Summary
Orexins (also known as hypocretins) are recently discovered neuropeptides made exclusively in hypothalamic neurons that have been shown to be important in narcolepsy/cataplexy and arousal. Here, we conducted behavioral, anatomical and neurophysiological studies that show that a subset of these cells, located specifically in lateral hypothalamus (LH), are involved in reward processing and addictive behaviors. We found that Fos expression in LH orexin neurons varied in proportion to preference for morphine, cocaine or food. This relationship obtained both in drug naïve rats and in animals during protracted morphine withdrawal, when drug preference was elevated but food preference was decreased. Recent studies showed that LH orexin neurons that project to ventral tegmental area (VTA) have greater Fos induction in association with elevated morphine preference during protracted withdrawal than non-VTA-projecting orexin neurons, indicating that the VTA is an important site of action for orexin’s role in reward processing. In addition, we found that stimulation of LH orexin neurons, or microinjection of orexin into VTA, reinstated an extinguished morphine preference. Most recently, using a self-administration paradigm we discovered that the Ox1 receptor antagonist SB-334867 (SB) blocks cocaine-seeking induced by discrete or contextual cues, but not by a priming injection of cocaine. Neurophysiological studies revealed that locally applied orexin often augmented responses of VTA dopamine (DA) neurons to activation of the medial prefrontal cortex (mPFC), consistent with the view that orexin facilitates activation of VTA DA neurons by stimulus-reward associations. We also recently showed that orexin in VTA is necessary for learning a morphine place preference. These findings are consistent with results from others showing that orexin facilitates glutamate-mediated responses, and is necessary for glutamate-dependent long-term potentiation, in VTA DA neurons. We surmise from these studies that LH orexin neurons play an important role in reward processing and addiction, and that LH orexin cells are an important input to VTA for behavioral effects associated with reward-paired stimuli.
Introduction
Orexin behavioral neurobiology
Orexin A and orexin B (also known as hypocretin 1 and hypocretin 2) are recently discovered peptides that are produced from a prepro-orexin molecule made solely in hypothalamic neurons. Since their nearly simultaneous discovery by de Lecea et al and Sakurai et al in 1998 (de Lecea et al., 1998; Sakurai et al., 1998), considerable work has been done to characterize this new peptide neurotransmitter system. Sakurai et al. (1998) also characterized 2 receptors for the orexin system, termed Ox1 and Ox2. The Ox1 receptor binds orexin A with 30 nM affinity but has much lower affinity for orexin B, whereas the Ox2 receptor binds both orexin peptides with similar high affinity. The orexin neurons give rise to a highly divergent system of fiber projections that spans the entire neuraxis, including innervation in the cerebral cortex, hippocampus, thalamus, midbrain, and spinal cord (Peyron et al., 1998; Sutcliffe and de Lecea, 2002). Likewise, the two orexin receptors are widely distributed in the CNS, but are regionally selective (Lu et al., 2000; Marcus et al., 2001; Trivedi et al., 1998).
Great interest was focused on this system in the year after its discovery, when again two groups virtually simultaneously reported that dysfunction in the orexin system is strongly associated with narcoleptic symptoms in animals (Chemelli et al., 1999; Lin et al., 1999). Subsequent work in humans verified that narcoleptic patients (particularly those with cataplexy) have little orexin in their CSF, and lack most or all orexin neurons (Nishino, 2007; Nishino et al., 2000). With these compelling findings, the prevailing view of orexin function focused on arousal and maintenance of the waking state. Supporting this view were findings that major targets of orexin projections are classic brain arousal nuclei such as the locus coeruleus (Peyron et al., 1998; Sutcliffe and de Lecea, 2002), and that orexin application typically strongly activates these cells (Brown et al., 2001; Eriksson et al., 2001; Horvath et al., 1999; Ivanov and Aston-Jones, 2000; Korotkova et al., 2003).
However, a potential role for orexins in reward processing was evident from one of the first publications of their discovery. Sakurai and colleagues reported that administration of orexin A or orexin B into the lateral ventricle produced feeding in rats, which prompted them to name the new peptides “orexins”, meaning appetite stimulants (Sakurai et al., 1998). The first report of a possible role for orexins in addiction appeared in 2003, with findings that orexin neurons play a role in opiate withdrawal (Georgescu et al., 2003). These and other findings prompted us to examine a possible role for this novel neuropeptide system in reward processing and drug abuse. As reviewed below, our studies along with those from others now indicate that orexins play a prominent role in conditioned responses to stimuli associated with food and drug rewards. This reward-associated function of the orexin system may be separate from its role in maintenance of the waking state, and mediated by a separate population of (laterally located) orexin neurons.
Orexins and reward processing
Orexin neurons are activated by reward-associated stimuli
Our report in 2005 was the first to demonstrate that orexin neurons in lateral hypothalamus (LH) play an active role in reward processing and drug abuse (Harris et al., 2005). We used a conditioned place preference (CPP) paradigm to measure Fos activation in orexin neurons associated with preference expressed for drug or natural rewards. We found that rats conditioned with morphine, cocaine or food in a CPP paradigm exhibited substantially increased Fos staining in LH orexin neurons on the drug-and food-free CPP test session (Fig. 1, Table 1). Notably, this Fos induction in LH orexin neurons was in close proportion to the degree of preference that animals exhibited during the CPP test day (r = 0.72 to 0.90, p<.01). Moreover, this behavioral correspondence with Fos induction was selective for orexin neurons in the lateral part of the orexin cell field (in LH), and was not found for orexin neurons outside LH (e.g., perifornical, PeF, or dorsomedial hypothalamus, DMH), nor for non-orexin neurons within LH (Table 1). Note also that LH orexin neurons have a lower baseline (constituitive) level of Fos expression, and show a larger increase in Fos with the CPP test, than PeF or DMH orexin neurons.
Figure 1.
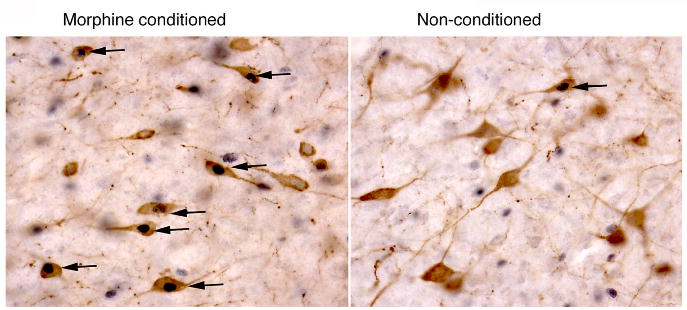
High-power photomicrograph of the LH showing the double labeling of orexin (brown cytoplasm) and Fos protein (black nuclei) in morphine-conditioned and non-conditioned animals, as indicated. Black arrows indicate double-labeled cells. Taken from (Harris et al., 2005).
Table 1. Orexin-Fos double labeling.
