Abstract
Similar to patients with ventromedial prefrontal cortex (VMPC) lesions, substance abusers show altered decision-making, characterized by a tendency to choose the immediate reward, at the expense of negative future consequences. The somatic-marker model proposes that decision-making depends on neural substrates that regulate homeostasis, emotion and feeling. According to this model, there should be a link between alterations in processing emotions in substance abusers, and their impairments in decision-making. A growing evidence from neuroscientific studies indicate that core aspects of addiction may be explained in terms of abnormal emotional/homeostatic guidance of decision-making. Behavioural studies have revealed emotional processing and decision-making deficits in substance abusers. Neuroimaging studies have shown that altered decision-making in addiction is associated with abnormal functioning of a distributed neural network critical for the processing of emotional information, and the experience of “craving”, including the VMPC, the amygdala, the striatum, the anterior cingulate cortex, and the insular/somato-sensory cortices, as well as non-specific neurotransmitter systems that modulate activities of neural processes involved in decision-making. The aim of this paper is to review this growing evidence, and to examine the extent of which these studies support a somatic-marker theory of addiction. We conclude that there are at least two underlying types of dysfunctions where emotional signals (somatic-markers) turns in favor of immediate outcomes in addiction: (1) a hyperactivity in the amygdala or impulsive system, which exaggerates the rewarding impact of available incentives, and (2) hypoactivity in the prefrontal cortex or reflective system, which forecasts the long-term consequences of a given action.
Keywords: Decision-making, Addiction, Somatic states, Craving, Ventromedial Prefrontal Cortex, Amygdala, Insula, Dopamine, Serotonin
1. Introduction
Various models have been applied to the phenomenon of compulsive drug use, but in so far as they are rooted in neuropsychology, compulsive drug use can be described as a condition associated with dysfunctional brain mechanisms that subserve the ability to make decisions. Thus the decision-making capacity of drug abusers is seen as similar to that of patients with mesial orbitofrontal/ventromedial prefrontal cortex lesions (VMPC) characterized by marked obliviousness to the long term consequences of their decisions, and failure to learn from repeated mistakes. Such patients tend to preserve normal intelligence, memory and other complex cognitive functions, but their ability to experience and express emotions normally, and their social behaviour, undergo marked changes. These patients begin to make choices that often lead to financial losses, loss in social standing, and even loss of family and friends. By comparing the cognitive and behavioural profiles of VMPC lesion patients and those of individuals with substance abuse or dependence problems, an argument has been made that individuals with drug abuse or dependence problems are afflicted with a decision-making impairment reminiscent of that of VMPC patients, such that when confronted with a decision that involves a conflict between an immediate reward but a long term negative consequence, these patients tend to choose the immediate reward, at the expense of severe negative future consequences. Thus individuals with substance abuse or dependence problems share with VMPC patients a certain degree of “myopia” for the future. Furthermore, like VMPC patients, these individuals seem unaware that they have a problem; they tend to deny it, minimize it, or have a hard time explaining their behavior (Verdejo-García and Pérez-García, 2008).
The aim in this article is to apply a “somatic marker” model of addiction to explain the “myopia for the future” manifested in the behavioural decisions of many individuals with a history of chronic drug use. We will first outline the neural framework of the somatic marker model and the scientific evidence that support its validity. Second, we will review the neuropsychological, pharmacological, structural and functional imaging studies on drug addiction, and we will illustrate how the wide range of findings from such studies can be understood in terms of the proposed model of addiction.
2. A somatic marker model of drug addiction
2.1. Overview
The somatic marker framework originally proposed by Damasio (1994) provides a systems-level neuroanatomical and cognitive framework for decision-making, and for choosing according to long-term outcomes rather than short-term ones. The term “somatic” refers to the collection of body- and brain-related responses that are hallmarks of affective and emotional responses. Somatic markers are a special instance of feelings generated from emotions and feelings that have been connected by learning to anticipated future outcomes of certain scenarios. When a negative somatic marker is juxtaposed to a particular future outcome the combination functions as an alarm bell. When a positive somatic marker is juxtaposed instead, it becomes a beacon of incentive. This is the essence of the Somatic Marker Hypothesis (Damasio, 1994).
We attribute drug users’ difficulty to make advantageous decisions in real-life to a defect in the neural circuitry that subserves the action of this affective/emotional mechanism (somatic markers). More specifically, the conflict of whether to decide to take a drug or not is resolved when somatic signals triggered by either the impulsive neural system, in which the amygdala is a key structure, or the reflective neural system, in which the mesial orbitofrontal/VMPC is a key structure, prevail. The amygdala detects or recognizes the environmental features that are potential sources of immediate pleasures, or satisfaction of homeostatic needs, such as an immediate stress or withdrawal relief (Koob and Le Moal, 2005; Sinha and Li, 2008). In turn, this triggers responses in other brain areas that may become translated into feelings of desire, anticipation, and urges to seek the drug right at that moment. It is important to note that the operation of the reflective system, in which the VMPC is a key structure, is dependent on the integrity of two sets of neural systems: one is critical for working memory and its executive processes (inhibition, planning, cognitive flexibility), in which the DLPFC is a critical neural substrate. The other system is critical for processing emotions, in which the insular cortex, and the posterior cingulate (precuneate area) are key structures (Bechara and Van der Linden, 2005). The VMPC serves the role of coupling these two systems together. Damage or dysfunction of either of these systems, including the DLPFC, can indirectly alter the normal function of the VMPC, and thus impair the normal operation of the reflective system. In prior studies, we have shown that impaired working memory and other executive functions in substance abusers can also lead to poor decision-making capacity, which is known to primarily depend on the integrity of the VMPC (Bechara and Martin, 2004; Verdejo-García and Pérez-García, 2007a, b). However, it is important to note that the relationship between decision-making and working memory (and its executive processes) is asymmetrical in nature, i.e., poor decision-making, linked to the VMPC, can occur independent of any working memory deficits; however, poor working memory compromises decision-making (Bechara and Van der Linden, 2005).
Thus during the process of pondering decisions, the immediate and future prospects of an option may trigger numerous affective/emotional (somatic) signals that conflict with each other. However, at the end of the process, an overall positive or negative signal emerges. The mechanism that determines the valence of this dominant signal has been suggested to be consistent with the principles of natural selection (Bechara, 2005; Paulus, 2007). In other words, stronger signals gain selective advantage over weaker ones until a winner takes all, and an overall, more dominant, pattern of affective signaling emerges, which then can act on appropriate neural systems to modulate feeling, cognition and the behavioral decision as to whether to seek the drug or not. There are at least two underlying types of dysfunctions where this overall signal turns in favor of immediate outcomes: (1) a hyperactivity in the amygdala or impulsive system, which exaggerates the rewarding impact of available incentives, and (2) hypoactivity in the prefrontal cortex or reflective system, which forecasts the long term consequences of a given action. Individuals with substance dependence may be afflicted with either one or both of these dysfunctions.
2.2. Induction of Somatic States: Role of the Amygdala, Insula, and Prefrontal Cortex
Somatic states can be induced from (1) primary inducers, and (2) secondary inducers. Primary inducers are innate or learned stimuli that induce pleasurable or aversive (somatic) states automatically and obligatorily. The actual encounter of a drug by a drug user is an example of primary inducers. We have argued that the amygdala is a critical substrate in the neural system necessary for triggering somatic states from primary inducers. This somatic state is evoked via effector structures such as the hypothalamus and autonomic brainstem nuclei that produce changes in internal milieu and visceral structures along with other effector structures such as the ventral striatum (which includes the nucleus accumbens), periacqueductal gray (PAG), and other brainstem nuclei, which produce changes in facial expression and specific approach or withdrawal behaviors (Bechara et al., 2003). A recent event-related fMRI study has shown that cocaine abusers display increased activation of the amygdala and the ventral striatum in response to “unseen” cocaine images presented outside the attention time-window (roughly <33 miliseconds) (Childress et al., 2008). This pre-attentive response has been also linked to amygdala activity in phobics exposed to “unseen” images of the phobic object (Öhman et al., 2001). Both cases reflect the automaticity of the emotional signals triggered by the amygdala to content-specific primary inducers.
When a drug user encounters drug cues, affective/emotional (somatic) signals triggered by these cues may remain unconscious, or they may become subjectively experienced as a feeling of desire, anticipation, or urge to take the drug. It has been suggested that the insula plays a key role in translating the raw physiological signals that are the hallmark of a somatic state into what one subjectively experiences as a feeling (Damasio, 1994; Craig, 2002). However, additional neural regions have also been suggested to be important for this process, and this includes the adjacent somatosensory cortices (referred to as S2 and S1), as well as the cingulate cortex, and specifically the posterior cingulate (or precuneate region) (Damasio, 1994). This same neural system is also important for the development of somatic state patterns that may be evoked at a future time when recalling a previous emotional experience. In other words, after a somatic state has been triggered by a primary inducer and experienced at least once, a pattern for this somatic state is formed. The subsequent presentation of a stimulus that evokes memories about a specific primary inducer will then operate as a secondary inducer. Secondary inducers are presumed to re-activate the pattern of somatic state belonging to a specific primary inducer. For example, recalling or imagining the experience of a drug re-activates the pattern of somatic state belonging to the actual previous encounter of that drug. This notion has been elaborated in the I-RISA model of addiction (Goldstein and Volkow, 2002), which posits that the enactment of secondary inducers activates the orbitofrontal and cingulate cortices producing an increase of the craving sensation and possibly a decrease in inhibitory control. However, the somatic state generated by the recall or imagination of using a drug (secondary inducer) is usually fainter than one triggered by an actual use of that drug (primary inducer).
