Abstract
Cue-induced drug-seeking in rodents progressively increases after withdrawal from cocaine, suggesting that cue-induced cocaine craving incubates over time. Here, we explored the role of the medial prefrontal cortex (mPFC, a brain area previously implicated in cue-induced cocaine seeking) in this incubation. We trained rats to self-administer cocaine for 10 d (6 h/d, infusions were paired with a tone-light cue), and then assessed after 1 or 30 withdrawal days the effect of exposure to cocaine cues on lever presses in extinction tests. We found that cue-induced cocaine-seeking in the extinction tests was higher after 30 withdrawal days than after 1 day. The time-dependent increases in extinction responding were associated with large (ventral mPFC) or modest (dorsal mPFC) increases in ERK phosphorylation (a measure of ERK activity and an index of neuronal activation). After 30 withdrawal days, ventral but not dorsal injections of muscimol+baclofen (GABAa+GABAb receptor agonists that inhibit neuronal activity) decreased extinction responding. After 1 withdrawal day, ventral but not dorsal mPFC injections of bicuculline+saclofen (GABAa+GABAb receptor antagonists that increase neuronal activity) strongly increased extinction responding. Finally, muscimol+baclofen had minimal effect on extinction responding after 1 day, and in cocaine-experienced rats, ventral mPFC injections of muscimol+baclofen or bicuculline+saclofen had no effect on lever presses for an oral sucrose solution. The present results indicate that ventral mPFC neuronal activity plays an important role in the incubation of cocaine craving.
Keywords: Craving, drug cues, ERK, Extinction, Cocaine self-administration, Prefrontal cortex, Reinstatement, Relapse
Relapse to cocaine use in humans can occur after prolonged abstinence and is often precipitated by exposure to craving-provoking cocaine-associated cues (O’Brien, 2005). In 1986, Gawin and Kleber (1986) hypothesized that cue-induced cocaine craving increases over the first several weeks of abstinence and remains high over extended periods (see (Kosten et al., 2005) for tentative support for this hypothesis in a clinical trial). We and others identified an analogous incubation phenomenon in rats: time-dependent increases in cue-induced cocaine-seeking over the first few months of withdrawal (Grimm et al., 2001; Lu et al., 2004c; Neisewander et al., 2000), a phenomenon we termed incubation of cocaine craving. This incubation was demonstrated in extinction (Hollander and Carelli, 2007; Lee et al., 2006; Lu et al., 2004b; Sorge and Stewart, 2005), cue-induced reinstatement (Grimm et al., 2001; Grimm et al., 2003; Mead et al., 2007), and acquisition of a new response (Di Ciano and Everitt, 2004) procedures. Incubation of reward craving was also demonstrated in rats trained to self-administer heroin (Shalev et al., 2001), methamphetamine (Shepard et al., 2004), alcohol (Bienkowski et al., 2004), sucrose solution (Grimm et al., 2005; Grimm et al., 2002), and food (Youtz, 1938).
We previously explored the role of amygdala extracellular signal-regulated kinases (ERK), a signaling pathway implicated in cocaine’s behavioral effects (Girault et al., 2007; Lu et al., 2006) in incubation of cocaine craving (Lu et al., 2005). We found that exposure to cocaine cues in extinction tests increases phosphorylated ERK (p-ERK, a measure of ERK activity) in central amygdala after 30 days but not 1 day of withdrawal from cocaine. After 30 withdrawal days, inhibition of central amygdala p-ERK by U0126 decreased cue-induced cocaine-seeking, while after 1 withdrawal day, stimulation of central amygdala p-ERK by NMDA increased cocaine-seeking, an effect reversed by U0126. These data indicate that in central amygdala, ERK activation rapidly induces changes in synaptic transmission events that acutely control cue-induced cocaine-seeking (Lu et al., 2006).
Here, we assessed whether ERK activity in medial prefrontal cortex (mPFC) plays a role in incubation of cocaine craving. There is evidence from both humans and animal studies that mPFC contributes to cocaine addiction (Jentsch and Taylor, 1999; Kalivas et al., 2005). In both humans (Childress et al., 1999; Maas et al., 1998) and rats (Ciccocioppo et al., 2001; Neisewander et al., 2000), exposure to cocaine cues increases mPFC neuronal activity. In studies using a reinstatement model of drug relapse (Epstein et al., 2006; Stewart and de Wit, 1987), acute inactivation of dorsal but not ventral mPFC neurons by tetrodotoxin or GABAa-GABAb receptor agonists (muscimol+baclofen) attenuates cocaine priming-, cocaine cue-, or stress-induced reinstatement of cocaine seeking (Capriles et al., 2003; Fuchs et al., 2005; McFarland and Kalivas, 2001; McLaughlin and See, 2003). Based on these results, and our findings on the role central amygdala ERK in incubation of cocaine craving (Lu et al., 2005), we hypothesized that dorsal mPFC ERK activity would contribute to incubation.
However, we found that exposure to cocaine cues in extinction tests increased p-ERK more strongly in ventral than in dorsal mPFC after 30 withdrawal days. Also, acute inhibition of p-ERK by U0126 in dorsal or ventral mPFC had no effect on enhanced responding to cocaine cues after 30 withdrawal days (data not shown) suggesting that acute activation of mPFC ERK does not mediate the incubation of cocaine craving. Therefore, we used local neuronal inactivation (the GABAa+GABAb receptor agonists, muscimol+baclofen) (McFarland and Kalivas, 2001) or activation (that GABAa+GABAb receptor antagonists, bicuculline+saclofen) procedures to further explore mPFC role in incubation of cocaine craving. In these experiments, we continued to use p-ERK as a neuronal activity marker (Thomas and Huganir, 2004) in order to verify the effectiveness (and anatomical specificity) of our pharmacological manipulations that were aimed at inhibiting or activating mPFC neurons. In this regard, results from several studies indicate that ERK activity controls the induction of commonly used neuronal activity markers (e.g. Fos, Zif268) that are induced by cocaine or cocaine cues (Girault et al., 2007; Lu et al., 2006; Mattson et al., 2005; Miller and Marshall, 2005; Valjent et al., 2000).
Methods
Subjects and apparatus
Male Long-Evans rats (350–400 g, Charles River) were maintained under a reverse 12-h light-dark-cycle. Food and water were available in the rats’ home cage. Procedures followed the guidelines of the “Principles of Laboratory Care” (NIH publication no. 86–23, 1996) and were approved by the local Animal Care Committee. Self-administration chambers were located inside sound-attenuating cabinets and controlled by a Med-Associates system. Each chamber had two levers located 9 cm above the floor. Presses on one (active, retractable) lever activated the infusion pump; presses on the other (inactive, stationary) lever were also recorded. Rats’ catheters were connected via a modified cannula (Plastics One) to liquid swivels (Instech) with PE-50 tubing.