The percentages of orexin-positive cells that were also Fos-positive are indicated for each group in the LH, PeF and DMH. The right column gives correlation coefficients for the comparisons between these percentages and the corresponding preference score in each animal. LH by group ANOVA F(3,39)=33, p<.01. The non-orexin Fos+ neurons in the LH are given as total counts not percentages.
| Groups: | Cell Types: | Percentage | Correlations | |
| Fos+ | R: | |||
| Morphine | Orx LH | 48±2* | .72 | p<.01* |
| Conditioned | NonOrx LH | 55±6 | .30 | p=.34 |
| N=12 | Orx PFA | 62±2 | .04 | p=.91 |
| Orx DMH | 67±4 | −.11 | p=.71 | |
| Food | Orx LH | 50±3* | .87 | p<.01* |
| Conditioned | NonOrx LH | 47±5 | .20 | p=.64 |
| N=8 | Orx PFA | 42±3 | .26 | p=.54 |
| Orx DMH | 47±6 | −.16 | p=.71 | |
| Cocaine | Orx LH | 52±5* | .90 | p<.01* |
| Conditioned | NonOrx LH | 78±7 | .51 | p=.20 |
| N=8 | Orx PFA | 67±3 | .41 | p=.32 |
| Orx DMH | 74±3 | .50 | p=.20 | |
| Non-conditioned | Orx LH | 17±2 | .11 | p= .81 |
| NonOrx LH | 43±6 | .30 | p=.53 | |
| N=15 | Orx PFA | 52±4 | .42 | p=.36 |
| Orx DMH | 59±4 | .02 | p=.96 | |
| Naïve | Orx LH | 15±1 | ||
| N=6 | NonOrx LH | 29±8 | ||
| Orx PFA | 52±3 | |||
| Orx DMH | 57±6 | |||
| Novelty conditioned | Orx LH | 18±2 | .09 | p=.86 |
| NonOrx LH | 50±1 | −.52 | p=.31 | |
| N=6 | Orx PFA | 56±3 | .02 | p=.97 |
| Orx DMH | 63±5 | .42 | p=.43 | |
significantly different from other groups, p<.05 Orx = orexin positive neurons. Taken from (Harris et al., 2005).
These findings prompted us to examine whether activation of LH orexin neurons plays a role in driving the associated preference. We tested this using systemic administration of the Ox1 receptor antagonist, SB-334867 (SB). Results indicated that, indeed, this antagonist given before the test session significantly attenuated expression of a morphine CPP (Harris et al., 2005).
Orexin neurotransmission in VTA drives reinstatement of drug-preference
We reasoned that the above findings might indicate a role for conditioned activation of orexin neurons in driving reinstatement of extinguished drug-seeking. To test this, we conditioned rats for morphine CPP, and then extinguished their morphine preference by repeatedly exposing them to the CPP environment without drug reward. After achieving extinction of preference (which typically required 1–3 weeks), we microinjected the Y4 receptor agonist rat pancreatic polypeptide (rPP) into LH to stimulate orexin neurons. We used rPP because Campbell and colleagues (Campbell et al., 2003) showed previously that this compound preferentially stimulates Fos induction in orexin neurons when injected into LH. We found that rPP microinjected into LH produced a robust reinstatement of preference for the previous (extinguished) morphine-paired side (Fig. 2). This effect was specific to LH, as injections dorsal to LH, or medial to LH among non-LH orexin neurons, were not effective in reinstating preference (Harris et al., 2005). This reinstatement with LH rPP was also specific for orexin neurotransmission, as it was completely blocked by systemic pretreatment with SB.
Figure 2.
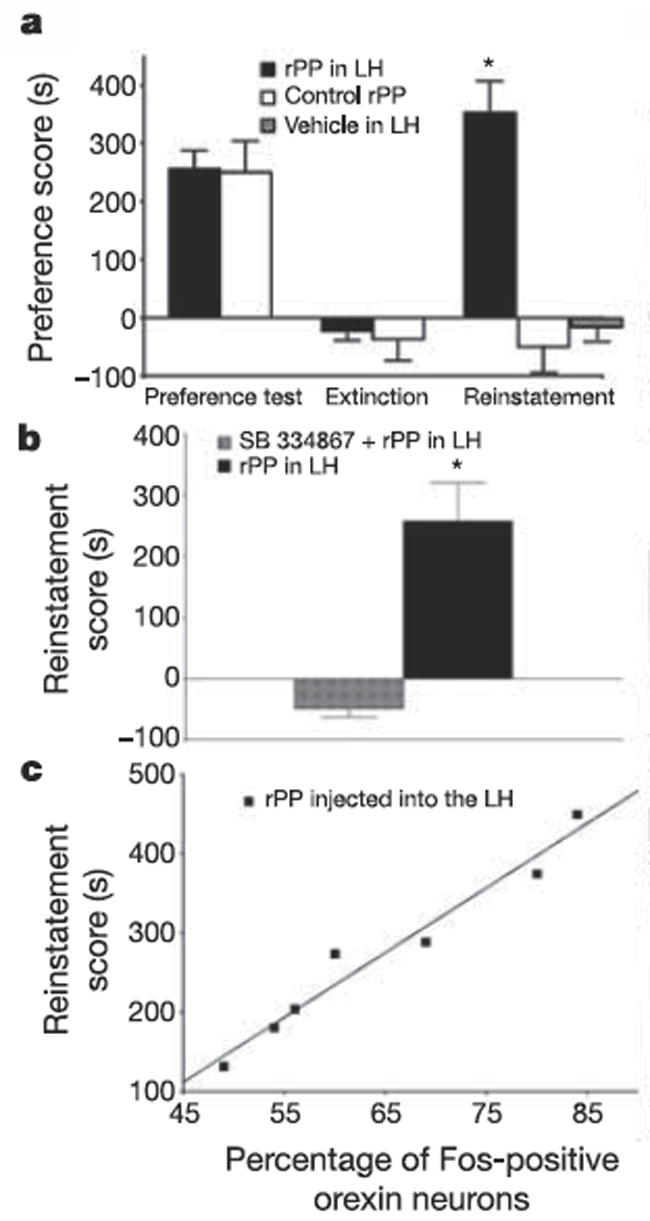
Activation of lateral hypothalamus orexin neurons by rPP reinstated an extinguished preference for morphine. a, Preference scores are shown for both rPP- (150 nM) and vehicle-injected groups (mean ± s.e.m. in morphine-paired side minus saline-paired side) during the initial conditioning test, after extinction and during the reinstatement test. b, The selective orexin A antagonist, SB 334867 (20–30 mg kg), blocked reinstatement by rPP (n = 8). Data were included only if rPP injection into lateral hypothalamus on the following day (without the antagonist pretreatment) produced reinstatement of preference. c, Plot of correlation between reinstatement scores and percentages of lateral hypothalamus orexin neurons that were Fos activated in rPP reinstated animals. Taken from (Harris et al., 2005).
The orexin system projects widely throughout the CNS, so there are many possible targets where orexin might act to produce this reinstatement of preference. One site that seemed likely was the ventral tegmental area (VTA), where dopamine (DA) neurons that play a critical role in reward and reinforcement mechanisms are located. We tested the VTA as a site of orexin action in reinstatement of preference, and found that microinjections of orexin directly into VTA robustly elicited morphine preference in animals that had previously been extinguished (Harris et al., 2005). Together with the preceding results, these findings provide strong evidence that orexin projections from LH to VTA play an important role in expression of drug preference, and may also be involved in relapse of drug-seeking following extinction.