Provided that somatic state representations in the insula (and related somatosensing cortices) develop normally, triggering somatic states from secondary inducers becomes dependent on cortical circuitry in which the mesial orbitofrontal/ventromedial prefrontal cortex (or what we define here as the VMPC) plays a critical role. While in primary induction the amygdala couples sensory features of the environmental cues to effector structures involved in the triggering of somatic states, in secondary induction the VMPC couples (1) recalled or imagined scenarios supported by neural systems important for memory, such as the DLPC (for working memory) and the hippocampus, to (2) neural systems involved in the representations of somatic states, such as the insula, somatosensory cortices, and posterior cingulate/precuneate region. Thus secondary inducers are entities generated by “thoughts” and “memories” of the primary inducer. This process is more reflective and focuses more on affective/emotional responses associated with anticipated outcomes (i.e., the reflective system) (Bechara, 2005). The recall or imagination of a drug experience, or reflecting on the awful feeling of hang over and drug withdrawal, are all examples of secondary inducers. There is growing evidence that the VMPC becomes dysfunctional in chronic drug users (Franklin et al., 2002; Matochik et al., 2003; Volkow et al., 2003). Moreover, functional neuroimaging studies have demonstrated prominent VMPC activation when drug users are exposed to drug-related cues (Wang et al., 1999), or when they undergo pharmacological challenges that mimic the effect of the drug of choice (Adinoff et al., 2001). These results establish a link between chronic drug use and VMPC function, which is known to be critical for making decisions that are advantageous in the long term.
In light of more recent evidence showing that strokes that damage the insula tend to literally wipe out the urge to smoke in individuals previously addicted to cigarette smoking (Naqvi et al., 2007), we may have to refine the specific role played by the insula in addiction. Anatomically, the insula is well connected to both the impulsive system (where the amygdala is a key structure) and the reflective system (where the VMPC is a key structure). However, as indicated earlier, the insula, and especially the anterior insula, is also anatomically organized in such a way that it receives signals from the entire viscera (i.e., the body proper). Thus incentive stimuli (e.g., drug cues) can generate motivation in the individual and instigate approach responses in relation to themselves through the “impulsive system”. However, internal factors associated with deprivation states (such as withdrawal) are viewed as a “gate” that determines how effective the incentive input is in exciting the motivational circuits related to the impulsive system. This view regarding the importance of these homeostatic states in addiction has begun to draw attention (e.g., see [Paulus, 2007] for a review). This process appears to depend on the insula. Feedback loops arising from the body, reflecting the status of the viscera and homeostasis, and mediated through the insula, will adjust the strengths of activities within the impulsive and reflective systems, thereby sensitizing the impulsive system, and potentially over-riding the inhibitory control of the reflective system. An additional possibility is that increased insula signaling may “hijack” the function of the reflective system, in such a way that it forces it to formulate plans for action to seek and procure drugs in order to satisfy urgent body needs (e.g., see [Contreras et al., 2007]), instead of forecasting the negative consequences of such actions, and controlling the impulsive system.
2.3. Operation of Somatic States: Role of the Striatum, Anterior Cingulate, and Supplementary Motor Area
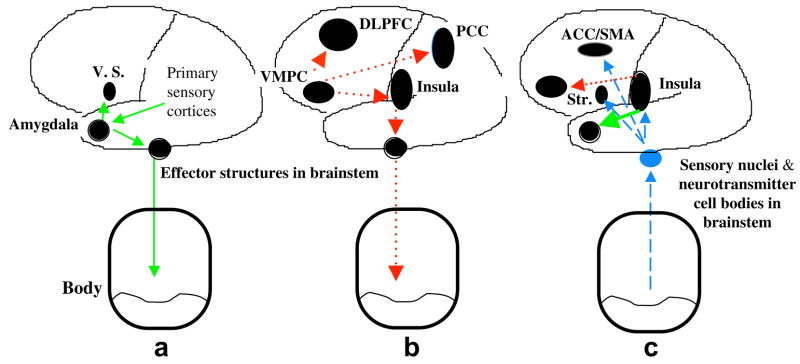
During the pondering of a decision, somatic states are triggered by primary (drug cues) or secondary inducers (thoughts about drugs and potential negative consequences associated with drug use) (See Figure 1, a and b). Once induced, they participate in at least two important functions (see Figure 1, c):
Figure 1.
A schematic model of somatic state activation and decision-making. (a) This is the neural circuitry (in green) that represents the impulsive system, in which the amygdala is a trigger structure for emotional (somatic) states from primary inducers. It couples the features of primary inducers, which can be processed subliminally (e.g., via the thalamus) or explicitly (e.g., via primary sensory cortices), with effector structures that trigger the emotional/somatic response. However, the amygdala is also directly connected to the ventral striatum (V.S.) and its trigger can also activate classical motivational systems associated with approach of drug related cues.
(b) This is the neural circuitry (in red) that represents the reflective system, in which the ventromedial prefrontal cortex (VMPC) is a trigger structure for emotional (somatic) states from secondary inducers. It couples systems involved in memory to systems involved in processing emotions, so that memories or thoughts about drug cues are linked to their emotional attributes. Memories, information, or knowledge held temporarily in working memory, and manipulated by the executive processes of working memory are dependent on the dorsolateral prefrontal cortex (DLPFC), but these working processes also include the ventrolateral prefrontal cortex as well as the lateral region of the orbitoforntal cortex. The hippocampus is also engaged, at least in situations where the memory of a scenario exceeds 40 seconds, which is the maximum capacity of the short term memory mediated by the DLPFC). Structures involved in representing previous feeling states include the Insula and surrounding somatosensory cortices, as well as the posterior cingulate cortex (PCC) and the precuneate cortex. This coupling mediated by the VMPC can also lead to the triggering of somatic states (red lines).
(c) Somatic signals triggered simultaneously by the impulsive system and reflective system compete. We argue that this competition actually occurs in the body proper, although some authors have challenged the validity of this peripheral link (e.g., see [Dunn et al., 2006] for a critique). Even if this peripheral link proves to be invalid, neuroanatomical evidence supports neural competition at the next link of the circuitry, the neurotransmitter cell bodies in the brainstem. Whether the competition of hailing somatic signals via the impulsive and reflective systems occurs in the body or the brainstem, a “winner takes all” somatic signal emerges, either positive or negative, and this resultant ascending feedback somatic signal (blue lines) participate in two functions: in one it provides a substrate for feeling the emotional state, through the insula and surrounding somatosensory cortices; in the other, it provides a substrate for biasing decisions through motor effector structures such as the striatum (Str.), and the anterior cingulate cortex (ACC) and adjacent Supplementary Motor Area (SMA). Another highly candidate region involved in this complex motor preparation is the Cerebellum (not shown in this diagram).
In light of more recent evidence on the role of the insula in cigarette smoking, we propose that the insula has a more specific role: Especially during withdrawal or drug deprivation, homeostatic signals arising in the body are perceived in the insula as feelings of urges, which in turn act on the impulsive system and sensitize it (green line), whereas its action on the reflective system is to inhibit it or “hijack” it. It remains to be determined the mechanism by which the insular activity sensitizes the impulsive system. One possibility, for example, is that neuronal signals from the insula activate the ventral tegmental area and increase the mesolimbic dopamine surge, thereby heightening the motivation to seek drugs. Such a possible mechanism can connect the insula to the classic neural systems that have been implicated in addiction (i.e., the mesolimbic dopamine system).
(i) Feeling the somatic state
From several recent studies, the insular cortex has emerged as a key neural substrate involved in translating the raw physiological signals associated with an emotion into what becomes a subjectively experienced as a feeling (Craig, 2002; Damasio, 1994). This can include the subjective feeling that accompanies drug use (not action related), or perhaps what has been referred to as the “liking” effect of drug use (Berridge and Robinson, 1995), and also the subjective feeling associated with the action of seeking, obtaining, and consuming the drug, i.e., the feeling of an urge or desire for the drug; or what has been referred to as the “wanting” effect of drug use (Berridge and Robinson, 1995). Although the insula may be a necessary structure for translating visceral signals into a subjective feeling, evidence suggests that it may not be the only structure that mediates this function. For instance, studies on the subjective experience of pain has shown that in addition to the insular and adjacent somatosensory cortices, the discomfort and the desire to avoid the pain, so called “pain affect”, was also attributed to the supracallosal sector of the anterior cingulate (Rainville et al., 1997). Similarly, studies have revealed changes in activity in the insular and somatosensory cortices in association with the “high”, or euphoric experience of acute doses of opiate and stimulant drugs (Breiter et al., 1997; Risinger et al., 2005; Langleben et al., 2008; Sell et al., 2000). Furthermore, human lesion studies suggested that damage to the insula can drastically interrupt addiction to cigarette smoking, presumably by wiping out the feeling of the urge to smoke (Naqvi et al., 2007). However, other studies using functional neuroimaging have shown that the anterior cingulate is also important for the feeling associated with drug craving (Childress et al., 1999; Daglish et al., 2001, 2003). Although the anterior cingulate may compliment the insula in generating subjective feelings, it is important to note that the anterior sector of the cingulate (as opposed to its posterior and retrosplenial sectors) is anatomically in close proximity to the Supplementary Motor Area (SMA). Furthermore, a wide range of functional neuroimaging studies has linked this anterior cingulate region to conflict monitoring and motor response selection (Botvinick et al., 1999; Kerns et al., 2004; Pardo et al., 1990). Given that craving is a feeling connected with the action of seeking, obtaining, and consuming the drug (i.e., wanting), the question arises as to whether the anterior cingulate is related to the feeling associated with craving, or perhaps to the tendency to act on that feeling, i.e., to select response options and generate plans for action and behaviour.