Intravenous and intracranial surgery
Rats were anesthetized with sodium pentobarbital+chloral hydrate (60+25 mg/kg, i.p.). In Exp. 1 rats were implanted with intravenous catheters using a procedure previously described (Crombag et al., 2002; Shaham et al., 1996). In Exp. 2–4, rats were implanted with intravenous catheters+guide cannulae (23-gauge, Plastics One) 1.0–1.5 mm dorsal to the dorsal or ventral mPFC. Coordinates of the intended infusion sites were AP 2.8 mm, ML 0.6 mm, and DV −3.8 and −5.6 mm for dorsal or ventral PFC, respectively (Swanson, 1998). Cannula placements are shown in Figures 2–4. The analgesic buprenorphine (NIDA), an opiate, (0.1 mg/kg, s.c.) was injected after surgery to decrease post-surgical pain. During recovery (7–10 d) and training, catheters were flushed every 24–48 h with sterile saline+the antibiotic Gentamicin (0.08 mg/ml) to prevent infections.
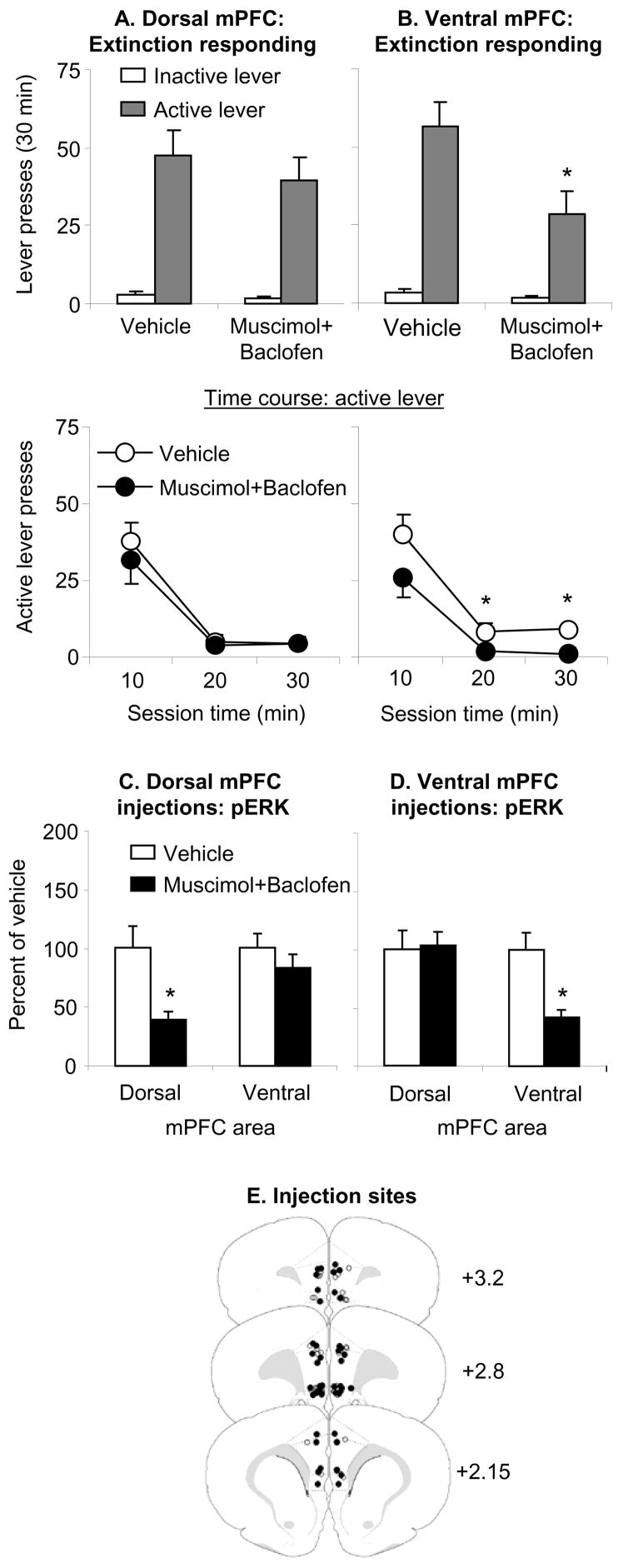
Figure 2. Effect of dorsal and ventral mPFC muscimol+baclofen injections on day 30 extinction responding.
(A–B) Test for cocaine seeking after dorsal or ventral mPFC injections of vehicle or a mixture of muscimol+baclofen (0.03+0.3 nmol/side). Data are mean±SEM number of non-reinforced presses on the previously active lever and on the inactive lever during the extinction tests. (C–D) p-ERK levels in the dorsal and ventral mPFC after dorsal or ventral mPFC injections of vehicle or muscimol+baclofen. Data are depicted as a percent of the mean±sem of the vehicle condition. Ventral but not dorsal mPFC injections of muscimol-baclofen significantly decreased extinction responding; both ventral and dorsal mPFC injections of muscimol+baclofen significantly decreased local p-ERK levels. (E) Approximate anatomical location of the injectors for vehicle (open circles) and muscimol+baclofen (closed circles). Numbers indicate the approximate anterior-posterior distance in mm from Bregma. * Different from vehicle, p<0.05 (n=8–12 per group).
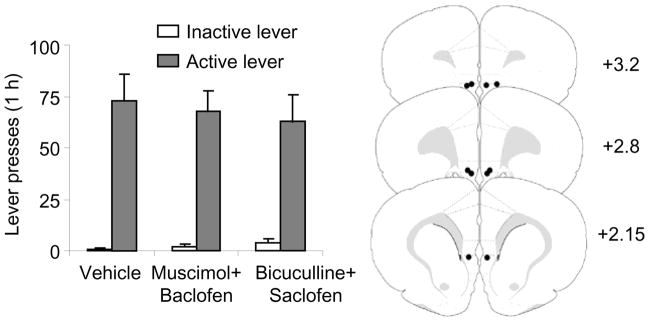
Figure 4. Effect of ventral mPFC muscimol+baclofen or bicuculline+saclofen injections on sucrose self-administration.
Data are mean±SEM number of responses on the active and inactive levers after bilateral injections of vehicle, muscimol+baclofen (0.03+0.3 nmol/side), and bicuculline+saclofen (50+50 ng/side) (n=5). No significant effects were observed.