Orexin neurotransmission is necessary for cue-induced, but not for cocaine-induced, reinstatement of cocaine-seeking in self-administering rats
We recently conducted a set of experiments using the self-administration paradigm to determine whether orexin is involved in stimulus-cocaine interactions and relapse of cocaine-seeking (Smith et al., 2007). Rats self-administered intravenous cocaine (0.2 mg/infusion) in the presence of discrete tone and light cues during daily 2-hour sessions. Animals were then given extinction trials, in which lever presses no longer produced cocaine infusions or cues. After animals extinguished lever responding, we elicited reinstatement of cocaine-seeking via cue presentation or a cocaine prime (10 mg/kg, ip). We found that systemic administration of 20 – 30 mg/kg SB significantly attenuated cue-induced reinstatement of cocaine-seeking, as compared to reinstatement following vehicle injection in the same animals (Fig. 3). In a within-subjects design, SB or vehicle was also administered prior to a late extinction trial when responding had tapered off to an average of less than 10 presses per 2-hour session. SB had no effect on lever responding in this extinction trial, indicating that the reinstatement effect was not due to general effects of the antagonist on locomotion or arousal.
Figure 3.
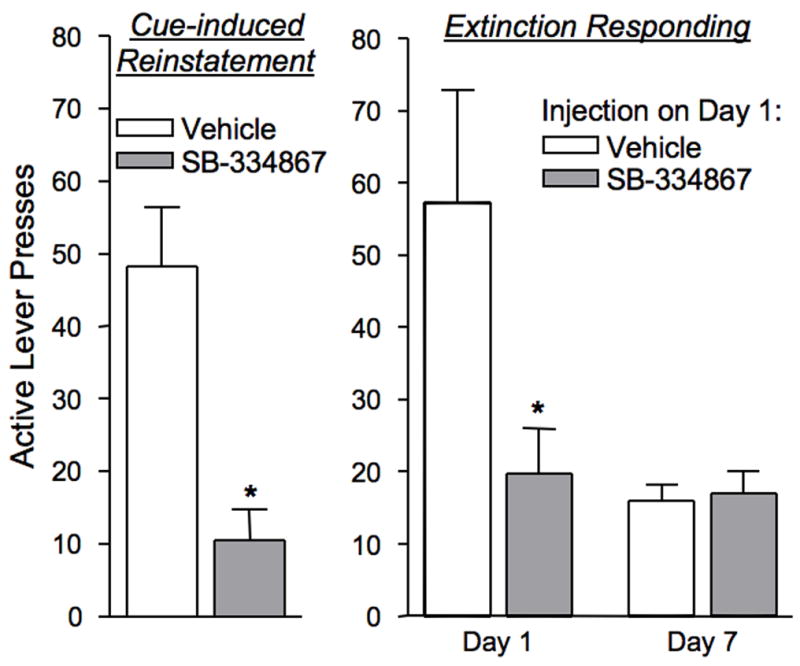
SB-334867 (30 mg/kg, ip) attenuated cue- and context-elicited cocaine-seeking. Left graph - Pretreatment with SB-334867 significantly reduced cue-induced reinstatement of lever-pressing as compared to vehicle pretreatment in the same animals (*p<0.01). Right graph -Animals given SB-334867 prior to the first extinction session had significantly reduced lever-pressing as compared to animals given vehicle (*p<0.05). Extinction days 1 and 7 are significantly different for animals given vehicle prior to the first extinction session (p<0.05), whereas extinction days 1 and 7 are not different for animals given SB-334867 prior to the first extinction session.
In contrast to the effectiveness in blocking cue-induced reinstatement of cocaine-seeking, 10 or 30 mg/kg SB did not attenuate reinstatement of responding induced by a priming injection of cocaine. Animals similarly reinstated lever-pressing after a cocaine prime whether pretreated with vehicle or the antagonist. These results suggest that antagonism of the Ox1 receptor does not block the memory of stimulus properties of cocaine, but acts more selectively to block certain aspects of drug-seeking.
To investigate mechanisms underlying this selectivity, we administered SB during other stages of drug-taking and drug-seeking in the cocaine self-administration paradigm. Administration of 30 mg/kg SB during established self-administration produced no significant effects on the number of lever presses or drug infusions as compared to self-administration the day before and after, when no pretreatment was given. However, we saw a large effect when SB was given prior to the first day of extinction (Fig. 3). Typically, animals engage in a particularly high amount of drug-seeking during the first extinction session despite being unrewarded by drugs or cues; this is taken to represent contextually-driven drug-seeking. Pretreatment with 30 mg/kg SB significantly attenuated this early extinction effect, as compared to animals that were pretreated with vehicle on that day. During the subsequent extinction days, when no pretreatment with SB was given, the animals that received the antagonist on Extinction Day 1 showed a burst of extinction responding on Day 2, and then showed a tapered gradual extinction of responding across days, similar to animals that initially received vehicle. Thus, the effect of SB on extinction responding was limited to the day on which it was administered. These findings have a marked similarity to the reinstatement results; that is, drug-seeking elicited by external triggers, such as cues or context associated with cocaine, was vulnerable to orexin receptor antagonism, whereas drug-seeking elicited by a cocaine prime, or direct self-administration of cocaine, was not susceptible to SB.
Taken together, these self-administration findings have led us to hypothesize that SB only affects behaviors that rely on increased activation of VTA DA cells, including cue- or context-elicited drug-seeking. SB alone does not alter the spontaneous activity of DA neurons, but does reduce excitatory effects on DA neurons, such as those caused by orexin and antipsychotics (Borgland et al., 2006; Massi et al., 2007; Rasmussen et al., 2007). Acutely, cocaine increases DA release via direct actions on DA terminals, and does not require increased activation of DA cells in the VTA. As discussed in more detail below, we propose that a primary mechanism of action for orexin to augment drug-seeking behavior is to increase glutamate responsiveness of VTA DA neurons (consistent with previous physiological results; Borgland et al., 2006).
Orexin neurons are functionally dichotomous
As reviewed above, orexin neurons appear to be involved both in arousal and in reward. We found that the reward functions are associated primarily with orexin cells in LH, whereas others provided evidence that arousal-related processes were more associated with orexin neurons in the DMH and PeF. We reviewed and integrated such information in a recent article, and concluded that orexin neurons are functionally dichotomous (Harris and Aston-Jones, 2006a). In this view, orexin neurons of DMH and PeF activate with stress and arousal, whereas those in LH activate with reward-related stimuli. For example, Estabrooke et al (Estabrooke et al., 2001) reported that LH, but not PeF or DMH, orexin neurons are Fos-activated during waking compared to sleep. Fadel et al. (Fadel et al., 2002) found that neuroleptics that cause weight gain preferentially activate LH, rather than more medial, orexin neurons, consistent with our data for LH involvement in reward processes. In addition, morphine withdrawal activates Fos in DMH and PeF but not in LH orexin neurons (Sharf et al., 2008), whereas chronic ethanol consumption increased the area of orexin mRNA expression in LH, but not in more medial hypothalamic areas (Lawrence et al., 2006). We found that footshock stimulation induced Fos in PeF and DMH, but not in LH, orexin neurons (Harris et al., 2005). These and other results also lead us to propose that the role of orexin reported in stress-induced reinstatement of cocaine-seeking by Boutrel et al (Boutrel et al., 2005) involves activation of noradrenergic and CRF neurons by stress-responsive orexin neurons in DMH and PeF, but not by LH orexin cells (Harris and Aston-Jones, 2006a).