(ii) Biasing behavioural decisions
In order for somatic signals to influence decisions, they must act on appropriate neural systems. One target for somatic state action is the striatum. The striatum has been functionally subdivided into two parts: One is the ventral striatum, and in connection with the dopamine projection that it receives from the ventral tegmental area (known as the mesolimbic dopamine projection), this region has strongly been implicated in the motivation and drive associated with the seeking of drug reward (Robinson and Berridge, 1993, 2003). The other is the dorsal striatum, which has strongly been linked to habits and learning of implicit actions, which are all characteristics of the phenomenon of addiction (Everitt and Robbins, 2005). Evidence suggests that in the striatum, the operation of somatic states is implicit, i.e., the subject learns to select a correct response, but without awareness of whether the response is correct. Using “the weather forecast task,” Knowlton et al., 1996) showed that normal and amnesic subjects implicitly learned to predict the weather without awareness of the complex rules governing performance of the task. The behavioral guidance that occurred without awareness of the rules of the task was absent in subjects with Parkinson disease (PD), who did poorly on this task. These findings have been further replicated in PD using different implicit learning tasks (Perretta et al., 2005; Wilkinson and Jahanshahi, 2007). In addition, patients whose brain damage involves both medial temporal lobes, a portion of the orbital prefrontal cortex and the anterior cingulate, but spare the striatum/basal ganglia completely, demonstrated covert, but not overt, learning of affective valences (Tranel and Damasio, 1993). These studies suggest that the striatum is both necessary (Knowlton et al., 1996) and sufficient (Tranel and Damasio, 1993) to modify behavior through the influence of somatic states at a covert (implicit) level. This supports the notion that this region plays a role in “knowledge without awareness”. This is consistent with several investigations that suggested that the amygdala-ventral striatum system is important for drug stimulus-reward (incentive) learning (Knowlton et al., 1996; White, 1996), and the control of drug-related cues over behaviour (Cador et al., 1989; Knowlton et al., 1996). Consistent with this notion, a recent fMRI experiment showed prominent amygdala, ventral striatum and ventral pallidum activation to cocaine images presented at an implicit level in cocaine dependent individuals. Furthermore, the degree of amygdala and ventral pallidum activation significantly predicted “off-scanner” conscious emotional evaluation of these same images.
Other relevant target structure for somatic state action on behaviour is the supracallosal sector of the anterior cingulate, and adjacent supplementary motor area (SMA). In the supracallosal sector of the anterior cingulate, and perhaps the adjacent supplementary motor area (SMA), the biasing mechanism of response selection is conscious or explicit, i.e., there is “action with awareness of what is right or wrong”; the decisions are “voluntary” or “willful”, and guided by knowledge, awareness, and premeditation. Evidence shows that the anterior cingulate and adjacent regions (i.e., SMA) play a role in the implementation of “voluntary” or “willful” decisions; decisions that are guided by “knowledge with awareness”, or in response selection when a wide range of novel choices is required, and when the response selection is driven by conscious/explicit knowledge (Bechara and Damasio, 2005). This conceptualization is consistent with the studies on drug abuse. Indeed, a number of studies have indicated that these areas are often involved in the experience of craving, which is related to the action of seeking the drug (Childress et al., 1999; Daglish et al., 2001).
There are other neural sites where ascending somatic signals exert influence on cognition. At the level of the lateral orbitofrontal and dorsolateral prefrontal region, the biasing mechanism of somatic states is explicit, but it is at the level of “thought” or “memory”, and not behavioral action. In other words, as one is deliberating on several options and scenarios held in their working memory, the biasing effect of somatic states is to endorse some options and reject other ones, before any of these options are translated into actions.
2.4. The biological nature of somatic markers: Role of neurotransmitter systems
Although the Damasio view has insinuated that different emotions and bodily states (or somatic states) are characterized by a unique signature of visceral responses (see (Rainville et al., 2006 for an example), the fact remains that the preponderance of evidence do not seem to support this depiction (e.g., see Cacioppo et al., 1993, 2000 for reviews). However, the cumulative evidence suggesting that there are no physiological response profiles that differentiate discrete emotions is not necessarily fatal to the concept of the somatic marker hypothesis, as interoception from visceral responses is still likely to be playing a role (see the discussion of the somatovisceral afference model of emotion – SAME – which was first proposed in 1992 to explain precisely how the undifferentiated visceral responses might produce immediate, discrete, and indubitable emotions (Cacioppo et al., 1993, 2000). Thus somatic markers can be viewed as becoming differentiated at the level of the central nervous system, and not necessarily in the periphery, although peripheral visceral input still plays a key role.
Once somatic states from primary and/or secondary inducers are induced in the body, a large number of channels convey body information to the central nervous system (e.g., spinal cord and vagus nerve). Evidence suggests that the vagal route is especially critical for relaying somatic signals to the brain (Martin et al., 2004). Although research in this area is still in progress, early evidence suggests that the biasing action of somatic states on behavior and cognition is mediated by the release of neurotransmitters. Indeed, the cell bodies of the neurotransmitter dopamine (DA), serotonin (5-HT), noreadrenaline (NA), and acetylcholine (Ach) are located in the brainstem; the axon terminals of these neurotransmitter neurons synapse on cells and/or terminals all over the cortex (Blessing, 1997). When somatic state signals are transmitted to the cell bodies of dopamine neurons, for example, the signalling influences the pattern of dopamine release at the terminals, such as in the nucleus accumbens or other regions. In turn, changes in dopamine release will modulate synaptic activities of neurons subserving behaviour and cognition within the reflective system. This chain of neural mechanisms provides a way for somatic states to exert a biasing effect on decisions (see Nieuwenhuis et al., 2004). Thus somatic markers do not cause behaviours or decisions; they only modulate and bias the mechanisms that support these functions. In other words, decisions can still be made in the complete absence of somatic states, except that the decisions are likely to be disadvantageous, and driven primarily by what the impulsive system is in favour.
The suggestion that changes in neurotransmitter levels can influence decisions is supported by several findings. A dietary reduction of dopamine function has been shown to alter decision-making performance in a double-blind placebo controlled trial in healthy volunteers (Sevy et al., 2006). Furthermore, buprenorphine but not methadone has been shown to improve decision-making in the framework of opioid substitution treatment (Pirastu et al., 2006). Manipulation of noradrenaline levels has failed to modulate complex decision-making (O’Carroll and Papps, 2003), whereas reduction of serotonin levels through tryptophan depletion has yielded mixed results (Rogers et al., 2003; Talbot et al., 2006).
3. Empirical Support for the Somatic Marker Model
The first line of empirical support comes from experiments that examined the decision-making capacity of individuals with substance dependence problems using laboratory instruments that measure decision-making, such as the Iowa Gambling Task (IGT). The neural circuitry that is critical for processing emotions (or somatic state activation) overlap considerably with that subserving decision-making, as measured by complex laboratory decision-making tasks, such as the IGT. In other words, performance on this task is impaired by damage to various key structures that make up somatic marker circuitry. Alternatively, performance of this task activates neural components of the somatic marker circuitry in functional neuroimaging studies. In a PET activation study, which examined patterns of brain activation during IGT performance in healthy participants (Ernst et al., 2002), the authors observed that decision-making was associated with increased activation in the VMPC, anterior cingulate, parietal/insular cortices and the amygdala, predominantly on the right side. Later imaging studies have confirmed and extended these findings, and implicated additional neural regions, e.g., the striatum (Verney et al., 2003), and the nucleus accumbens (Mathews et al., 2004), in processes that are critical for decision-making.
In substance dependent individuals (SDI), Bolla et al. (2003) used oxygen labelled PET to examine brain activation in 25-day abstinent cocaine dependent patients while performing the Iowa Gambling Task (IGT). Group analyses showed increased activation during IGT performance in the right orbitofrontal cortex, and less activation in the left dorsolateral cortex of cocaine patients, with regard to healthy subjects. Activation of the orbitofrontal cortex was directly correlated with better performance on both groups, and negatively correlated with amount of cocaine used in the patient group. In another study, Ersche et al. (2005) tested current opiate and amphetamine users and ex-users in the Cambridge Gamble task. SDI and matched healthy comparison participants were subjected to oxygen labelled PET while performing this decision-making task. Results revealed that drug abusers performing the risk task showed increased activation of the left orbitofrontal cortex and decreased activation of the right dorsolateral cortex (identical localization but reversed lateralization with respect to the results of the Bolla et al. study). No significant differences were found between the current users consuming pharmacologically different components (amphetamine vs. opiates). A more recent study scanned three different groups including polydrug abusers with pathological gambling, polydrug abusers without pathological gambling and healthy controls while performing a fMRI version of the IGT (Tanabe et al., 2007). Results were broadly consistent with the somatic marker framework; both groups of drug abusers showed reduced task related activation in the VMPC, the key structure to trigger somatic states associated with long-term prospects, when compared to controls. In addition, only non-gambling drug abusers had reduced superior frontal and frontal pole activation in comparison with gambling drug users and controls. This finding was supported by behavioral results, where both gamblers and controls outperformed drug abusers for the IGT learning curve.
Other studies have examined patterns of brain regional activation in abstinent methamphetamine abusers and young stimulant recreational users using a two-choice prediction task (Paulus et al., 2002, 2003, 2007). This task also taps into decision-making function under conditions of uncertainty, by requiring the prediction of an uncertain outcome, which can be predicted correctly (success) or incorrectly (failure). However, unlike the IGT or Cambridge Gamble tasks, this task does not involve incentive evaluation of rewards and punishments. Overall, the behavioural results of these studies demonstrated a more rigid stimulus driven decision-making pattern in the methamphetamine group, as opposed to a more outcome driven pattern in the healthy participants. Imaging patterns in methamphetamine individuals while performing the two-choice prediction task showed decreased activation of the orbitofrontal, dorsolateral, insular and inferior parietal cortices; orbitofrontal activation was inversely correlated with duration of methamphetamine use (Paulus et al., 2003). This decreased activation was particularly observed in low error-rate phases of the task (i.e., when the subjects successfully predicted the correct outcomes). Interestingly, the patterns of brain activation during this task were strong predictors of amphetamine relapse in a 1-year follow-up study (Paulus et al., 2005). In a later study that tested young stimulant recreational users employing the same task, results showed that these users were less able to flexibly modify their choice pattern in response to the frequency of errors, which was related to the degree of insular activation (Paulus et al., 2007).