Drugs and intracranial injections
Cocaine-HCl (NIDA) was dissolved in saline. Muscimol (Tocris, Ellisville, MO; 0.03 nmol/side)+baclofen (Tocris, 0.3 nmol/side) or bicuculline methiodide (Sigma, St. Louis, MO; 50/side)+saclofen (Sigma, 50 ng/side) mixtures were also dissolved in saline. The doses of the different drugs are based on previous reports (Herremans et al., 1996; Jasmin et al., 2003; McFarland and Kalivas, 2001). Bicuculline+saclofen and muscimol+baclofen (0.5 μl/side) were injected within 5–10 min prior to the test sessions with 30 gauge injectors (Plastics One, Roanoke, VA) over 1 min; injectors were left in place for 1 min after injections. For Exp. 2–3, injector placements were checked during the analysis of p-ERK immunoreactivity. For Exp. 4, the rats were deeply anesthetized at the end of the experiment, and then they were decapitated and their brains were removed. Coronal sections (30 μm) were sliced on a cryostat and stained with Cresyl Violet. The brains were then verified for cannulae placement under a light microscope.
Immunohistochemistry and quantification of p-ERK positive cells
Immediately after the extinction tests, the rats were anesthetized with isoflurane and perfused transcardially with 4% paraformaldehyde. The brains were removed and post-fixed for 2-h before being transferred to 30% sucrose in 0.1 m sodium phosphate, pH 7.4, for 48 h at 4°C. The brains were frozen in powdered dry ice and stored at −80°C. Coronal sections (30 μm) were cut in a cryostat, collected in Tris buffered saline (TBS) (0.025M Tris, 0.5M NaCl, pH 7.5) for slices to be stained immediately or to be placed in cryoprotectant [20% glycerol+2% dimethylsulfoxide (DMSO) in 0.1 M sodium phosphate, pH 7.4], and stored at −80°C.
Free-floating sections were rinsed 3 times for 10 min each in TBS, incubated for 15 min in 0.2% Triton X-100 in TBS, and then washed 3 times for 10 min each in TBS. Then, sections were incubated overnight at 4°C in 1:400 dilution of p-ERK rabbit polyclonal antibody (Cell Signaling, Beverly, MA) in TBS 0.2% Triton X-100. Sections were washed 3 times for 10 min each in TBS and incubated with an anti-rabbit polyclonal antibody conjugated to Alexa 488 at a dilution of 1:200. Sections were washed again 3 times 10 min each with TBS and mounted on chrom-alum coated slides. After drying, slides were coverslipped using Vectashield (Vectorlabs, Burlingame, CA).
Images of dorsal and ventral mPFC were captured with a CCD camera (Coolsnap Photometrics, Tucson, AZ) and Zeiss Axioscop-2 microscope (Zeiss, Jena, Germany) and identified using the Swanson atlas (Swanson, 1998). The sampling areas (0.38mm2 per hemisphere) for dorsal mPFC consisted of the anterior cingulate and prelimbic dorsal areas, while the sampling area for ventral mPFC consisted of the ventral prelimbic and infralimbic areas (Fig. 1). Borders of the anterior cingulate, prelimbic, and infralimbic areas were derived from Van Eden and Uylings (1985) and personal communication with Dr. Uylings. Both hemispheres were counted and 1–3 coronal sections per sample were used. p-ERK positive cells (cells containing dendritic, cell body, and nuclear staining) were manually counted under 20x magnification by an observer blind to experimental conditions.
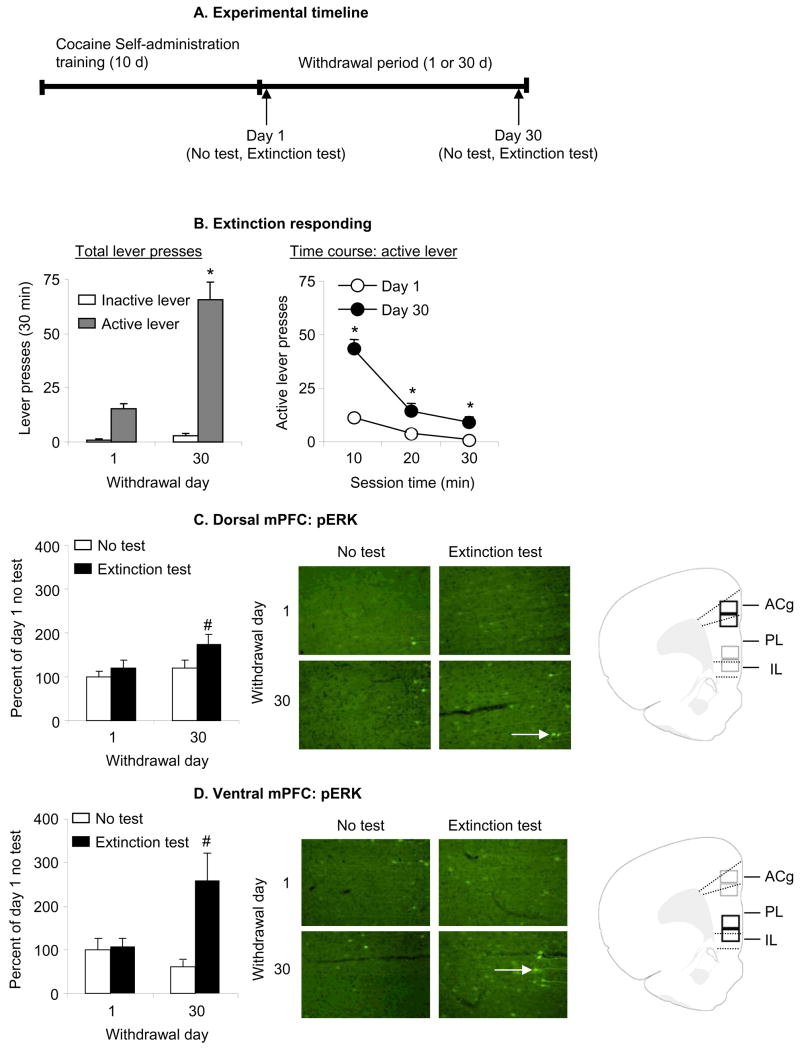
Figure 1. Effect of cue-induced cocaine seeking in extinction tests on p-ERK levels.