The dichotomy of orexin function implies that orexin neurons differ in their input-output connections according to reward vs arousal roles. There is already evidence along these lines: Fadel and colleagues found that VTA- and mPFC-projecting orexin cells originate preferentially from the LH (Fadel et al., 2002; Fadel and Deutch, 2002). Yoshida and colleagues reported that PeF/DMH orexin neurons are innervated by other hypothalamic regions involved in homeostatic and arousal-related drive states, whereas LH orexin neurons are preferentially targeted by brainstem areas involved in autonomic and visceral processing, and by reward-related areas such as the VTA and NAc shell (Yoshida et al., 2006). We are conducting studies to further examine this possibility (Richardson et al., 2007; Sartor and Aston-Jones, 2008); some are briefly reviewed below.
Altered hedonic processing during protracted withdrawal: possible role of LH orexin neurons
We previously reported that chronic exposure to cocaine or morphine, followed by protracted forced abstinence, resulted in dramatically altered preferences for drug and natural rewards. Thus, preference for morphine or cocaine increased, and for food or novelty decreased, at 2 or 5 weeks following protracted forced abstinence from chronic morphine or cocaine (Aston-Jones and Harris, 2004; Harris et al., 2001; Harris and Aston-Jones, 2001). This increased preference for drug, and decreased interest in natural rewards, is similar to reports by addicts (Jaffe, 1990) and could be a source of difficulty in maintaining prolonged abstinence from drugs after previous chronic exposure.
We examined the neural substrates of this altered hedonic processing during protracted withdrawal by examining Fos expression on the preference test day. We hypothesized that brain areas with altered Fos that mirrored altered preferences for drug and natural rewards would be good candidates for structures that might underlie the altered hedonic processing during protracted withdrawal. Thus, we sought brain areas where Fos expression was higher than normal when withdrawn animals were tested for drug preference, and where Fos expression was lower than normal when withdrawn animals were tested for food or novelty preference. Our studies to date revealed 3 such areas: the basolateral amygdala, nucleus accumbens shell, and LH (Aston-Jones and Harris, 2004). Notably, the LH region that contained altered Fos expression in this study coincided with the area that contains orexin neurons. Subsequent analysis of LH sections with Fos co-stained for orexin revealed that, indeed, Fos activation in orexin neurons in LH was higher than normal for animals in protracted morphine withdrawal tested for drug preference, but lower than normal for animals in protracted morphine withdrawal tested for food preference – ie, the Fos expression in LH orexin neurons closely mirrored the altered preferences that resulted from protracted drug withdrawal (Fig. 4). This result is consistent with our previous finding that Fos expression in LH orexin neurons correlates closely with preferences for morphine, cocaine or food reward (Harris et al., 2005), and indicates that LH orexin neurons may play a significant role in the altered hedonic processing that occurs during protracted drug withdrawal. Other of our recent studies indicate that neural systems in addition to LH orexin neurons may also be involved in this altered hedonic processing during protracted withdrawal as well (Harris et al., 2007a; Harris and Aston-Jones, 2007).
Figure 4.
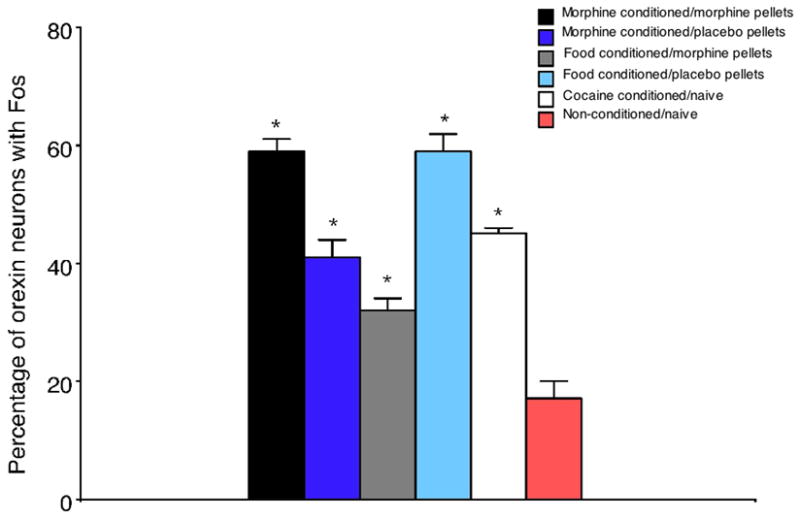
Fos expression in LH orexin neurons as a function of treatments, as indicated. Morphine- and food-conditioned animals were given morphine or placebo pellets for 2 weeks, and pellets were removed and animals remained in their home cages for 5 weeks before CPP conditioning, as in our previous publications (Aston-Jones and Harris, 2004; Harris and Aston-Jones, 2003c). Note that a higher percentage of LH orexin neurons exhibited Fos in withdrawn animals than in placebo-pelleted animals subjected to morphine CPP. Conversely, a lower percentage of LH orexin neurons exhibited Fos in withdrawn animals than in placebo pelleted animals subjected to food CPP. Cocaine CPP in naïve rats (no prior drug treatment) also increased Fos expression in LH orexin neurons.
Topography of orexin projections to reward-related brain areas
The functional dichotomy reviewed above also implies that subpopulations of orexin neurons differ in their projection targets, so that some are involved in arousal whereas others are more concerned with reward and reinforcement. We have begun to address this issue using tract-tracing to retrogradely label orexin neurons from various targets, combined with Fos to identify orexin neurons that are activated by a CPP preference test in previously-naïve or morphine-dependent animals (Richardson et al., 2007). Rats received a unilateral injection of the retrograde tracer wheat germ agglutinin-colloidal gold (WGA-Au) in VTA. Animals were made morphine-dependent for 2 weeks by subcutaneously implanting two morphine pellets 7 days after WGA-Au microinjections; other rats were given placebo pellets. CPP conditioning began two weeks after pellet removal; conditioning and testing were identical to our previously published reports for morphine (Byrne et al., 2003). Results showed that dependent animals exhibited an enhanced preference for the morphine-associated environment on the CPP test day compared to placebo-pelleted rats, as in our previous studies (Harris and Aston-Jones, 2003c). A higher percentage of LH orexin neurons that exhibit Fos were retrogradely labeled from VTA in dependent than in non-dependent animals (26.4 ± 4.0% versus 11.4 ± 5.4%, p<0.05). The number of Fos-activated LH orexin neurons that project to VTA was significantly correlated with the intensity of conditioned preference in dependent animals (R=0.743, p<0.05). In addition, a greater percentage of retrogradely labeled orexin neurons were Fos-activated in dependent than in non-dependent animals (27.2 ± 4.0% versus 14.9 ± 5.0%, p=.06, n = 6 per group); this difference was not found for non-retrogradely-labeled neurons. In addition, the number of VTA-projecting orexin neurons that were Fos-activated was significantly correlated with the intensity of conditioned preference in dependent animals (R=0.740, n=6/group, p<0.05).Thus, as preference scores increased in dependent rats, the percentage of Fos-activated, VTA-projecting orexin neurons increased. There was no such correlation with preference found for Fos+ LH orexin neurons that were not retrogradely labeled from VTA, nor was this correlation significant in non-dependent animals.