Consistent with the somatic-marker model, we predict that addiction is a condition in which (1) the reflective system becomes weakened, and the triggering of somatic states is altered; this can arise from dysfunctions in any of the neural components of the reflective system, including working memory and its related executive processes (i.e., the DLPFC and also the ventrolateral region of the prefrontal cortex and extending inferiorly into the lateral region of the orbitofrontal cortex), the emotional systems responsible for holding representations of somatic states (e.g., posterior cingulate, insula and somatosensory cortices), or the VMPC region, which couples the two systems together (in the VMPC region we are including the mesial orbitofrontal region); (2) the impulsive system becomes sensitized; this can arise from over-activity in the amygdala system, which ultimately translates into increased activity of the dopamine system, and specifically the mesolimbic dopamine projections to the nucleus accumbens/ventral striatum; or (3) a combination of the two conditions. As indicated earlier, in light of more recent evidence showing that strokes involving the insula are associated with increased likelihood of wiping out the urge to smoke (Naqvi et al, 2007), sensitization of the impulsive system may be mediated by the insula, where internal factors associated with deprivation states (such as withdrawal) are translated into urges, and adjustment in the relative strengths of the impulsive and reflective systems.
In the remaining sections we will review the evidence that examined various aspects of the addictive syndrome and we will highlight how the results from such a variety of studies fit and support the predictions made by the somatic marker model of addiction.
3.1. Behavioural and Physiological Studies
If decision-making is a process guided by emotions (Bechara, 2004; Damasio, 1994), then there should be a link between abnormalities in expressing emotions and experiencing feelings in substance dependent individuals (SDI) on the one hand, and impairments in decision-making on the other hand.
The most frequently used paradigm to assess decision-making is the Iowa Gambling Task (IGT) (Bechara et al., 2001; Ernst et al., 2003; Grant et al., 2000; Monterosso et al., 2001; Petry et al., 1998), which was initially developed to investigate the decision-making defects of neurological patients with VMPC damage, and to provide empirical support for the somatic-marker hypothesis. This task factors a number of aspects: immediate rewards and delayed punishments, risk, and uncertainty of outcomes (Bechara, 2003). The task has been described in detail elsewhere (Bechara et al., 1994, 2000); briefly, in this task, the participants have to choose between decks of cards that yield high immediate gain but larger future loss (long-term disadvantageous decks), and decks that yield lower immediate gain but a smaller future loss (long-term advantageous decks). A number of studies that used this paradigm have shown impairments in decision-making performance among alcohol, cannabis, cocaine, opioids, MDMA and methamphetamines abusers (Bechara et al., 2001; Fein et al., 2004; Grant et al., 2000; Hanson et al., 2008; Quednow et al., 2007; Verdejo-García et al., 2007a,b; Whitlow et al., 2004). Decision-making deficits have also been reported in populations who are at high risk for drug abuse, such as drug-naive individuals with higher familiar density of alcohol abuse (Fein and Chang, 2008; Lovallo et al., 2006), adolescents with externalizing behaviour disorders (Ernst et al., 2003), or adolescent binge drinkers (Johnson et al., 2008; Goudriaan et al., 2007). Interestingly, impaired decision-making has been observed also in individuals with Antisocial Personality Disorder (APD) (Mazas et al., 2000; van Honk et al., 2002), a psychiatric disorder that is robustly associated with substance dependence, and which involves severe disturbances in emotion processing (Blair and Utah, 2000); and pathological gambling, a form of behavioral addiction where the neurotoxic effects of drugs are minimized (Goudriaan et al., 2006).
Additional evidence for impaired decision-making in SDI stems from studies that used different decision-making paradigms, including tasks of delay discounting (Kirby et al., 1999; Monterosso et al., 2001), tasks that involve betting, such as the Cambridge Gamble task (Rogers et al., 1999), and probabilistic choice tasks (Heiman and Dunn, 2002; Paulus et al., 2002, 2003). Although the behavioural results from these studies reveal evidence for impaired decision-making among SDI, they do not necessarily link poor decision-making capacity to any abnormality in emotional processing. However, other psychophysiological studies have attributed these decision-making impairments to defective processing of affective/emotional signals (or somatic markers) (Bechara et al., 1997; Crone et al., 2004). Furthermore, cognitive modelling analyses (Stout et al., 2004, 2005) strongly support the notion that affective/emotional factors associated with the processing of reward and punishment play a significant role in the decision-making performance of SDI on the IGT.
More specifically, in a series of studies using the IGT, the performance of SDI was compared to that of patients with damage to the VMPC (Bechara and Damasio, 2002; Bechara et al., 2002). These studies also included physiological measures of autonomic activity before and after making a choice in the IGT. The physiological responses triggered after making the choice and seeing the outcome (i.e., gain or loss of a certain amount of money) were called (i) reward/punishment responses; and those generated before making the choice were called (ii) anticipatory response, i.e., responses triggered during the time the participant was pondering from which deck to chose. Good performance in the IGT has been shown to be linked to the development of these anticipatory emotional responses, which in this case were changes in the skin conductance response (SCR), especially before selecting cards from the disadvantageous decks (A and B). It was suggested that these anticipatory emotional responses help guide decision-making away from disadvantageous choices (i.e., avoid decks A and B, and choose from decks C and D) (Bechara et al., 1997). In a study that used the IGT to measure behavioural decisions, and the skin conductance response to measure anticipatory and reward/punishment responses in SDI, the behavioural and physiological results revealed that there were at least two different subgroups within this SDI population (Bechara and Damasio, 2002). One subgroup of SDI (a minority of the sample) showed a behavioural profile similar to that of healthy participants, i.e., they selected more cards from the advantageous decks. They also showed a physiological profile similar to healthy participants, in that they began to trigger anticipatory SCRs before selecting cards from the bad decks. By contrast, another subgroup of SDI (a majority of the sample) exhibited behavioural and physiological profiles that were different from healthy participants, and more similar to patients with VMPC damage, i.e., they chose disadvantageously on the task, and they failed to acquire anticipatory SCRs.
In a subsequent study, a variant version of the IGT was used, in which they reversed the order of reward and punishment contingencies, so that the advantageous decks yielded high immediate punishment but even higher future reward, and the disadvantageous decks had lower immediate punishment, but even lower long-term reward (Bechara et al., 2002). The combination of the behavioral results from the original and variant tasks, in conjunction with physiological responses recorded during the performance of both tasks, helped discriminate between two other sub-groups: those who were hypersensitive to immediate reward and those who were insensitive to long-term consequences. In this way, they identified three sub-populations of SDI: one small sub-population of SDI that was indistinguishable from healthy participants, a second small subpopulation that was indistinguishable from VMPC lesion patients, and a third larger sub-population of SDI that was different from the other two; these SDI exhibited signs of hypersensitivity to reward, as evidenced by impaired performance on the original IGT, normal performance on the variant version, and abnormally high reward SCR in both tasks. Interestingly, these subpopulations did not differ in terms of basic neuropsychological abilities or clinical characteristics such as severity of drug use. A subsequent study has replicated this pattern of behavioral and physiological response using the Cambridge Gamble Task paradigm (Fishbein et al., 2005) that was designed to isolate different components of the IGT (see Clark and Robbins, 2002). In this study, polydrug users selected more risky choices in the high-risk conditions of the task, and failed to generate increased SCR responses when making riskier decisions with regard to healthy participants. Similar results have been obtained in a sample of pathological gamblers with negligible exposure to alcohol and drugs (Goudriaan et al., 2006).
It is important to note here that the problem in one of the subpopulations of SDI is not apparently linked to a primary dysfunction in the VMPC (or reflective) system itself, but rather an overactivity in the impulsive system, thereby exaggerating the incentive impact of reward stimuli. This deficit is still consistent with the proposed somatic marker model of addiction, except that the deficit is related to a different part of the neuronal circuitry. The one surprising finding is that one subpopulation of SDI seemed normal and did not exhibit any signs of “somatic marker” deficits. As a result, one argument can be made that the proposed somatic marker model does not explain all instances of addiction. We argue that the somatic marker model should explain all instances of addiction, except that this particular subgroup should not be considered as true addicts, although they meet the DSM-IV diagnostic criteria for drug dependence. We have suggested the use of the term “functional addicts” since this particular subgroup seem functional in their real-life (Bechara et al., 2002). Nonetheless, further research should be conducted to investigate if the emotional/decision-making profile of this subgroups is associated with better prognosis of their dependence problems.
Importantly, decision-making deficits are strong predictors of clinical outcome and relapse in addiction. Recent studies in alcohol and opiate users have demonstrated that poor performance on the IGT and the Cambridge Gamble Task predicts treatment retention and the probability of relapse (Bowden-Jones et al., 2005; Passetti et al., 2008). In addition, imaging studies have revealed that patterns of brain activation within the neural substrates of the somatic marker model can prospectively predict drug use relapse as far as one year after cessation of drug use (Grüsser et al., 2004; Paulus et al., 2005).