(A) Outline of experimental timeline. Four groups of rats self-administered cocaine for 6 h/d for 10 d. On withdrawal days 1 or 30, the rats were either tested or not tested for cocaine seeking in extinction tests, and their brains were removed for p-ERK analysis. (B) Test for cue-induced cocaine seeking: data are mean±sem number of lever-presses on the previously active lever and on the inactive lever during the tests for cocaine seeking performed under extinction conditions after 1 day or 30 days of withdrawal. During the test sessions, cocaine was not available and lever presses resulted in the delivery of the tone-light cue previously paired with cocaine. All rats were chronically housed in the self-administration chambers during training; the rats in the 30 days withdrawal groups were housed in their home cage in the animal facility after the self-administration training. Non-reinforced lever responding after 30 withdrawal days was higher than after 1 withdrawal day. * Different from Day 1, p<0.01, n = 9–11 per group). (C-D) p-ERK levels in dorsal or ventral mPFC on day 1 or 30 of withdrawal in the test or no test conditions; data are depicted as percent of mean Day 1 No Test condition. p-ERK positive cells are indicated with white arrows. Rats in the Extinction Test condition were trained to self-administer cocaine and were exposed to the cocaine cues in a 30 min extinction test after 1 or 30 withdrawal days. Rats in the No Test condition were trained to self-administer cocaine and were not exposed to the cocaine cues after 1 or 30 withdrawal days. Exposure to cocaine cues in the extinction tests increased the number of p-ERK positive cells (white arrows) in both dorsal and ventral mPFC after 30 days but not 1 day of withdrawal; this effect was more pronounced in ventral mPFC than in dorsal mPFC. Abbreviations: ACg, Anterior cingulate cortex; PL, Prelimbic cortex; IL, infralimbic cortex. # Different from the other 3 groups, p<0.05.
Behavioral procedures
The experiments consisted of three phases: self-administration training, withdrawal period (1 or 30 d), and tests for cue-induced cocaine-seeking under extinction conditions.
Training phase
Rats were chronically housed in the self-administration chambers during training. They were trained to self-administer cocaine (0.75 mg/kg/infusion; 0.10 ml/infusion over 2.3 sec) during six daily 1-h sessions, separated by 5 min, over 10 d under a fixed-ratio-1 40-sec timeout reinforcement schedule. These training conditions were based on our previous studies on incubation of cocaine craving (Grimm et al., 2003; Lu et al., 2004a; Lu et al., 2004b; Lu et al., 2007). Active lever-presses activated the infusion pump and led to the delivery of a 5-sec tone-light cue. Sessions started at the onset of the dark cycle and began with insertion of the active lever and illumination of a red houselight that remained on during the sessions. At the end of each 1-h session, the houselight turned off and the active lever retracted. To facilitate acquisition of lever-presses for cocaine, food was removed from the chambers during the 6-h sessions of the first 3–5 training days. Once acquisition was stable, food was freely available. Water was freely available for all days of training. The number of cocaine infusions/h was limited to 20. The groups to be tested in the different experiments were matched for their cocaine intake.
Withdrawal phase
After the training phase, rats to be tested after 30 withdrawal days were returned to the animal facility and handled 3 times/week; these rats were brought to the self-administration chambers 1 d prior to the extinction tests. The rats tested after 1 withdrawal day remained in the self-administration chambers after training.
Extinction tests
The 30-min extinction tests in the presence of the cocaine-associated cues were conducted after 1 or 30 withdrawal days. The experimental conditions were the same as in training except that active lever-presses were not reinforced with cocaine. Tests started at the onset of the dark cycle and began with the insertion of the active lever and the illumination of the red houselight that remained on for the duration of the session. Active lever presses during testing resulted in contingent presentations of the tone-light cue previously paired with cocaine infusions.
Exp. 1: Effect of cue-induced cocaine seeking in extinction tests on p-ERK immunoreactivity
The purpose of Exp. 1 was to assess whether exposure to cocaine cues in extinction causes time-dependent increases in neuronal activity in the dorsal and ventral mPFC, as assessed by pERK immmunoreactivity. We trained rats to self-administer cocaine and then divided them into 4 groups (n=10–11 per group) in a 2 (withdrawal day: 1, 30) × 2 (extinction test: no, yes) factorial design in which the rats either underwent the 30-min extinction test or were left undisturbed in the self-administration chambers. Immediately after testing (or no testing), the rats were anesthetized, transcardially perfused, and their brains were collected for determination of p-ERK levels.
Exp. 2: Effect of mPFC muscimol+baclofen injections on day 30 extinction responding
In Exp. 2 we assessed the role of ventral and dorsal mPFC in the time-dependent increases in cocaine seeking after withdrawal by determining whether decreasing local neuronal activity by injections of muscimol+baclofen would decrease cocaine seeking after prolonged withdrawal from cocaine. We used 4 groups of rats (n=8–10 per group) that were trained to self-administer cocaine and then underwent 30 min extinction tests after 30 withdrawal days. For 2 groups, we injected muscimol+baclofen or its vehicle into dorsal mPFC, while for 2 other groups we injected muscimol+baclofen or vehicle into ventral mPFC. Immediately after testing, the rats were anesthetized, transcardially perfused, and their brains were collected for determination of p-ERK levels. In this experiment (and in Exp. 3) we measured p-ERK in order to verify the effectiveness of our experimental manipulations on neuronal activity in mPFC.
Exp. 3: Effect of mPFC bicuculline+saclofen injections on day 1 extinction responding
In Exp. 2 we found that ventral but not dorsal mPFC muscimol+baclofen injections, which inhibit neuronal activity, significantly decreased enhanced cocaine-seeking after 30 withdrawal days. Here, we assessed the role of ventral mPFC in the time-dependent increases in cocaine-seeking after withdrawal by determining whether increasing neuronal activity using bicuculline+saclofen in this brain area on day 1 withdrawal will increase cocaine seeking. We used 4 groups of rats (n=5–6 and 12–13 for dorsal and ventral mPFC, respectively) that were trained to self-administer cocaine and underwent 30 min extinction tests after 1 withdrawal day. For 2 groups, we injected bicuculline+saclofen or its vehicle into ventral mPFC, while for the other 2 groups we injected bicuculline+saclofen or its vehicle into dorsal mPFC. Immediately after testing, the rats were anesthetized, transcardially perfused, and their brains were collected for determination of p-ERK levels.
Exp. 4: Effect of ventral mPFC injections of muscimol+baclofen on day 1 extinction responding and effect of ventral mPFC injections of muscimol+baclofen and bicuculline+saclofen on sucrose self-administration
The purpose of Exp. 4 was to explore the specificity of the behavioral effects of our drug injections into ventral mPFC. We first assessed the effect of muscimol+baclofen injections on day 1 extinction responding. We then assessed the effect of muscimol+baclofen or bicuculline+saclofen injections on oral sucrose self-administration. We used 2 groups of rats (n=8–11) that were trained to self-administer cocaine and then underwent 30 min extinction tests after 1 withdrawal day; these rats were injected with muscimol+baclofen or its vehicle into ventral mPFC prior to the extinction tests. We then trained 5 of these rats, and 2 additional rats that were trained to self-administer cocaine but were not given an extinction test, to lever press for 10% oral sucrose. For these rats, food was restricted to maintain 85–90% of the rats’ free-feeding weight (~20 g of food/d) and sucrose self-administration began three days later. The rats were trained over 8 d (1 h/d) to lever press for 10% sucrose solution (FR-1 reinforcement schedule, 20 sec time-out, 0.2 ml per reward delivery over 4 sec on days 1–3, 0.4 ml over 8 sec on day 4, and 0.6 ml over 12 sec on days 5–8). After the rats displayed stable lever presses (less than 15% of mean responding over 3 d) for 0.6 ml per reward delivery, we examined the effect of vehicle, muscimol+baclofen, and bicuculline+saclofen injections into ventral mPFC on sucrose self-administration using a counterbalanced within-subjects design. Injections were given every 48 h and regular sucrose self-administration sessions (with no injections) occurred between the test sessions.