This study extends past studies by demonstrating that Fos activation in VTA-projecting LH orexin neurons correlates with the intensity of reward during protracted withdrawal, and supports the view that the VTA is an important site of action of LH orexin neurons in reward processing and drug abuse. This study also confirms our previous finding that prior morphine exposure enhances the preference for drug-associated environments (Harris and Aston-Jones, 2003a), and that animals that undergo prolonged forced abstinence have a higher degree of activation in LH orexin neurons that project to the VTA than control animals. Studies are underway to determine if this phenomenon is exclusive to LH orexin neurons or if enhanced activation of PeF or DMH orexin neurons that project to the VTA is also observed. Additionally, it will be determined if the activation of LH orexin neurons that project to other areas less directly linked with reward (e.g., locus coeruleus, tuberomammillary nucleus) is also enhanced after morphine preference testing.
Orexin modulates glutamate responses in VTA DA neurons
Glutamate transmission in VTA is important for several aspects of drug abuse. Cocaine causes release of glutamate in VTA (Kalivas and Duffy, 1995), and glutamate inputs to VTA are critical for reinstatement of extinguished drug-seeking behavior, including that evoked by cocaine or conditioned cues (Bossert et al., 2004; Sun et al., 2005; Vorel et al., 2001). Several studies have demonstrated that a single injection of cocaine induces long-term potentiation (LTP) in VTA DA neurons that is dependent upon NMDA receptor activation (Borgland et al., 2004; Borgland et al., 2006; Thomas and Malenka, 2003; Ungless et al., 2001). Consistent with these results, we showed that glutamate transmission and plasticity in VTA is important for learning a cocaine CPP, and for learning or expressing a morphine CPP (Harris and Aston-Jones, 2003b; Harris et al., 2004). A recent study extended the LTP results by showing that repeated cocaine injections produced larger LTP in DA neurons than single injections (Liu et al., 2005), suggesting that the stronger abuse potential with repeated cocaine experience may result in part from plasticity in these cells.
Recent work also indicates that orexin interacts in an important manner with glutamate function in VTA DA neurons, and that this interaction may be significant for drug abuse behavior. Borgland et al (2006) demonstrated that cocaine-induced plasticity in VTA DA neurons (described above) depends critically upon orexin inputs. They found that the Ox1 antagonist SB, when co-administered with cocaine, blocked the glutamate-dependent LTP in midbrain DA neurons. That study also showed that orexin administration to midbrain slices produced a late-phase glutamate-dependent LTP in DA neurons, and that this was due primarily to the insertion of new NMDA receptors in the synapse which then facilitated an AMPA receptor-mediated LTP. Finally, they showed that SB injected either systemically or directly into the VTA blocked the development of locomotor sensitization following repeated cocaine injections. These findings provide important new information indicating that orexin in VTA plays an important role in synaptic plasticity of DA neurons. As described below, additional work by others and our group indicates that this plasticity is important behaviorally as well.
Orexin modulates responses of VTA DA neurons to PFC activation
As described above, glutamate inputs and plasticity in VTA are critical for learning stimulus-drug associations (Harris and Aston-Jones, 2003b; Harris et al., 2004). Also, orexin increases responses and plasticity of VTA DA neurons evoked by glutamate inputs (Borgland et al., 2006). However, these previous studies did not specify which glutamate inputs are modulated by orexin. Other studies showed that glutamate inputs to VTA originate from several sources, including PFC, bed nucleus of the stria terminalis (BNST), subthalamic nucleus and pedunculopontine tegmental nucleus (Carr and Sesack, 2000; Geisler and Zahm, 2005; Georges and Aston-Jones, 2002b; Kita and Kitai, 1987; Rinvik and Ottersen, 1993; Sesack and Pickel, 1992). We reasoned that inputs representing stimulus information that becomes associated with drug administration might provide glutamate inputs to VTA that would be modulated by orexin.
Several lines of evidence indicate that mPFC inputs to VTA are important to the function of the DA system, including its role in reward and drug abuse. The mPFC provides direct glutamate innervation of DA and GABA neurons in VTA (Carr and Sesack, 2000; Sesack et al., 2003; Sesack et al., 1989; Sesack and Pickel, 1992), and regulates the release of DA in nucleus accumbens (Karreman and Moghaddam, 1996; Taber et al., 1995; You et al., 1998), which plays a central role in drug abuse (Kalivas and McFarland, 2003; Kalivas and Volkow, 2005; Koob, 1999; Wolf et al., 2004). The mPFC is a critical region for goal-directed behaviors and impulse control. It receives highly processed information from several brain areas, and is involved in complex cognitive and behavioral processes (Bubser and Schmidt, 1990; Granon et al., 1994; Kolb, 1984; Muir et al., 1996; Watanabe, 1996). In addition, the mPFC has been shown to be important in drug abuse and, specifically, in reinstatement of cocaine-seeking behavior (Kalivas and McFarland, 2003; McFarland et al., 2004; McFarland and Kalivas, 2001). In humans, imaging studies have revealed decreased metabolic activity in mPFC during drug withdrawal (Goldstein and Volkow, 2002) and large increases in activity in the mPFC following exposure to drug cues (Childress et al., 1999; Grant et al., 1996; Maas et al., 1998). Therefore, mPFC afferents to VTA are a potential source of VTA glutamate that is involved in reinstatement, learning and expression of drug-seeking behavior. Because our results indicate that orexin neurons are stimulated by cues that reinstate extinguished drug-seeking (Harris et al., 2005), we anticipate that orexin will be released onto VTA DA neurons at about the same time that mPFC inputs representing stimulus-response information are arriving at these cells. We hypothesize that the conditioned orexin input from LH would augment responses to glutamate (NMDA receptor-mediated) inputs from mPFC in a manner similar to that described by Borgland et al. (Borgland et al., 2006), serving to increase responding of VTA DA neurons to mPFC inputs.
We tested the influence of orexin on prefrontal projections to the VTA by recording the activity of DA neurons in isoflurane-anesthetized rats (Moorman and Aston-Jones, 2007). Responses were evoked from DA neurons by stimulating the prelimbic/infralimbic areas of the mPFC. Through the use of a second pipette glued to the recording pipette (Akaoka and Aston-Jones, 1991; Georges and Aston-Jones, 2002a), we delivered orexin A directly to the site of the recorded neuron. Using different combinations of microstimulation and orexin delivery during recording, we were able to ascertain the influence of orexin on mPFC-evoked responses in DA neurons.
Stimulation of the prelimbic/infralimbic regions in mPFC in the absence of orexin evoked short-latency (<50 ms) responses in 40% of putative DA neurons. Approximately 22% of DA neurons exhibited short-latency evoked responses within the range of monosynaptic projections (<25 ms (Thierry et al., 1983)). Orexin A (1.4 μM, 60 nl) applied directly to recorded neurons (in the absence of mPFC stimulation) produced strong increases in firing rate (in 58% neurons) and bursting (in 28% neurons) (Moorman and Aston-Jones, 2007), consistent with more global applications of orexin in slice (Korotkova et al., 2003) and in vivo (Muschamp et al., 2007).