There are relatively few studies that examined emotional perception and experience, and its relationship to decision-making, in SDI. Most studies on emotional perception have focused on analyzing possible alterations in the processing of emotional facial expressions in long-term substance abusers, which seem to vary depending on drug of choice (Hoshi et al., 2004; Kemmis et al., 2007; Kornreich et al., 2001, 2003). Cocaine abusers have particular difficulties in recognizing emotional expressions of fear (Kemmis et al., 2007; Verdejo-García et al., 2007). In the first study, recently abstinent regular cocaine users had lower recognition hits for expressions of fear than occasional cocaine users and healthy controls. In the latter study, cocaine polysubstance users abstinent for at least four months had lower recognition hits for expressions of fear, surprise, and the global recognition score (including all emotions). Furthermore, the fear recognition and global scores were positively correlated with decision-making performance in the IGT, supporting the link between emotion processing and decision-making. In alcoholics, several studies have revealed significant alterations in the processing of facial expressions, although the range of emotions affected is still controversial. One study showed that alcohol dependent individuals showed specific impairments for recognizing facial expressions portraying happiness and anger (Kornreich et al., 2001). These alterations were characterized as overestimation of the intensity of the emotion displayed. By contrast, other studies showed that overestimation of the intensity of emotion in facial expressions reported by alcoholics related mainly to the facial expression of fear (Townshend and Duka, 2003); the degree of this overestimation correlated with the number of prior detoxifications. In this study, alcoholics also presented with difficulties in distinguishing between the facial expressions of anger and disgust. The significant alteration in anger and fear processing among SDI was supported by a recent fMRI study, where alcoholics showed lower task-related activation in several regions of the somatic marker neural system, including the subgenual anterior cingulate cortex (which is included in what we define here as the VMPC region), the insular cortex, the amygdala, and the striatum, when exposed to angry and fearful facial expressions (Salloum et al., 2007). Alcohol abusers also have problems in identifying prosody in sentences with incongruent semantic content, and in matching affective prosody with facial expression (Uekermann et al., 2005). In opiate users, results have shown a generally slower reaction time and poorer emotion recognition accuracy (Kornreich et al., 2003; Martin et al., 2006). Other studies have analyzed the effects of controlled acute doses of different drugs on the perception of emotions. These studies have shown that acute low doses of alcohol and MDMA can improve the recognition of emotional facial expressions in current users, although recognition accuracy significantly decreased during the following days (Hoshi et al., 2004; Kano et al., 2002). Detrimental effects of acute drug doses on the recognition of emotions in facial expressions have also been reported using ketamine (Abel et al., 2003). These results indicate that SDI are impaired in the recognition of facial expressions portraying different emotions, including fear, anger, disgust, and happiness. The poorer recognition of facial emotional expressions can affect SDI’s interpretation of social cues, so that they can be less able to manage and regulate emotions, and to make decisions and solve problems of an interpersonal or social nature. In this sense, their poor ability to recognize facial emotional expressions has been attributed to several aspects of their addictive behaviors, such as diminished empathy, increased levels of aggression (Hoshi et al., 2004), and a higher frequency of relapse and ensuing alcohol detoxification (Townshend and Duka, 2003). In particular, poor recognition of fear expressions, which is thought to depend on the amygdala, can be associated with impaired conditioning of fear responses to drug related environments, increasing the probability of relapses.
Finally, very few studies have examined the emotional experience of SDI. The most frequently used paradigm in the study of the experience of emotions in SDI is the presentation of affective images that induce emotional states, such as the International Affective Picture System (IAPS). The IAPS consists of a large set of images classified according to their normative values in three relevant dimensions: valence (indicating if the emotional response induced is pleasant or unpleasant), arousal (if the emotional response induced is arousing or relaxing), and control (if the emotional response induced can/can not be controlled by the subject). Gerra et al. (2003) used this paradigm to analyze the neuroendocrine response of SDI and healthy participants to experimentally induced pleasant and unpleasant emotions. Their results showed that in response to unpleasant images, SDI showed decreased activity in several neuroendocrine markers, including norepinephrine, cortisol, and adrenocorticotropic hormone levels. Similar results have been obtained using a different response modality, the subjective affective response to IAPS images (Aguilar de Arcos et al., 2005). SDI showed a more flattened response pattern to both pleasant and unpleasant images. SDI scored as less positive the images considered by normal participants to be very pleasant and arousing. SDI also scored as less negative the images considered by normal participants to be highly unpleasant and arousing. A subsquent study used the same IAPS images to examine the emotional experience of current heroin users enrolled in a clinical trial and using heroin as a prescribed treatment. It was observed that these current heroin users had even lower arousal response to pleasant affective images when compared to both abstinent opioid users and healthy individuals (Aguilar de Arcos et al., 2008). The fact that SDI showed such a flattened emotional response to pleasant affective images may suggest that they also have a diminished emotional response to natural reinforcers in general, except for drugs, which begin to exert exaggerated rewarding effects. This notion is strongly supported by imaging studies on craving in drug addiction, which show that drug related stimuli are able to strongly activate brain regions involved in emotional evaluation and reward processing (Garavan et al., 2000; George et al., 2001; Grant et al., 1996; Kilts et al., 2001, 2004; Wang et al., 1999; Wexler et al., 2001). In contrast, the same brain regions show blunted activation to other natural reinforcing stimuli such as food or sex (Garavan et al., 2000). Although these studies are consistent with Somatic Marker Model in that neural systems supporting affective and emotional processing become altered among SDI, the proposed model does not really explain the specificity of these alterations. In other words, the proposed model does not really explain why, for example, the trigger structures for somatic states begin to exaggerate the reward signals elicited by drug related cues, at the expense of blunting the reward signals from non-drug related rewards. However, the implications of these differences are such that somatic states associated with natural reinforcers may not be strong enough to bias decisions in SDI, while strong somatic states associated with the prospect of abusing drugs become so powerful to drive decisions towards drug use.
3.2. Neuroimaging and Neuropharmacological Studies
As mentioned earlier, it has been suggested that the mechanism by which somatic states influence decision-making is via non-specific neurotransmitter systems (Bechara, 2003; Bechara and Damasio, 2005; Blessing, 1997). The goal of this section is to review neuroimaging and neuropharmacological findings that provide support for this notion.
3.2.1. Structural and Functional Neural Abnormalities Associated with Substance Addiction
Franklin et al. (2002) were the first to use a focal structural analysis of images from the brain scans of crack-cocaine dependent individuals. They used voxel-based-morphometry analyses (VBM) from magnetic resonance imaging (MRI) in a sample of cocaine dependent individuals. They found significant decrements in grey matter concentrations (ranging from 5% to 11%) in a number of neural regions considered critical for the operations of the somatic-marker circuit, including the VMPC bilaterally, the anterior insular cortex, bilaterally, in addition to changes in some temporal cortices, and also in the right anterior cingulate cortex, which is also a target region in the somatic marker neural circuitry. Interestingly, these reductions in grey matter volume were not significantly correlated with measures of severity of drug dependence. Matochik et al. (2003) used VBM analyses to examine grey and white matter composition of the brains of abstinent cocaine dependent individuals. However, these authors focused on the analysis of tissue composition in the frontal lobe, and its main structural subdivisions: dorsolateral, cingulate, and orbitofrontal regions. Their results showed significant grey matter decrements in the lateral prefrontal cortex, cingulate gyrus/cortex, and medial and lateral aspects of the orbitofrontal cortex, predominantly in the right hemisphere. All these findings reflect changes in areas that are known as critical components of the somatic marker circuitry (Figure 1). This study also failed to report significant correlations between indices of severity of drug abuse and reductions in grey matter volume, although the years of cocaine use were associated with lower tissue density in the inferior white matter adjacent to the frontal cortex. In a later study, Makris et al. (2004) specifically measured the volume of the amygdala on both sides using segmentation-based morphometric analysis in cocaine dependent individuals. They found decreased absolute volume, primarily in the right amygdala (23% volume reductions, although total volume was also decreased); and absence of laterality asymmetry in the cocaine group. The reduced volumetric measures of the amygdala were not correlated with measures of drug use severity or co-morbid psychiatric conditions, and these volume reductions were present in all cocaine dependent individuals, including those who used the drug for a short time period (1–2 years of abuse). Furthermore, Fein et al. (2006) showed that long-term abstinent alcoholic patients who had impaired performance on the IGT also had significant reductions of grey matter in the amygdala. Alcohol users also have structural abnormalities, and reductions of the left-right assymetry in the insular cortex (Jung et al., 2007). Interestingly, gray matter density in the insula and the anterior cingulate cortices differentiated alcohol abstainers vs relapsers in a follow-up imaging study (Cardenas et al., 2007). Although all these studies reveal structural abnormalities in neural structures known to be critical for somatic state activation (or processing emotions), the critical question that remains unanswered, is whether some degree of dysfunction preceded the substance abuse condition (Glahn et al., 2007), or whether these abnormalities were the consequences of the abuse of these drugs.
Other studies have focused on white matter microstructure (Bartzokis et al., 1999; Lim et al., 2002). White matter abnormalities were consistently found in pathways that connect regions thought to be critical components of the somatic-marker circuit. For instance, Bartzokis et al. (1999), in a MRI study; they compared male cocaine dependent individuals to healthy participants. The results revealed age-related, higher incidence of white matter lesions within the insular cortex of the cocaine group. Using diffusion tensor MRI, Lim et al. (2002) analyzed the white matter composition of the different subregions of the prefrontal cortex in cocaine dependent individuals. Their results also showed altered white matter microstructure in the inferior frontal regions. These abnormalities seem to reflect disruption of functional connectivity between the VMPC and a number of paralimbic regions involved in the processing of emotional/somatic states, such as the insular cortex. These results have been recently replicated by Lyoo et al. (2004), who found white matter abnormalities in prefrontal and insular regions in cocaine dependent individuals. A recent study using diffusion tensor imaging have also demonstrated that cocaine dependent individuals present with white matter abnormalities in the anterior region of the corpus callosum, and that the severity of these abnormalities correlate with measures of impulsivity (Moeller et al., 2005). It is important to note that deficits in impulse control are often observed in both SDI and neurological patients with lesions in target regions of the somatic-marker circuit, and that these problems in impulse control may contribute to their poor decision-making abilities. Therefore, future studies should investigate the relationship between structural abnormalities and behavioural domains associated with decision-making in SDI. Another important question to be addressed in future studies is to conduct longitudinal studies to determine whether underlying brain alterations in decision-making and impulse control are pre-existing characteristics, or perhaps they are a consequence of drug abuse.
Alterations in neural regions that represent critical components of the somatic-marker circuit have also been shown in functional imaging studies, such as functional abnormalities in the frontal, parietal, and subcortical (including paralimbic) regions of the brains of SDI. Chang et al. (2002), using perfusion MRI, analyzed the regional cerebral blood flow of long-term abstinent methamphetamine dependent individuals. Their results showed decreased regional CBF in the insular cortices and inferior frontal regions, bilaterally, and in the right lateral parietal region. Nonetheless, methamphetamine dependent individuals showed increased regional CBF in the occipital, and right dorsal/posterior parietal regions. This pattern of alterations in functional activity was attenuated in the female methamphetamine abusers, relative to male drug abusers. The authors interpreted this finding as reflecting a neuroprotective effect of estrogens, in females, on the potential neurotoxic action of methamphetamine. Volkow et al. (2001) also demonstrated higher cortical (mainly parietal), and lower subcortical (thalamus and striatum) metabolism in abstinent methamphetamine abusers, although no significant gender differences were reported in this sample of drug abusers. Thus the issue of gender and vulnerability to drug abuse remains unsettled, and the question should be addressed in future research.