Results
The rats demonstrated reliable cocaine self-administration and there were no significant differences between rats in Exp. 1–4 (p>0.05). Mean±sem 10-d daily cocaine intake (infusions/6 h) for Exp. 1–4 was 63.7±4.0 (n=41), 58.0±2.3 (n=39), 56.2±3.2 (n=36), and 58.4±2.8 (n=19), respectively. Eighteen rats were excluded because of misplaced cannulae or suspected neuronal damage near the cannulae; one rat was excluded because unusually high number of lever responding on day 1 extinction in the vehicle condition (67 lever presses/30 min >3 SD from its group mean, Exp. 4).
Exp. 1: Effect of cue-induced cocaine seeking in extinction tests on p-ERK immunoreactivity
The number of non-reinforced active lever-presses in the extinction tests (the operational measure of cue-induced cocaine seeking) was higher after 30 withdrawal days than after 1 day; this effect was strongly associated with increased p-ERK levels in ventral mPFC and modestly associated with increased p-ERK levels in dorsal mPFC (Fig. 1). We analyzed lever presses with ANOVA, using the between-subjects factor of Withdrawal Day (1, 30) and the within-subjects factor of Lever (active, inactive). This analysis revealed significant effects of Withdrawal Day (F1,19=43.5, p<0.01), Lever (F1,19=43.5, p<0.01), and Withdrawal Day by Lever (F1,19=39.9, p<0.01). The analysis of the time course of active lever responding (10 min intervals) revealed significant effects of Withdrawal day (F1,19=42.1, p<0.01), Session time (F2,38=57.7, p<0.01), and Withdrawal day by Session time (F2,38=17.9, p<0.01). The significant interaction is due to the fact that the slope of the within-session extinction curve was steeper after 30 withdrawal days than after 1 day. This pattern of results suggests that the increase in lever presses after 30 days of withdrawal is due to initial increased responsiveness to cocaine cues rather than increased within-session resistance to extinction.
We analyzed dorsal or ventral p-ERK cell counts (per mm2) with ANOVA, using the factors of Withdrawal Day and Extinction test (yes, no). Analysis of ventral mPFC revealed significant effects of Extinction test (F1,37=7.9, p<0.01) and Withdrawal Day by Extinction test (F1,37=6.8, p<0.05), but not of Withdrawal day (p>0.1). The analysis of dorsal mPFC revealed significant effects of Withdrawal Day (F1,37=4.6, p<0.05), and Extinction test (F1,37=5.9, p<0.05), but not Withdrawal Day by Extinction test (p>0.2). Post-hoc (Fisher PLSD tests) group differences are presented in Fig. 1.
Exp. 2: Effect of mPFC muscimol+baclofen injections on day 30 extinction responding
Ventral but not dorsal mPFC injections of muscimol+baclofen significantly decreased extinction responding after 30 withdrawal days; both ventral and dorsal mPFC injections of muscimol+baclofen significantly decreased local p-ERK levels (Fig. 2). We analyzed lever presses, using the between-subjects factor of Muscimol+Baclofen (vehicle, drug mixture), and the within-subjects factor of Lever. Analysis of ventral mPFC injections revealed significant effects of Lever (F1,19=48.5, p<0.01), Muscimol+Baclofen (F1,19=6.2, p<0.05), and Lever by Muscimol+Baclofen (F1,19=5.2, p<0.05). Analysis of dorsal mPFC injections revealed a significant effect of Lever (F1,4=77.6, p<0.01) but not Muscimol+Baclofen or Lever by Muscimol+Baclofen (p values>0.4). For ventral mPFC, analysis of the time course of active lever responding (10 min intervals) revealed significant effects of Session time (F2,38=41.2, p<0.01), Muscimol+Baclofen (F1,19=5.7, p<0.05), but not Session Time by Muscimol+Baclofen (p>0.4). For dorsal mPFC, the time course analysis revealed significant effects of Session time (F2,28=35.3, p<0.01), but not Muscimol+Baclofen or Session Time by Muscimol+Baclofen (p>0.4). Finally, ventral mPFC muscimol+baclofen injections decreased p-ERK levels in ventral (F1,19=18.3, p<0.01) but not dorsal (p>0.1) mPFC, while dorsal mPFC muscimol+baclofen injections decreased p-ERK levels in dorsal (F1,14=9.7, p<0.01) but not ventral (p>0.1) mPFC.