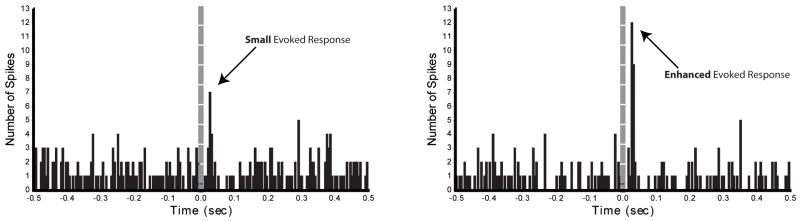
Our main interest was to test whether orexin application would facilitate mPFC-evoked responses in DA neurons, possibly indicating a circuit substrate for orexin-induced facilitation of glutamate responses, or plasticity, in VTA necessary for stimulus-drug conditioning. We tested the influence of mPFC stimulation on DA neurons when given before, during or following local orexin application. Results showed that for most cells mPFC-evoked responses following orexin application were enhanced. An example of such an orexin-enhanced mPFC-evoked response is shown in Fig. 5. In contrast, we observed an equivalent number of neurons exhibiting enhanced and diminished mPFC-evoked responses (45% each) when tested during orexin application. We speculate that the difference between effects of orexin on responses to mPFC stimulation during vs after orexin application may be due to different time courses of orexin effects on DA vs GABA neurons in VTA. That is, Borgland et al. (2006) found that orexin produced a long-lasting potentiation of glutamate-mediated responses in DA neurons, but others found that orexin produced only a transient increase in glutamate responses in presumed GABA neurons (which may in turn inhibit DA neurons) (Korotkova et al., 2003). Thus, effects of orexin on DA neurons may outlast those on inhibitory GABA interneurons, which could result in the effects observed here.
Figure 5.
Responses of a single DA neuron evoked with mPFC stimulation before and after application of orexin A to the recorded neuron. Left panel shows the responses of a DA neuron evoked with 1 mA stimulation of the mPFC. Right panel shows the responses of the same neuron to the same stimulation approximately 5 min following delivery of 60 nl of 1.4 μM orexin A locally onto the recorded neuron. Dashed line is the onset of stimulation.
Orexin likely facilitates additional excitatory inputs to VTA also. Several other areas send (in some cases relatively strong) glutamatergic projections to the VTA, including the LH (Geisler et al., 2007). Indeed, it has been shown that orexin neurons co-express orexin and glutamate (Rosin et al., 2003); the co-release of these transmitters could produce enhanced excitatory drive from the LH itself. The influence of orexin on other glutamatergic inputs remains to be tested. However, we speculate that complex stimulus information is relayed to VTA from mPFC, and therefore that orexin-facilitated mPFC drive on dopamine neurons is particularly relevant for learning stimulus-drug associations and for stimulus-induced reinstatement of extinguished drug-seeking. This may underlie the observations that activation of both the mPFC and the LH orexin system are critical in learning stimulus-drug associations and in reinstatement of drug-seeking.
LH orexin neurons are critical for learning morphine preference
A recent study (Narita et al., 2006) showed that microinjection of SB into VTA during conditioning reduced acquisition of a morphine CPP, confirming that orexin in VTA is critical for learning this stimulus-drug relationship, just as glutamate is (reviewed above). These studies suggest a critical behavioral role of the glutamate-dependent plasticity in DA neurons that is regulated by orexin inputs. We examined this issue using lesion methods and examined specifically the role of LH orexin projections to the VTA in learning stimulus-drug relationships (Harris et al., 2007b). We made bilateral neurotoxic lesions of LH neurons, or we made a neurotoxic lesion of LH neurons unilaterally, and then injected the orexin antagonist SB into the contralateral VTA just preceding each of 3 morphine CPP conditioning trials. Results were similar with both manipulations, and are illustrated for the contralateral disconnection procedure in Fig. 6. These manipulations both prevented learning the CPP, as evidenced by no preference expressed on the subsequent CPP test day. Normal preference for the morphine-paired side appeared in animals in which either a neurotoxic lesion was outside of LH, or the contralateral microinjection of SB was outside of VTA. These results are consistent with those of other recent studies (Narita et al., 2006), and indicate that LH orexin is not only involved in conditioned reward processes, but also in plasticity in the VTA associated with learning stimulus-drug relationships.
Figure 6.
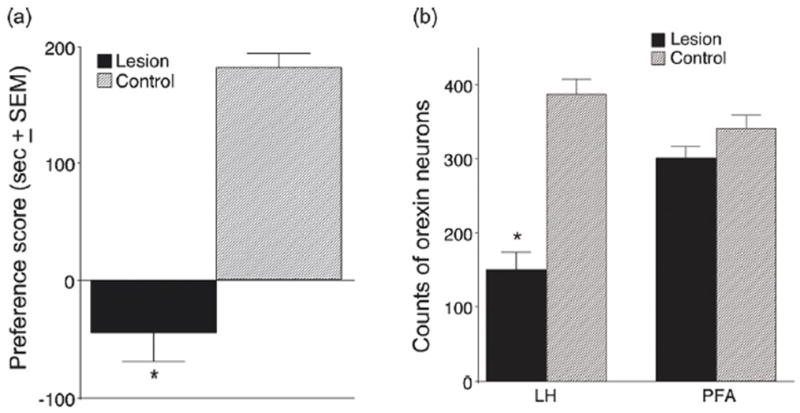
(a) Conditioned place preference (CPP) scores and (b) orexin neuronal cell counts for animals given unilateral NMDA injections in the LH and microinjections of SB 334867 in the contralateral VTA during CPP training. (a) Preference scores were calculated by subtracting the time spent in the morphine-paired chamber during the preconditioning day from the time spent in that chamber on the test day (i.e., post-conditioning). Control animals received vehicle instead of NMDA in the LH and received the same SB injections in the contralateral VTA. Scores represent group mean±SEM. (b) Neuronal cell counts of surviving orexin neurons from six adjacent 40 um-thick sections at the level of the neurotoxin injection from animals with effective lesions. Control refers to the number of orexin neurons found on the non-lesioned side in the same slices (*P < .01, n = 9). Taken from (Harris et al., 2007b).
To test whether orexin is also necessary for the acquisition of cocaine-associated cues during self-administration, we used a Pavlovian conditioned-cues paradigm previously described by See and colleagues (See, 2005). Although acquisition of self-administration typically occurs across several sessions, the Pavlovian conditioned-cues paradigm has the advantage of confining cue acquisition to a single session. Animals were trained to self-administer cocaine (0.2 mg/infusion) in the absence of cues. After five days of stable responding, animals were exposed to a single Pavlovian conditioning session, in which no levers were extended and the animals received passive infusions of cocaine paired with discrete tone and light cues. The number of infusions was based on the self-administration for each animal in previous sessions. The Pavlovian session was followed by five more days of self-administration without cues. Following extinction of lever pressing in the absence of cocaine, drug-seeking was robustly reinstated by the cocaine-paired cues. Administration of 30 mg/kg SB prior to the Pavlovian acquisition trial had no effect on subsequent expression of cue-elicited reinstatement of lever responding. That is, animals pretreated with SB or vehicle showed similar reinstatement responding, indicating that orexin is not necessary for acquiring cocaine-cue associations in this paradigm. In contrast, 30 mg/kg SB in these same animals significantly reduced the expression of conditioned-cue-elicited drug-seeking when administered prior to the subsequent reinstatement trial (Smith and Aston-Jones, unpublished). This corresponds to our results described above showing that cue-induced reinstatement of cocaine-seeking requires orexin transmission.