In a large sample of ecstasy abusers, functional abnormalities were found, bilaterally, in the cingulate cortices, amygdala, striatum, and hippocampus (Obrocki et al., 2002). Although no significant relationships were observed between severity of ecstasy use and brain regional functional activity, earlier exposure to ecstasy was associated with decreased functional activity in the amygdala and striatum. However, one should interpret with caution the effects of ecstasy on cognition since the drug is almost always co-abused with marijuana, thus rendering it difficult to determine whether the deficits are linked to ecstasy or marijuana use.
Overall, findings from both structural and functional imaging studies consistently indicate that several key neural substrates in the circuitry of somatic state activation and decision-making are affected in SDI. Decreased grey matter volumes have been detected in regions involved in the triggering of somatic markers, i.e. the amygdala and VMPC, and in the covert and overt processing of emotional (somatic) signals in the brain, i.e. the striatum, insular/anterior cingulate cortices (Franklin et al., 2002; Makris et al., 2004; Matochik et al., 2003). Additionally, studies of brain white matter of individuals addicted to substances showed abnormalities in white matter pathways that connect the VMPC to several other limbic structures involved in core aspects of somatic state activation and decision-making (Bartzokis et al., 1999; Lim et al., 2002; Lyoo et al., 2004).
One interesting and consistent finding among all these studies was the asymmetry of these abnormalities in terms of their presence in the right, as opposed to the left, hemisphere (Franklin et al., 2002; Makris et al., 2004; Matochik et al., 2004). Such a finding is congruent with the suggested predominant role of the right hemisphere in somatic state activation and decision-making (Clark et al., 2003; Tranel et al., 2002). Finally, it is also striking that almost none of these studies found significant correlations between abnormalities in some morphological and structural indices of the brain, and various measures of the severity of drug abuse, including age of onset, frequency of drug use, peak use, or amount of money spent in illegal drugs. This lack of correlations may suggest that these brain abnormalities, to some extent, precede the onset of drug use, thus predisposing the individual to drug addiction (Bechara, 2005). Nonetheless this is a very controversial issue, since some studies have been able to correlate brain imaging and drug use measures (Obrocki et al., 2002). More research is needed to settle the issue about the relationship between drug use patterns and alterations in certain brain structures and functions.
3.2.2. Neuropharmacological Changes Associated with Substance Addiction
Several studies have demonstrated that pharmacological manipulations of the dopamine, serotonin, and noradrenaline systems can significantly affect different aspects of emotional experience (Murphy et al., 2002) and decision-making (Sevy et al., 2006; Rogers et al., 2004) in healthy participants, as measured by various experimental paradigms.
In humans, there is a compelling evidence for a major role of dopamine (DA) in the increased sensitization of the incentive motivational properties of drugs of abuse (see Volkow et al., 2004 for a review). This increased sensitization to the reinforcing effects of drugs tends to facilitate the continuous administration of drugs of abuse, even when they lose their pleasurable hedonic effects (i.e., wanting vs. liking) (Robinson and Berridge, 1993, 2003), thus contributing to the reinstatement of drug consumption after protracted abstinence (Fresnois et al., 2005; Heinz et al., 2004; Lu et al., 2004; Wang et al., 1999). Recent theoretical accounts (Everitt et al., 1999, 2002) have proposed that during the sensitized state, there is an enhanced DA mediated response to the incentive motivational value of drugs in the striatum and the amygdala. This enhanced response coincides with a weakened activity with the prefrontal cortex, which reflects a weakened inhibitory control of the prefrontal cortex to the hyperactive amygdala-striatum system. This weakens the capacity of the individual to self-regulate the drug seeking behaviour, thus leading to a persistent and compulsive use of drugs, irrespective of the long-term negative consequences.
In support of this view, a number of studies using PET have shown persistent reductions of dopamine receptors at the level of the striatum in SDI, including alcohol, heroin, cocaine and methamphetamine dependent individuals (Volkow et al., 1996, 1997). Interestingly, related studies have demonstrated an association between striatal dopamine post-synaptic receptor densities and the metabolic activity of the orbitofrontal cortex in cocaine and methamphetamine addicts (Volkow et al., 2001; Wang et al., 2004). This association is proposed to reflect a higher sensitivity of the orbitofrontal cortex to dopamine modulation stemming from limbic structures involved in emotional signalling and reward processing. Also it may reflect the mechanism by which altered somatic signals may bias decision-making in SDI towards immediate reward. This notion is consistent with experiments showing increased striatal dopamine response to craving cues (Volkow et al., 2008). The reductions of dopamine receptor densities tend to recover in certain brain regions, such as the thalamus, but the reductions seem to be long lasting at the level of the striatum (Wang et al., 2004).
Abnormal DA functions in several neural regions known to be components of the somatic-marker circuit have been reported in studies showing decreased DA transporter density in the striatum (including nucleus accumbens) and prefrontal cortex of cocaine, methamphetamine and heroin dependent individuals (Volkow et al., 1997; Sekine et al., 2001; Shi et al., 2008). Other studies revealed reduced occupancy of striatal DA receptors in response to the addition of competitive radioligands (e.g. [11C]raclopride) and mimetic drugs like methylphenidate (Schlaepfer et al., 1997; Volkow et al., 1999, 2002a, b) or amphetamine (Martinez et al., 2007). These findings suggest that there are significant alterations in DA release in several neural structures, considered as key components of the somatic-marker neural circuitry, in SDI. Some investigators have interpreted these alterations as reductions in DA activity, which may explain the blunted response of SDI to a variety of natural reinforcers, beside drugs, which has been reported in several neuroimaging (Garavan et al., 2000; Martin-Soelch et al., 2001) as well as subjective self-report studies (Aguilar de Arcos et al., 2005, 2008). Furthermore, this has been used toexplain the sensitized response of addicted individuals to the reinforcing effects of drugs of abuse, since they are probably the only powerful stimuli that are capable of inducing reward (Heinz et al., 2004). However, the issue of whether reduced DA receptors really means reduced dopamine release, or in fact the opposite, i.e., increased dopamine release has been debated, and there has been a strong argument in favour of the possibility that decreased DA receptors relates to increased DA release and hyperdopmaniergic activity, which gives rise to a sensitized motivational response (e.g., see Robinson and Berridge, 1993, 2003).
The serotonin (5HT) system has also been implicated in mediating the biasing effects of somatic-markers on cognition, including decision-making (Bechara et al., 2001). Nonetheless, studies of the role of 5HT in addiction have been far less extensive in comparison to DA. Some studies have proposed that low 5HT activity contributes significantly to the dysphoric, or anhedonic, state associated with abstinence from drugs of abuse (Kish et al., 2001). Other studies showed that low 5-HT activity is associated with decreased prefrontal activity, which is necessary for exerting inhibitory or impulse control over behaviour (Robbins, 2000). Thus low 5-HT and prefrontal activity observed in SDI may relate not only to compulsive drug use, but also several to other co-morbid conditions, such as their depressed mood, or antisocial behaviour (Roiser and Sahakian, 2004; Schreckenberger et al., 1999).
3.3. Somatic Markers and Neural Correlates of Craving
People still disagree on how craving should be defined (Franken, 2003; Tiffany, 1999). We will define craving as the accompanied emotional state that is produced by emotionally competent stimuli that are associated with the reinforcing effects of drugs of abuse (Bechara, 2003). To study craving in the laboratory, a number of studies have used different strategies to induce craving. These strategies include the visual presentation of the drug, or drug related paraphernalia, through images (pictures), or videos; the auditory presentation of audio-taped scripts containing autobiographical experiences related to drug use; or the direct infusion of the drug itself. The aim of this section is to review the evidence showing an overlap between the neural structures that represent critical components of the somatic marker neural circuitry, and neural structures activated during states of craving in substance abusers.
Grant et al. (1996) used PET to study craving in recently abstinent cocaine abusers. In the scanner, participants were shown videos of drug related paraphernalia, and cocaine self-administration. A pattern of significantly increased activation associated with craving was detected primarily in frontal (VMPC and dorsolateral –DLPC), parietal, temporal, and striatal regions. The correlation analyses showed that the subjective response of craving was associated with changes in the activation of the amygdala in cocaine abusers. A follow up study by the same group (Bonson et al., 2002) used auditory presentation of drug cues, i.e., a script describing sensations associated with being “high” on cocaine. The results revealed increased activation in the DLPC, orbitofrontal cortex, amygdala and adjacent entorhinal cortices. The correlation analyses showed that the subjective response of craving correlated with the degree of activity within these neural regions.
Numerous subsequent PET studies in cocaine abusers have also revealed a significant overlap between the neural regions activated during craving and those considered as components of the somatic marker neural circuitry. Wang et al. (1999) detected higher metabolism in the orbitofrontal and insular cortices of abstinent cocaine abusers after an interview in which participants were allowed to manipulate drug paraphernalia. Furthermore, activation of the left insular cortex was significantly correlated with self-reported craving. Similarly, Childress et al. (1999) found amygdalar and anterior cingulate activation in cocaine abusers exposed to a drug-related video.
In two subsequent PET studies, Kilts et al. (2001, 2004) examined the craving response of cocaine dependent men and women to auditory scripts describing events from their own previous drug experience. These studies reveal that there are important gender differences in regional patterns of brain activation in relation to drug cue induced craving. However, irrespective of these differences, the general neural circuitry engaged during craving, in both males and females tend to reveal activation within neural regions that are considered critical components of the neural circuitry underlying somatic state activation and decision-making.