Exp. 3: Effect of mPFC bicuculline+saclofen injections on day 1 extinction responding
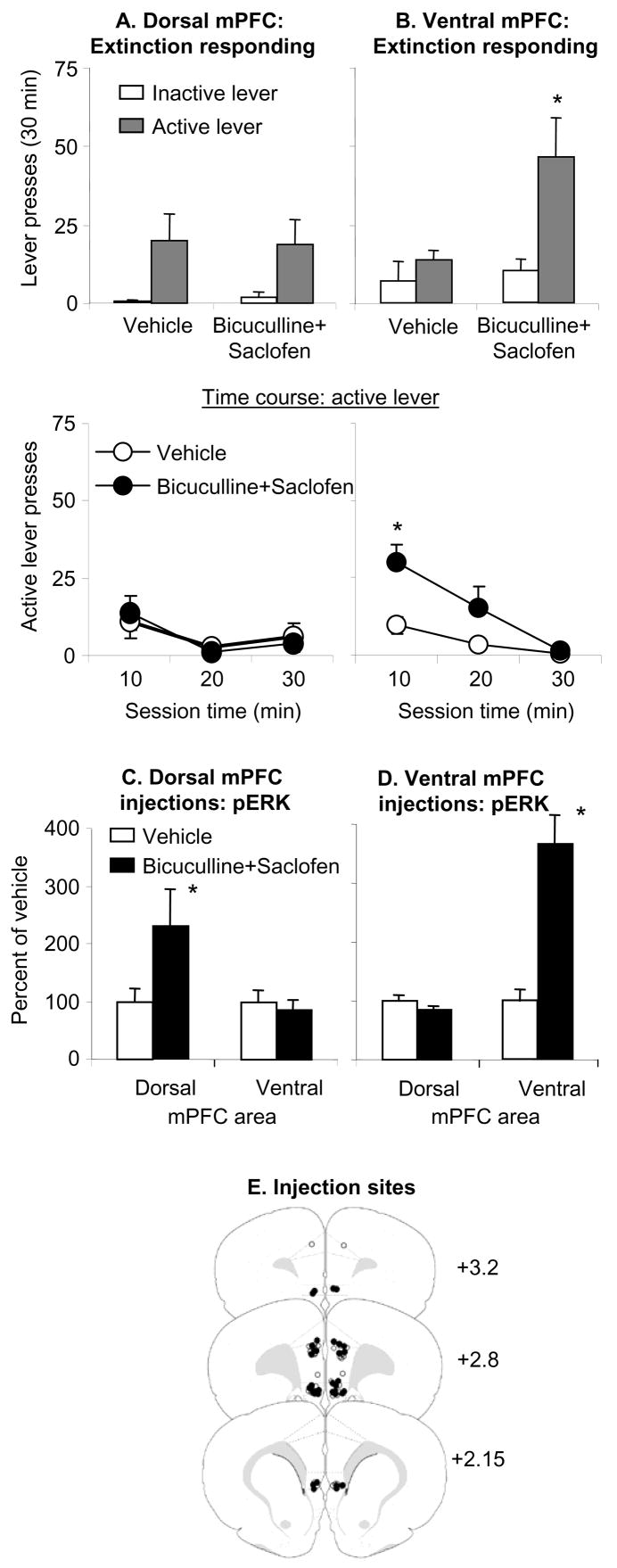
Ventral but not dorsal mPFC injections of bicuculline+saclofen increased extinction responding after 1 withdrawal day; both ventral and dorsal mPFC injections of bicuculline+saclofen increased local p-ERK levels (Fig. 3). We analyzed lever presses using the between-subjects factor of Bicuculline+Saclofen (vehicle, drug mixture) and the within-subjects factor of Lever. Analysis of ventral mPFC injections revealed significant effects of Lever (F1,23=11.9, p<0.01), Bicuculline+Saclofen (F1,23=4.4, p<0.05), and Lever by Bicuculline+Saclofen (F1,23=5.5, p<0.05). The significant interaction is due to the fact that the slope of the within-session extinction curve was steeper after bicuculline+saclofen injections than after vehicle injections. Analysis of dorsal mPFC injections revealed a significant effect of Lever (F1,9=13.0, p<0.01), but not of Bicuculline+Saclofen or Lever by Bicuculline+Saclofen (p values>0.4). For ventral mPFC, analysis of the time course of active lever responding (10 min intervals) revealed significant effects of Session time (F2,46=16.4, p<0.01), Bicuculline+Saclofen (F1,23=6.2, p<0.05), and Session Time by Bicuculline+Saclofen (F2,46=4.3, p<0.05). For dorsal mPFC, the time course analysis of active lever responding revealed significant effects of Session time (F2,18=5.8, p<0.05), but not Bicuculline+Saclofen or Session Time by Bicuculline+Saclofen (p>0.4). Finally, ventral mPFC Bicuculline+Saclofen injections increased p-ERK levels in ventral (F1, 22=22.7, p<0.01) but not dorsal (p>0.1) mPFC, while dorsal mPFC Bicuculline+Saclofen injections increased p-ERK levels in dorsal (F1,9=5.6, p<0.05) but not ventral (p>0.1) mPFC.
Figure 3. Effect of dorsal and ventral mPFC bicuculline+saclofen injections on day 1 extinction responding.
(A–B) Test for cocaine seeking after dorsal or ventral mPFC injections of vehicle or a mixture of bicuculline+saclofen (50+50 ng/side). Data are mean±SEM number of non-reinforced presses on the previously active lever and on the inactive lever during the extinction tests. (C–D) pERK levels in the dorsal and ventral mPFC after dorsal or ventral mPFC injections of vehicle or bicuculline+saclofen. Data are depicted as a percent of the mean±SEM of the vehicle condition. Ventral but not dorsal mPFC injections of bicuculline+saclofen increased extinction responding; both ventral and dorsal mPFC injections of bicuculline+saclofen increased local p-ERK levels. (E) Approximate anatomical location of the injectors for vehicle (open circles) and (closed circles) bicuculline+saclofen. Numbers indicate approximate anterior-posterior distance in mm from Bregma. * Different from vehicle, p<0.05 (n= n=5–6 and 12–13 for dorsal and ventral mPFC groups, respectively).
Exp. 4: Effect of ventral mPFC injections of muscimol+baclofen on day 1 extinction responding and effect of ventral mPFC injections of muscimol+baclofen and bicuculline+saclofen on sucrose self-administration
Day 1 extinction responding
Ventral mPFC injections of muscimol+baclofen had minimal effect on extinction responding after 1 withdrawal day. The analysis revealed a significant effect of Lever (F1,17=29.5, p<0.01) but not Muscimol+Baclofen or Lever by Muscimol+Baclofen (p values>0.4). The mean±SEM number of active and inactive lever presses in the vehicle and the muscimol+baclofen conditions were 15.5± 4.6, and 0.1± 0.1, and 12.8± 2.8, and 0.3± 0.1, respectively (n=8–11).
Sucrose self-administration
Ventral mPFC injections of muscimol+baclofen or bicuculline+saclofen had minimal effect on sucrose self-administration (Fig. 4). We analyzed lever presses using the within-subjects factors of Drug Condition (vehicle, muscimol+baclofen and bicuculline+saclofen) and Lever. This analysis revealed a significant effect of Lever (F1,4=52.3, p<0.01) but not Drug Condition or Lever by Drug Condition (p values>0.4).
Discussion
The present results implicate ventral mPFC activity in the incubation of cocaine craving, the progressive increase in cue-induced cocaine-seeking after withdrawal. Exposure to cocaine cues in extinction tests increased ventral mPFC p-ERK immunoreactivity (a neuronal activity marker) after 30 days but not 1 day of withdrawal. Inhibition of ventral mPFC neuronal activity by muscimol+baclofen decreased cue-induced cocaine-seeking after 30 days but not 1 day of withdrawal, while local stimulation of neuronal activity by bicuculline+saclofen increased cocaine-seeking after 1 day of withdrawal. In contrast, a role of dorsal mPFC in incubation of cocaine craving has not been established in our study. Exposure to cocaine cues modestly increased dorsal mPFC p-ERK immunoreactivity after 30 days of withdrawal. However, inhibition of dorsal mPFC neurons by muscimol+baclofen injections did not significantly decrease cue-induced cocaine seeking after 30 days of withdrawal, and stimulation of these neurons by bicuculline+saclofen injections did not increase cocaine seeking after 1 day of withdrawal.
