. New York: Wiley; 1980. [Google Scholar]
- 3.Roth M, Lewitt-Bentley A, Michel H, Deisenhofer J, Huber R, Oesterhelt D. Nature (London) 1989;340:659–662. [Google Scholar]
- 4.Møller J V, le Maire M. J Biol Chem. 1993;268:18659–18672. [PubMed] [Google Scholar]
- 5.Iliopoulos I, Olsson U. J Phys Chem. 1994;98:1500–1505. [Google Scholar]
- 6.Magny B, Iliopoulos I, Zana R, Audebert R. Langmuir. 1994;10:3180–3187. [Google Scholar]
- 7.Petit F, Audebert R, Iliopoulos I. Colloid Polym Sci. 1995;273:777–781. [Google Scholar]
- 8.Thomas J L, Barton S W, Tirrell D A. Biophys J. 1994;67:1101–1106. doi: 10.1016/S0006-3495(94)80575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikawa T, Akiyoshi K, Sunamoto J. Macromolecules. 1994;27:7654–7659. [Google Scholar]
- 10.March J. Advanced Organic Chemistry: Reactions, Mechanisms and Structure. New York: Wiley; 1985. pp. 372–374. [Google Scholar]
- 11.Wang T K, Iliopoulos I, Audebert R. Polym Bull. 1988;20:577–582. [Google Scholar]
- 12.Seigneuret M, Neumann J-M, Rigaud J-L. J Biol Chem. 1991;266:10066–10069. [PubMed] [Google Scholar]
- 13.Plusquellec D, Chevalier G, Talibart R, Wróblewski H. Anal Biochem. 1989;179:145–153. doi: 10.1016/0003-2697(89)90215-7. [DOI] [PubMed] [Google Scholar]
- 14.Pierre Y, Breyton C, Kramer D, Popot J-L. J Biol Chem. 1995;270:29342–29349. doi: 10.1074/jbc.270.49.29342. [DOI] [PubMed] [Google Scholar]
- 15.Verméglio A. Biochim Biophys Acta. 1977;459:516–524. doi: 10.1016/0005-2728(77)90050-0. [DOI] [PubMed] [Google Scholar]
- 16.Garavito R M, Rosenbusch J P. Methods Enzymol. 1986;125:309–328. doi: 10.1016/s0076-6879(86)25027-2. [DOI] [PubMed] [Google Scholar]
- 17.Breyton C, Tribet C, Olive J, Recouvreur M, Popot J-L. In: Photosynthesis: From Light to Biosphere. Mathis P, editor. Vol. 2. Dordrecht, The Netherlands: Kluwer; 1995. pp. 591–594. [Google Scholar]
- 18.Huang D, Everly R M, Cheng R H, Hetmann J B, Schägger H, Sled V, Ohnishi T, Baker T S, Cramer W A. Biochemistry. 1994;33:4401–4409. doi: 10.1021/bi00180a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cramer W A, Soriano G M, Ponomarev M, Huang D, Zhang H, Martinez S E M, Smith J L. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:477–508. doi: 10.1146/annurev.arplant.47.1.477. [DOI] [PubMed] [Google Scholar]
- 20.Miercke L J W, Ross P E, Stroud R M, Dratz E A. J Biol Chem. 1989;264:7531–7535. [PubMed] [Google Scholar]
- 21.Stoeckenius W, Bogomolni R A. Annu Rev Biochem. 1982;52:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- 22.Schafmeister C E, Miercke L J W, Stroud R A. Science. 1993;262:734–738. doi: 10.1126/science.8235592. [DOI] [PubMed] [Google Scholar]