Together, these experiments indicate that orexin is necessary for learning morphine-stimulus associations and preference, but not for learning the cocaine-stimulus relationships required for cue-evoked reinstatement of cocaine-seeking behavior. There are several procedural differences between these two learning paradigms that might underlie these different results for SB administration, including CPP vs self-administration. However, we note that these results are consistent with the finding that SB is ineffective on drug-seeking behaviors that include cocaine administration. Thus, one possibility, discussed in more detail below, is that orexin may be involved in morphine, but not in cocaine, conditioning because DA release following morphine requires VTA activation, whereas DA release following cocaine does not.
Discussion and hypothesis
Orexin is involved in cue- but not cocaine-induced relapse: an hypothesis
One interesting outcome of our recent studies with self-administration of cocaine is that SB did not block reinstatement of cocaine-seeking induced by cocaine itself, nor did this compound significantly affect established cocaine self-administration. This contrasts sharply with the ability of SB to block cocaine-seeking induced by cues, and to attenuate responding on the first day of extinction. What might explain this difference in SB effects on stimulus-elicited vs cocaine-elicited reinstatement of cocaine-seeking and maintenance of cocaine self-administration? We hypothesize that this is because the VTA is a major site of action for orexin in reward processes. Previous work established that DA release is an essential neural substrate for many types of motivated behavior (Wise, 2004). Orexin potentiates glutamate-mediated responses of VTA DA neurons, and it is possible that this orexin potentiation of responsiveness is a critical element in sufficient DA release to drive motivated behavior (eg., cocaine-seeking) for drug-associated stimuli. In contrast, cocaine itself elicits DA release without altering impulse activity of VTA DA neurons, so that effects of cocaine on behavior (such as cocaine-induced reinstatement or cocaine self-administration) do not require orexin transmission. This hypothesis is illustrated in Fig. 7, and is consistent with other previous results showing that SB can block or attenuate (i) ethanol self-administration, or cue-induced reinstatement or ethanol-seeking (Lawrence et al., 2006), (ii) expression of a morphine CPP (Harris et al., 2005), and (iii) stress-induced reinstatement of cocaine-seeking (Boutrel et al., 2005). In all of these cases, the drug or stimulus causes DA release by activating VTA DA neurons, and we hypothesize that therefore they require orexin transmission. This view is also consistent with the finding that intra-VTA SB attenuates development of locomotor sensitization to cocaine (Borgland et al., 2006), a process that requires plasticity in VTA DA neurons (Kalivas and Alesdatter, 1993; Sorg and Ulibarri, 1995).
Figure 7.
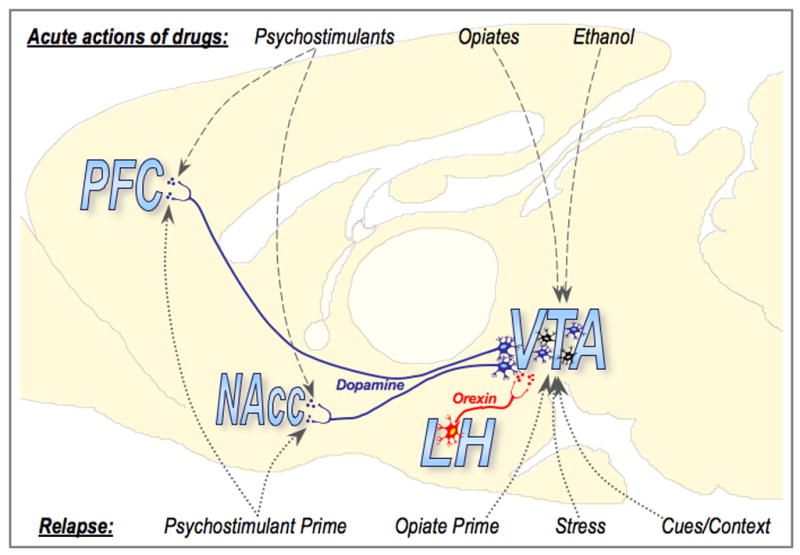
Diagram illustrating a proposed model for the role of orexin in drug-seeking and relapse. Orexin neurons in the lateral hypothalamus (LH) send direct and indirect projections to the ventral tegmental area (VTA). The VTA sends dopaminergic projections to several areas, including the prefrontal cortex (PFC) and nucleus accumbens (NAcc). Psychostimulants cause increased dopamine release in these target areas via direct actions at dopaminergic terminals, whereas opiates and alcohol cause increased dopamine release via actions in the VTA that result in increased dopaminergic cell activation. These differences in acute drug action might explain the ability of SB-334867 to attenuate only certain types of drug-seeking. SB-334867 attenuates increased activation of VTA dopaminergic neurons without effect on baseline firing. Therefore, relapse that depends upon increased VTA dopaminergic cell activation (i.e. opiate-, stress-, and cue-induced reinstatement, and context-elicited drug-seeking) is vulnerable to SB-334867. Relapse that does not require increased activation of VTA dopaminergic cells (i.e. cocaine-induced reinstatement) is unaffected by SB-334867. Illustration modified from Swanson (1992.
This hypothesis predicts that agents that act via glutamate release in VTA to motivate behavior would require orexin release in VTA. Studies examining the effects of local administration of SB in VTA on reinstatement behavior are underway to test this possibility. We also predict that SB should attenuate opiate self-administration, or opiate-induced drug-seeking behavior. Corresponding studies are also underway to test these ideas.
This view indicates that the VTA is an important site of action for orexin in reward-associated functions. LH orexin neurons are more prominently involved in reward processes than are more medial orexin neurons in PeF and DMH (Harris and Aston-Jones, 2006b). Also, we expect that LH orexin neurons that project to VTA may be more strongly activated by reward-related stimuli than non-VTA-projecting LH or PeF/DMH orexin neurons. Our preliminary results showing that VTA-projecting orexin neurons show elevated Fos in proportion to preference for a drug-associated environment, compared to non-VTA-projecting orexin neurons (described above), is consistent with this hypothesis. Additional work tracing inputs and outputs of orexin neurons is underway to further define the topography of connections for reward-associated orexin neurons.
Although most of our analysis of the orexin system to date has centered on drug reward, it is notable that these neurons are also Fos-activated by CSs associated with food reward. Results from Petrovich et al [Petrovich, 2002 #4291] show that amygdala inputs to hypothalamus regulate cue-induced feeding in sated animals. We speculate that orexin neurons may be involved in this circuit, and that the amygdala stimulates orexin neurons in response to food CSs, which in turn may play a role in conditioned overeating and obesity. Further studies are needed to evaluate this possibility.