Ventral mPFC muscimol+baclofen or bicuculline+saclofen injections had no effect on inactive lever-presses during testing or active lever presses for oral sucrose in rats previously trained to self-administer cocaine. Thus, it is unlikely that the effect of these injections on lever presses in the extinction tests is due to non-specific decreases or increases, respectively, in motor performance. However, this conclusion should be made with caution, because when the operational measure of drug seeking is lever responding, it is impossible to completely rule out that the effect of pharmacological manipulations on drug seeking is due to alterations in motor performance (for a discussion see Shalev et al., 2002). Below, we discuss our findings in reference to ventral mPFC role in cue-induced relapse to drug seeking and conditioned responses in extinction, and the brain circuitry of incubation of cocaine craving.
Role of ventral mPFC in cue-induced relapse to drug seeking
In the present study we identified a role of ventral mPFC in cue-induced cocaine seeking, as assessed in a single extinction test in the presence of discrete (tone-light) and contextual (self-administration chamber) cues previously associated with cocaine self-administration. These findings are surprising in view of results from studies using the reinstatement procedure where cue-induced drug seeking is typically assessed after 1–3 weeks of extinction of lever presses in the absence of the drug-paired (discrete or contextual) cues (Crombag et al., 2008; See, 2002). Using variations of this procedure, See and colleagues (Fuchs et al., 2005; McLaughlin and See, 2003) reported that reversible inactivation (muscimol+baclofen or tetrodotoxin) of dorsal but not ventral mPFC attenuates context-induced and discrete-cue-induced reinstatement of cocaine seeking. Additionally, in opposite direction to the present results, Peters et al. (2007) reported that muscimol+baclofen inactivation of the ventral mPFC potentiates spontaneous recovery of cocaine seeking 4 weeks after termination of extinction training (see also Rhodes and Kilcross (2004) for similar results in an appetitive (food) Pavlovian conditioning task). Spontaneous recovery refers to the resumption of the extinguished conditioned response that occurs after time has passed following the conclusion of extinction (Bouton and Swartzentruber, 1991). What might account for the different results between our studies and these previous studies regarding ventral and dorsal mPFC role in cue-induced cocaine seeking?
One issue to consider is that extinction training can reverse neuroadaptations (hypothesized to increase drug seeking) that normally occur after cocaine self-administration (Schmidt et al., 2001; Self et al., 2004; Sutton et al., 2003). Based on the work of Self and colleagues, and the findings that muscimol+baclofen inhibition of dorsal mPFC and accumbens core (both block discrete-cue-induced reinstatement after extinction) had no effect on early extinction responding after 2–3 weeks of withdrawal from cocaine, it has been hypothesized that the neurocircuitry of reinstatement after extinction is different from that controlling early extinction responding after withdrawal (Fuchs et al., 2006; Kalivas et al., 2006; Peters et al., 2007; See et al., 2007). Our negative results with muscimol+baclofen inactivation or bicuculline+saclofen stimulation of the dorsal mPFC are compatible with this hypothesis and with these previous results.
However, the degree to which the “differential neurocircuitry hypothesis” is relevant to the understanding of the neuronal mechanism of incubation of cocaine craving is unknown. As mentioned in the Introduction, this incubation has been demonstrated in both extinction (either in the presence or absence of discrete cues) and cue-induced reinstatement tests (Lu et al., 2004c). Furthermore, lever presses in extinction (without tone-light cue) and cue-induced reinstatement tests follow a similar time course after withdrawal and are highly correlated (Grimm et al., 2001; Lu et al., 2004c). These observations suggest that while different sets of cues induce cocaine seeking in these tests, they likely provoke a similar motivational state (craving) that incubates over time. Thus, to the degree that ventral mPFC is critical for incubation of cocaine craving, we predict that inactivation of this brain area would also decrease enhanced cue-induced reinstatement of cocaine seeking after prolonged withdrawal. Recent data of De Vries and colleagues (Van den Oever et al., 2008) tentatively support this prediction. After 21 withdrawal days, they observed heroin-cue-induced acute synaptic plasticity changes in ventral mPFC in rats exposed to either a single extinction session or cue-induced reinstatement tests after extinction. At this withdrawal time point, there is evidence for incubation of heroin craving (Shalev et al., 2001).
Another issue to consider is that in the reinstatement studies described above from the laboratories of Kalivas and See, the rats were trained to self-administer cocaine under limited access (2h/d) conditions, while in our study the duration of training was for 6 h/d (an extended access condition). There is evidence that limited and extended access to cocaine lead to different patterns of drug intake (non-escalated versus escalated intake over time) (Ahmed and Koob, 2005), and neuroadaptations in nucleus accumbens (Ferrario et al., 2005) and lateral hypothalamus (Ahmed et al., 2005). However, in our opinion it is unlikely that differences in cocaine intake during training can account for the different roles of ventral mPFC in cue-induced drug seeking in a single extinction test after prolonged withdrawal versus cue-induced reinstatement of cocaine seeking after extinction. Both cue-induced reinstatement and incubation of cocaine craving are reliably observed in rats with a history of limited and extended cocaine access (Kippin et al., 2006; Sorge and Stewart, 2005), and unlike the nucleus accumbens and lateral hypothalamus, the amount of cocaine intake during training has a minimal effect on cocaine-induced neuroadaptations in prefrontal cortex neurons (Ahmed et al., 2005; Ferrario et al., 2005).
Finally, an impressive body of work from fear conditioning studies of Quirk and colleagues indicates that ventral mPFC activity controls extinction learning: permanent lesions or reversible inactivation of ventral mPFC increase resistance to extinction, while electrical stimulation of ventral mPFC facilitates extinction responding (Milad and Quirk, 2002; Quirk and Mueller, 2008; Sierra-Mercado et al., 2006). This pattern of results appears contradictory to our findings where ventral mPFC inactivation decreased extinction responding while local stimulation increased this responding. In reconciling this apparent contradiction, it is important to make a distinction between two types of behaviors assessed in extinction studies. In fear conditioning studies, the ventral mPFC’s role has been primarily assessed in the recall of extinction learning in subsequent extinction sessions, while in our study the behavior of interest is the expression of the conditioned response in the initial extinction session. With this distinction in mind, our findings that inhibition of ventral mPFC decreased the expression of the response to cocaine cues in an initial extinction session are in agreement with several previous aversive and appetitive conditioning studies. Petrovich et al. (2007a, b) reported that permanent ventral mPFC lesions decrease initial extinction responding to food cues. Additionally, Quirk and colleagues, and other investigators, reported that reversible inactivation of ventral mPFC or local protein kinase A inhibition decrease conditioned freezing to fear cues in the initial extinction session (Akirav et al., 2006; Corcoran and Quirk, 2007; Mueller et al., 2008; Sierra-Mercado et al., 2006).