Using a different neuroimaging technique (functional magnetic resonance imaging, fMRI), Garavan et al. (2000) contrasted the responses of crack-cocaine dependent individuals to films containing natural outdoors, sexual explicit, and drug use related scenes. The drug-associated film induced increased activation of an extensive neural circuitry that included the prefrontal and parietal regions, temporal, insular, anterior and posterior cingulate cortices, and the striatum. A similar pattern of activation was observed in healthy participants when exposed to the film containing explicit sexual scenes (an emotionally competent natural reinforcing stimulus), which contrasted with the fainter activation of these regions in the cocaine abusers. These findings suggest that cocaine dependent individuals present with a reduced sensitivity to the rewarding properties of natural reinforcers, while at the same time, they present with an increased sensitivity to drug related stimuli. Although the somatic-marker model does not explain to the differential response to drug related stimuli versus non-drug stimuli or natural reinforcers, this abnormal capacity to process the emotional value of a stimulus has a significant impact on decision-making, in that it can shift the decision-making process towards short-term horizons, i.e., the seeking of drugs. In support, several fMRI studies have shown an exaggerated brain response to drug cues (George et al., 2001; Maas et al., 1999; Tapert et al., 2004; Wang et al., 1999; Wexler et al., 2004), even when they are presented at a pre-attentive level (Childress et al., 2008).
The brain activation studies on craving were not restricted to individuals who abused stimulant drugs, but similar findings were obtained from individuals addicted to opioids (Daglish et al., 2002, 2003; Langleben et al., 2008). A relevant finding from these studies was the correlation between length of abstinence and anterior cingulate activation, i.e. longer abstinence duration predicted larger cerebral blood flow changes in the anterior cingulate. This finding seems to suggest that the affective evaluation of drug related stimuli do not decrease. Rather it increases after prolonged abstinence, thus suggesting a persistent sensitization effect.
To mimic the acute dopamine response induced by cocaine, Volkow et al. (1999) injected two sequential doses of the drug methylphenidate to a sample of cocaine dependent individuals. The correlation analyses showed that changes in the subjective report of craving were significantly correlated with changes in the right striatum and orbitofrontal cortex. Further studies using raclopride traced PET in cocaine dependents have shown that craving was specifically associated with the rate of D2 receptor occupancy in the dorsal striatum (Volkow et al., 2006; Wong et al., 2007). Another pharmacological study showed that cocaine patients, compared to controls, had increased response to a procaine challenge in the right orbitofrontal, midfrontal, midtemporal, and parietal cortices, and in the brainstem (Adinoff et al., 2001). In contrast, saline administration was associated with deactivation of the orbitofrontal region in the cocaine patients. There was also a trend of a significant relationship between duration of cocaine abuse and right orbitofrontal cortex change rate in response to craving.
Another set of studies has directly employed the administration of the abuser’s drug of choice to evoke craving states. Breiter et al. (1997) administered repeated cocaine infusions to examine the “rush” and “craving” patterns of brain activation using fMRI in cocaine dependent patients. Their results showed a wide array of regional activations on regions involved in the ongoing evaluation of the emotional significance of emotionally competent stimuli, and the modification of behaviour through somatic signals (Fresnois et al., 2005; Kilts, 2001). Similarly, Kufahl et al. (2005) showed that cocaine users had significantly increased activation in the anterior prefrontal cortex, striatum, amygdala and ventral tegmental area after administration of a 20 mg/70 kg cocaine infusion. Using a cocaine “self-administration” paradigm during fMRI (i.e., participants were asked to press a buttom each time they wanted to receive a small cocaine dose, although the doses were indeed constantly administered at 5 minutes intervals) Risinger et al. (2005) also showed prominent activation of the orbitofrontal cortex, anterior cingulate, insula, striatum, thalamus and cerebellum which were correlated with self-reports of “high” and “craving”. In another study (Sell et al., 2000), a combination of heroin injection and visual presentation of films containing drug related scenes was used to induce craving in a sample of heroin dependent individuals undergoing methadone treatment. The correlation analyses showed that the subjective response of “urge to use heroin” was significantly associated with increased activation in the inferior frontal, right orbitofrontal, and insular cortices of the patients. The insula activation was significantly correlated with physiological measures of pulse rate, outlining its role in autonomic regulation. Significant activations were also observed in the brainstem in response to both the heroin injection and the drug-related film.
Together, these studies reveal a great overlap between the neural structures known to be critical components of the somatic-marker neural circuit, and the neural systems engaged in emotional states such as craving in substance abusers. Activities within the orbitofrontal cortex (included in what we define here as VMPC) and amygdala, two key areas for triggering somatic states, were shown to correlate with subjective self-reports of craving across several different studies (Adinoff et al., 2001; Bonson et al., 2002; Grant et al., 1996; Tapert et al., 2004). Areas involved in body mapping and emotional representation, as well as the generation of feelings, such as the insula and anterior cingulate, were also engaged during the experience of craving (Breiter et al., 1997; Daglish et al., 2001; Wang et al., 1999).
Most of the regions activated during the experience of craving induced by drug cues in SDI were also engaged in healthy participants when they were exposed to natural reinforcers, such as sex cues (Garavan et al., 2000). This suggests that the neural substrates that mediate craving in drug addicts have actually evolved to subserve natural emotional functions, such as those related to food and sex. Nonetheless, it is important to note that drug addicts present with some degree of dissociation between the emotional responses to drug versus non-drug related stimuli. In other words, drug addicts tend to trigger strong emotional responses (or somatic states) in response to drug related cues, but they trigger relatively weak somatic states in response to non-drug related, natural reinforcers.
4. Conclusion
The somatic-marker model of addiction proposes that the process of decision-making depends in many important ways on neural substrates that regulate homeostasis, emotion, and feeling. The evidence reviewed in this article shows that numerous studies in SDI point to abnormalities in key neural components of the neural circuitry necessary for somatic state activation and decision-making. According to this model, addiction is viewed as a condition in which the person becomes unable to choose according to long-term outcomes. Choosing according to long-term outcomes rather than short-term ones requires that the pain signals triggered by thoughts about the future negative consequences of seeking drugs dominate those triggered by the immediate rewarding consequences of consuming the drug. Based on the somatic marker model, we suggest that two broad types of conditions could alter this relationship and lead to defective decision-making, and the poor ability to resist the temptation of drug use. (1) One condition may involve a dysfunction in the VMPC system, which is critical for processing emotional (somatic) states from secondary inducers. This is a sort of a “reflective” system, which looses its ability to process and trigger somatic signals associated with future prospects, such as the negative consequences associated with drug use. (2) Another condition may involve hyperactivity in the amygdala system, which is critical for processing emotional (somatic) states from primary inducers. This is a sort of an “impulsive” system, which activity becomes altered in such a way that it diminishes the emotional/somatic impact of natural reinforcers, but it exaggerates the emotional/somatic impact of the immediate prospects of obtaining drugs. Thus drug cues acquire properties for triggering bottom-up, automatic, and involuntary somatic states through the amygdala. Once they do, this bottom-up somatic bias can modulate top-down cognitive mechanisms, in which the ventromedial prefrontal cortex is a critical substrate. If strong enough, this bottom-up influence can interfere or “hijack” the top-down cognitive mechanisms necessary for triggering somatic states about future outcomes. In other words, drugs acquire properties of triggering bottom-up, involuntary signals through the amygdala that modulate and bias top-down goal-driven attentional resources needed for the normal operation of the reflective system, which is critical for exerting control enabling an individual to resist the temptation to seek the drug.
There is evidence to suggest that somatic-markers may exert their biasing influence on decisions through the release of specific neurotransmitters (Bechara et al., 2001; Pirastu et al., 2006; Rogers et al., 2004; Sevy et al., 2006). Advances in the ability to prevent relapse and break the cycle of addiction may be made from pharmacological research aimed at understanding the modulatory influence of different neurotransmitter systems, such as DA, 5HT and NA, in the reflective/prefrontal system of SDI, which underlies their decision-making impairment and loss of control over their drug abuse behaviour. Although some evidence suggests that pharmacological interventions influence decision-making, we argue that pharmacological treatments alone, i.e., without cognitive and behavioural rehabilitation, may not be the most effective strategy for treating addiction. The idea is that poor prefrontal mechanisms of decision-making in SDI are in part related to learning in the presence of a deficiency in the neurotransmitters that modulate, unconsciously or consciously, the cognitive and emotional resources involved in decision-making. In other words, poor decision-making and poor learning to control certain behaviours are due in part to this deficiency. Reversal of this chemical deficiency alone is not sufficient for improving decision-making. The individual must re-learn how to think and behave in a particular situation related to drugs while treated with medications, which correct the deficiency in the neurotransmitter substrates of decision-making. Thus, only re-learning (i.e., rehabilitation) in the presence of normal pharmacology (i.e., drug treatment) is perhaps the most effective way to restore advantageous decisions in an addicted individual. The somatic-marker model of addiction has some implications for the clinical treatment of addiction. Since the model focuses on the existence of abnormal emotional regulation, intervention strategies aimed at improving emotional processing may be especially useful in the potential management or treatment of addiction.