Orexin and reward: a role in drug-seeking and relapse
The results reviewed above indicate that LH orexin neurons are stimulated in proportion to drug or food preference, that stimulation of LH orexin neurons drives reinstatement of an extinguished preference for morphine, and that orexin neurotransmission is needed for stimulus-induced (but not for cocaine-induced) reinstatement of extinguished cocaine-seeking. We propose that these results indicate a role for orexin systems in drug-seeking and addiction. Interestingly, human narcoleptics, who have few or no orexin neurons, rarely exhibit stimulant abuse and seeking despite the fact that they are treated for years with stimulants (Sakurai, 2007). Together, these findings indicate that LH orexin neurons may be stimulated by cues associated with drug acquisition and exposure, and that these neurons may be part of circuitry that is critically involved in drug abuse, and specifically in stimulus-induced drug relapse. Moreover, we propose that VTA is a critical region for orexin actions in reward-seeking behaviors. These findings are clinically significant, and indicate that development of compounds that specifically target LH orexin neurons, or orexin receptor actions in VTA DA neurons, may provide novel treatments for addiction and relapse. In particular, we propose that an orexin antagonist would reduce the propensity to relapse to drug-taking behavior, and would help maintain abstinence in addicts re-exposed to previous drug cues. Additional studies in this vein will advance our understanding of the role of orexin in addiction and lead to possible novel therapeutic targets.
Acknowledgments
This work was supported by PHS grants R37 DA06214, R01 DA017289, P50 DA015369, F31 DA019733, and T32 AA007474.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaoka H, Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47S1:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-Induced potentiation of synaptic strength in the ventral tegmental area: Electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. PNAS. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res. 1990;37:157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- Byrne R, Harris GC, Aston-Jones G. Glutamate input to the ventral tegmental area is necessary for both learning and expression of morphine place preference. Soc Neurosci Abstr. 2003;29:110.111. [Google Scholar]
- Campbell RE, Smith MS, Allen SE, Grayson BE, Ffrench-Mullen JM, Grove KL. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J Neurosci. 2003;23:1487–1497. doi: 10.1523/JNEUROSCI.23-04-01487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002a;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel glutamate input to midbrain dopamine neurons. Journal of Neuroscience. 2002b;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granon S, Vidal C, Thinus-Blanc C, Changeux JP, Poucet B. Working memory, response selection, and effortful processing in rats with medial prefrontal lesions. Behav Neurosci. 1994;108:883–891. doi: 10.1037//0735-7044.108.5.883. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, Altomare K, Aston-Jones G. Preference for a cocaine-associated environment is attenuated by augmented accumbal serotonin in cocaine withdrawn rats. Psychopharmacology. 2001;156:14–22. doi: 10.1007/s002130100693. [DOI] [PubMed] [Google Scholar]
- Harris G, Aston-Jones G. Altered motivation and learning following opiate withdrawal: Evidence for prolonged dysregulation of reward processing. Neurpsychopharmacology. 2003a;28:865–871. doi: 10.1038/sj.npp.1300122. [DOI] [PubMed] [Google Scholar]
- Harris G, Aston-Jones G. Critical role for ventral tegmental glutamate in cocaine conditioning. Neuropsychopharmacology. 2003b;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- Harris G, Aston-Jones G. Arousal and reward: A dichotomy in orexin function. Trends Neurosci. 2006a;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris G, Hummel M, Wimmer M, Mague S, Aston-Jones G. Elevations in nucleus accumbens FosB during cocaine abstinence correlates with divergent changes in reward function. Neuroscience. 2007a;147:583–591. doi: 10.1016/j.neuroscience.2007.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Harris G, Wimmer M, Randall-Thompson J, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007b;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology. 2001;24:75–85. doi: 10.1016/S0893-133X(00)00184-6. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003c;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006b;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behavioural Brain Research. 2007;176:251–258. doi: 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Sabrina D, Ivanov A, Aston-Jones G, Kilduff T, van den Pol AN. Strong hypocretin (orexin) innervation of the locus coeruleus activates noradrenergic cells. Journal of Comparative Neurology. 1999;415:145–159. [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Ralls TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. Pergamon; New York: 1990. pp. 522–573. [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci. 1995;15:5379–5388. doi: 10.1523/JNEUROSCI.15-07-05379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The Neural Basis of Addiction: A Pathology of Motivation and Choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol. 1987;260:435–452. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Research. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu Q-s, Pu L, Poo M-m. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37:335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Massi L, Moorman DG, Aston-Jones G. Responses of ventral tegmental area neurons to stimulation of orexin cell fields in vivo. Neuroscience Meeting Planner Online. 2007 Program No. 916.3. [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Modulation of ventral tegmental area neural responses to prefrontal stimulation by local orexin application in vivo. Neuroscience Meeting Planner Online. 2007 Program No. 916.2. [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S. The hypothalamic peptidergic system, hypocretin/orexin and vigilance control. Neuropeptides. 2007 doi: 10.1016/j.npep.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Hsu MA, Yang Y. The orexin-1 receptor antagonist SB-334867 blocks the effects of antipsychotics on the activity of A9 and A10 dopamine neurons: implications for antipsychotic therapy. Neuropsychopharmacology. 2007;32:786–792. doi: 10.1038/sj.npp.1301239. [DOI] [PubMed] [Google Scholar]
- Richardson KA, Knackstedt PT, Aston-Jones G. Orexin neurons that project to the ventral tegmental area are activated by morphine preference during protracted forced abstinence. Neuroscience Meeting Planner Online. 2007 Program No. 916.4. [Google Scholar]
- Rinvik E, Ottersen OP. Terminals of subthalamonigral fibres are enriched with glutamate-like immunoreactivity: an electron microscopic, immunogold analysis in the cat. J Chem Neuroanat. 1993;6:19–30. doi: 10.1016/0891-0618(93)90004-n. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior [see comments] Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sartor G, Aston-Jones G. Afferents that regulate lateral hypothalamic orexin neurons during reward-seeking behaviors. Neuroscience Meeting Planner Online. 2008 in press. [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with phaseolus vulgaris leucoagglutinin. Journal of Comparative Neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, DiLeone RJ. Orexin Mediates the Expression of Precipitated Morphine Withdrawal and Concurrent Activation of the Nucleus Accumbens Shell. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, See R, Aston-Jones G. The orexin-1 receptor antagonist SB-334867 blocks cue induced reinstatement of cocaine-seeking. Neuroscience Meeting Planner Online. 2007 Program No. 916.1. [Google Scholar]
- Sorg BA, Ulibarri C. Application of a protein synthesis inhibitor into the ventral tegmental area, but not the nucleus accumbens, prevents behavioral sensitization to cocaine. Synapse. 1995;20:217–224. doi: 10.1002/syn.890200305. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic Glutamate Receptors in the Ventral Tegmental Area Regulate Cocaine-Seeking Behavior in Rats. Neuropsychopharmacology. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Chevalier G, Ferron A, Glowinski J. Diencephalic and mesencephalic efferents of the medial prefrontal cortex in the rat: electrophysiological evidence for the existence of branched axons. Exp Brain Res. 1983;50:275–282. doi: 10.1007/BF00239191. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC. Synaptic plasticity in the mesolimbic dopamine system. Philos Trans R Soc Lond B Biol Sci. 2003;358:815–819. doi: 10.1098/rstb.2002.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vorel S, Liu X, Hayes R, Spector J, Gardner E. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Tzschentke TM, Brodin E, Wise RA. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vivo microdialysis study in freely moving rats. J Neurosci. 1998;18:6492–6500. doi: 10.1523/JNEUROSCI.18-16-06492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]