Implications of the present findings to the neuroanatomy of incubation of cocaine craving
At a circuit level, a question that arises from the present data on ventral mPFC role in the incubation of cocaine craving is how our findings fit with results from previous studies on the neuroanatomy of this incubation. As mentioned before, we previously found that incubation of cocaine craving involves glutamate-mediated increases in ERK activity in central amygdala (Lu et al., 2005; Lu et al., 2007). Thus, increased neuronal activity in both ventral mPFC (present study) and central amygdala appears critical for incubation of cocaine craving. However, while there are excitatory projections from ventral mPFC to central amygdala (McDonald et al., 1996), electrophysiological studies in anesthetized rats have shown that ventral mPFC stimulation inhibits central amygdala activity (Quirk et al., 2003). These data are incongruent with the notion that exposure to cocaine cues can simultaneously increase neuronal activity in ventral mPFC and central amygdala after prolonged withdrawal. However, electrophysiological data in anesthetized rats should be interpreted with caution, because brain neural activity patterns under anesthesia often do not accurately represent neuronal activity in the awake state (Kiyatkin, 1985; Kiyatkin and Rebec, 2001; Kreuter et al., 2004; Windels and Kiyatkin, 2006). Thus, the nature of the interaction between cocaine cue-induced increased activity in ventral mPFC and central amygdala during the tests for incubation of craving is currently unknown.
Recently, results from two studies implicate nucleus accumbens activity in the incubation of cocaine craving. Hollander and Carelli (2007) reported that in rats that demonstrated incubation of cocaine craving, exposure to cocaine cues increased neuronal activity in accumbens core but not shell after 30 days (but not 1 day) of withdrawal. Conrad et al. (2008) reported that incubation of cocaine craving is associated with a large increase (180–220%) in GluR1 AMPA surface expression in accumbens core and shell, suggesting the formation of calcium permeable GluR2-lacking receptors that are highly sensitive to excitatory stimulation (Cull-Candy et al., 2006). They provided electrophysiology evidence suggesting the formation of these receptors, and also demonstrated that accumbens core injections of NASPM, an antagonist of GluR2 lacking AMPA receptors (Blaschke et al., 1993), decreased enhanced extinction responding after 45 days (but not 1 day) of withdrawal. The relationship between these findings (potentially implicating accumbens core in incubation of cocaine craving) and our data (implicating ventral mPFC in this incubation) is unknown, because the predominant cortical projections to the core and shell are from the dorsal mPFC and ventral mPFC, respectively (Berendse et al., 1992; Voorn et al., 2004). However, critical anatomical experiments to rule out accumbens shell role in incubation of cocaine craving were not performed. Additionally, the ventral mPFC area inactivated in our study also partially innervates the medial part of accumbens core (Berendse et al., 1992; Voorn et al., 2004). Thus, we speculate that an excitatory projection from the ventral mPFC to the accumbens likely plays a role in the incubation of cocaine craving.
Concluding remarks
Our data indicate that the ventral but not dorsal mPFC plays a role in the incubation of cocaine craving. The present data are surprising in light of previous studies implicating the dorsal but not ventral mPFC in cue-induced reinstatement of cocaine seeking after extinction of the drug-reinforced responding. Interestingly, recent studies implicate the ventral mPFC in cue-induced relapse to heroin seeking (Rogers et al., 2008; Van den Oever et al., 2008), suggesting an important role of this brain area in drug relapse.
Acknowledgments
Research was supported by the National Institute on Drug Abuse, Intramural Research Program. The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript. We thank Sam Golden for excellent technical support, and Greg Quirk, Devin Mueller, and other members of the Quirk laboratory for their helpful comments on an early version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology. 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, Bogucka-Bonikowska A, Kostowski W. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Blaschke M, Keller BU, Rivosecchi R, Hollmann M, Heinemann S, Konnerth A. A single amino acid determines the subunit-specific spider toxin block of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor channels. Proc Natl Acad Sci U S A. 1993;90:6528–6532. doi: 10.1073/pnas.90.14.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clin Psychol Rev. 1991;11:123–140. [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress-and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci (USA) 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JR, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008 doi: 10.1038/nature06995. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Trans R Soc Lond B: Biol Sci. 2008 doi: 10.1098/rstb.2008.0090. accepted pending revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1007–1016. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47 Suppl 1:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of the cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans AH, Hijzen TH, Welborn PF, Olivier B, Slangen JL. Effects of infusion of cholinergic drugs into the prefrontal cortex area on delayed matching to position performance in the rat. Brain Res. 1996;711:102–111. doi: 10.1016/0006-8993(95)01404-7. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharamacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6:339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Chemoreactive properties of neurons of the medial thalamus in rats during urethane narcosis, wakefulness and restraint stress. Journal Higher Nerv Activity. 1985:35. [Google Scholar]
- Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience. 2001;102:565–580. doi: 10.1016/s0306-4522(00)00492-9. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, Poling J, Oliveto A. “Incubation” of cocaine relapse during a disulfiram clinical trial. CPDD, Annual Meeting Abstracts; 2005. p. 90. [Google Scholar]
- Kreuter JD, Mattson BJ, Wang B, You ZB, Hope BT. Cocaine-induced Fos expression in rat striatum is blocked by chloral hydrate or urethane. Neuroscience. 2004;127:233–242. doi: 10.1016/j.neuroscience.2004.04.047. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004a;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004b;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004c;47 Suppl 1:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. JNeurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Mead AN, Zamanillo D, Becker N, Stephens DN. AMPA-receptor GluR1 subunits are involved in the control over behavior by cocaine-paired cues. Neuropsychopharmacology. 2007;32:343–353. doi: 10.1038/sj.npp.1301045. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology. 2007 doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Gallagher M. Control of food consumption by learned cues: A forebrain-hypothalamic network. Physiol Behav. 2007;91:397–403. doi: 10.1016/j.physbeh.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci. 2007;27:6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11:611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21:RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology. 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking under opioid maintenance: the effects of opioid withdrawal, heroin priming and stress. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology. 2005;183:210–217. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H. Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 211–227. [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1998. [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Gouriounova M, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer ANM, Mansvelder HD, Smit AB, Spijker S, De Vries TJ. AMPA receptor plasticity is critical for conditioned relapse to heroin seeking. 2008 doi: 10.1038/nn.2165. Submitted. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. Dopamine action in the substantia nigra pars reticulata: iontophoretic studies in awake, unrestrained rats. Eur J Neurosci. 2006;24:1385–1394. doi: 10.1111/j.1460-9568.2006.05015.x. [DOI] [PubMed] [Google Scholar]
- Youtz REP. The change with time of a Thorndikian response in the rat. J Exp Psychol. 1938;23:128–140. [Google Scholar]