In this review we provide extensive evidence for the pertinence of applying the somatic-marker hypothesis to addiction, and we generate a somatic-marker theory of addiction that provides a number of testable predictions for future research. The main contribution of this model, with regard to previous models of addiction, is that it provides a systems-level neuroanatomical and cognitive framework for explaining the decision-making deficits of SDI, and their inability for choosing according to long-term outcomes rather than short-term ones. The model highlights the role of abnormal emotion processing in the poorer decision-making deficits of SDI. Despite these contributions, several specifications and limitations of the model should be mentioned. First, the somatic-marker hypothesis is an evolving theoretical framework still being empirically evaluated. For example, there are a number of compatible and alternative explanations for the abnormal performance of SDI and patients with VMPC lesions in the IGT, including working memory or reversal learning deficits (Fellows and Farah, 2005; Hinson et al., 2002). Additionally, some studies have challenged the notion that body-state feedback causally influences decision-making (Heims et al., 2004). Second, the somatic-marker model of addiction is not incompatible with previous theoretical models of addiction, including animal models of emotional response to drug cues (Panksepp et al., 2002), brain-based DA reinforcement models (Redish, 2004), and the incentive sensitization theory (Robinson and Berridge, 1993, 2002; see Lawrence et al., 2003 for review of these models). In particular, the somatic-marker model is compatible with the incentive sensitization theory, in the sense that both models predict a strong salience attribution for drug related stimuli. However, the somatic-marker model proposes that this salience attribution is modulated by the development of strong somatic-markers of emotive nature, which are able to bias decision-making towards drug abuse. The somatic-marker model is not specific to addiction. The model provides an account of how emotional states help guide decision-making towards long-term adaptive goals. Impaired decision-making and emotional dysregulation are core characteristics of substance dependence, but they are also prominent characteristics of a variety of disorders, such as pathological gambling, OCD or APD. Interestingly, these disorders are often concurrent with substance dependence, suggesting possible common underlying mechanisms. Further research is necessary to address how pre-existing genetic factors, and/or environmental influences, lead to neural functional abnormalities that may account for the development of these different disorders. An emerging line of research on pharmacogenetics is starting to yield evidence of a prominent role of certain functional polymorphisms in the neurobiological coupling between the “impulsive” and “reflective” systems (Heinz et al., 2005), or in the integrity of decision-making performance in the IGT (Roussos et al., 2008).
Acknowledgments
The research described in this article was supported by the following grants from the National Institute on Drug Abuse (NIDA): DA11779, DA12487, and DA16708, and grants MCYT BSO2003-07169 and SEJ2006-08278/PSIC from the Spanish Ministry of Education and Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel KM, Allin MPG, Kuchrska-Pietura K, David A, Andrew C, Williams S, Brammer MJ, Phillips ML. Ketamine alters neural processing of facial emotion recognition in healthy men: and fMRI study. Neuroreport. 2003;14:387–391. doi: 10.1097/00001756-200303030-00018. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Devous MD, Best SM, George MS, Alexander D, Payne K. Limbic responsiveness to procaine in cocaine-addicted subjects. Am J Psychiatry. 2001;158:390–398. doi: 10.1176/appi.ajp.158.3.390. [DOI] [PubMed] [Google Scholar]
- Aguilar de Arcos F, Verdejo A, Peralta MI, Sánchez-Barrera M, Pérez-García M. Experience of emotions in substance abusers exposed to images containing neutral, positive, and negative affective stimuli. Drug Alcohol Depend. 2005;78:159–167. doi: 10.1016/j.drugalcdep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Aguilar de Arcos F, Verdejo-Garcia A, Ceverino A, Montanez-Pareja M, Lopez-Juarez E, Sanchez-Barrera M, Lopez-Jimenez A, Perez-Garcia M. Dysregulation of emotional response in current and abstinent heroin users: negative heightening and positive blunting. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1110-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Goldstein IB, Hance DB, Beckson M, Shapiro D, Lu PH, Edwards N, Mintz J, Bridge P. The incidence of T2-Weighted MR imaging signal abnormalities in the brain of cocaine-dependent patients is age-related and region-specific. Am J Neuroradiol. 1999;20:1628–1635. [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Risky business: emotion, decision-making and addiction. J Gamb Stud. 2003;19:23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games Econ Behav. 2005;52:336–372. [Google Scholar]
- Bechara A, Damasio AR, Damasio H. Manipulation of dopamine and serotonin cause different effects on covert and overt decision-making. Abstrs Soc Neurosci. 2001:27. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision-making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. The role of the amygdala in decision-making. The Amygdala in Brain Function: Basic and Clinical Approaches. In: Shinnick-Gallagher P, Pitkanen A, Shekhar A, Cahill L, editors. Ann N Y Acad Sci. 2003. pp. 356–369. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bechara A, Van der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. The mind of an addicted brain: neural sensitization of wanting versus liking. Curr Dir Psychol Sci. 1995;4:71–76. [Google Scholar]
- Blair J, Utah F. Neurocognitive explanations of the antisocial personality disorders. Crim Behav Ment Health. 2000;10:66–81. [Google Scholar]
- Blessing WW. The Lower Brainstem and Bodily Homeostasis. New York: Oxford University Press; 1997. Anatomy of the lower brainstem; pp. 29–99. [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian JL, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl J, Kurian V, Ernst M, London E. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bowden-Jones H, McPhillips M, Rogers R, Joyce EM. Risk-taking on tests sensitive to ventromedial prefrontal cortex dysfunction predicts early relapse in alcohol dependency: a pilot study. J Neuropsychiatry Clin Neurosci. 2005;17:417–420. doi: 10.1176/jnp.17.3.417. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Berntson G, Larsen J, Poehlmann K, Ito T. The psychophysiology of emotion. In: Lewis M, Haviland-Jones J, editors. The handbook of emotion. 2. New York: Guiford Press; 2000. pp. 173–191. [Google Scholar]
- Cacioppo J, Klein DJ, Berntson GG, Hatfield E. The psychophysiology of emotion. In: Lewis M, Haviland JM, editors. The handbook of emotion. 1. New York: The Guiford Press; 1993. pp. 119–148. [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia. 2003;41:1474–1483. doi: 10.1016/s0028-3932(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Clark L, Robbins TW. Decision-making deficits in drug addiction. Trends Cogn Sci. 2002;6:361–363. doi: 10.1016/s1364-6613(02)01960-5. [DOI] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Crone EA, Somsen RJM, Van Beek B, Van der Molen MW. Heart rate and skin conductance analysis of antecedents and consequences of decision making. Psychophysiology. 2004;41:531–540. doi: 10.1111/j.1469-8986.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- Daglish MRC, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, Brewer C. Changes in cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001;158:1680–1686. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- Daglish MRC, Weinstein A, Malizia AL, Wilson S, Melichar JK, Lingford-Hughes A, Myles JS, Grasby P, Nutt D. Functional connectivity analysis of the neural circuits of opiate craving: “more” rather than “different”? Neuroimage. 2003;20:1964–1970. doi: 10.1016/j.neuroimage.2003.07.025. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error: Emotion, Reason, and the Human Brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: a critical evaluation. Neurosci Biobehav Rev. 2006;30:239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet J, Kimes AS, London ED. Decision-making in a risk-taking task: A PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision-making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJG, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine- and opiate-dependent individuals. Psychopharmacology. 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward: the role of amygdala and ventral striatal subsystems. Ann N Y Acad Sci (Advancing from the Ventral Striatum to the Extended Amygdala) 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug Alcohol Depend. 2008;92:141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fishbein D, Hyde C, Eldreth D, London ED, Matochik J, Ernst M, Isenberg N, Steckley S, Schech B, Kimes A. Cognitive performance and autonomic reactivity in abstinent drug abusers and nonusers. Exp Clin Psychopharmacology. 2005;13:25–40. doi: 10.1037/1064-1297.13.1.25. [DOI] [PubMed] [Google Scholar]
- Franken IHA. Drug craving and addiction: integrating psychological and neuropharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Fresnois F, Stinus L, DiBlasi F, Cador M, LeMoine C. A specific limbic circuit underlies opiate withdrawal memories. J Neurosci. 2005;25:1366–1374. doi: 10.1523/JNEUROSCI.3090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho J-K, Sperry L, Ross TJ. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Gerra G, Baldaro B, Zaimovic A, Moi G, Bussandri M, Raggi MA, et al. Neuroendocrine responses to experimentally-induced emotions among abstinent opioid-dependent subjects. Drug Alcohol Depend. 2003;71:25–35. doi: 10.1016/s0376-8716(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: a longitudinal study. Alcohol Clin Exp Res. 2007;31:928–938. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Psychophysiological determinants and concomitants of deficient decision making in pathological gamblers. Drug Alcohol Depend. 2006;84:231–239. doi: 10.1016/j.drugalcdep.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision-making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Grant S, London E, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RI, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Luciana M, Sullwold K. Reward-related decision-making deficits and elevated impulsivity among MDMA and other drug users. Drug Alcohol Depend. 2008 doi: 10.1016/j.drugalcdep.2008.02.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman GM, Dunn B. Decision biases and persistent illicit drug use: an experimental study of distributed choice and addiction. Drug Alcohol Depend. 2002;67:193–203. doi: 10.1016/s0376-8716(02)00071-6. [DOI] [PubMed] [Google Scholar]
- Heims HC, Critchley HD, Dolan R, Mathias CJ, Cipolotti L. Social and motivational functioning is not critically dependent on feedback of autonomic responses: neuropsychological evidence from patients with pure autonomic failure. Neuropsychologia. 2004;42:1979–1988. doi: 10.1016/j.neuropsychologia.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser-Sinopoli SM, Flor H, Braus DT, Buchholtz HG, Gründer G, Schreckenberger M, Smolka MN, Rösch F, Mann K, Bartenstein P. Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Somatic markers, working memory, and decision making. Cogn Affect Behav Neurosci. 2002;2:341–353. doi: 10.3758/cabn.2.4.341. [DOI] [PubMed] [Google Scholar]
- Hoshi R, Bisla J, Curran HV. The acute and sub-acute effects of ‘ecstasy’ (MDMA) on processing of facial expressions: preliminary findings. Drug Alcohol Depend. 2004;76:297–304. doi: 10.1016/j.drugalcdep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Xiao L, Palmer P, Sun P, Wang Q, Wei Y, Jia Y, Grenard JL, Stacy AW, Bechara A. Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia. 2008;46:714–726. doi: 10.1016/j.neuropsychologia.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YC, Jang DP, Namkoong K, Ku J, Kim JJ, Park S, Cho ZH, Kim YB, Lee E. Shape deformation of the insula in alcoholics: reduction of left-right asymmetry. Neuroreport. 2007;18:1787–1791. doi: 10.1097/WNR.0b013e3282f193b4. [DOI] [PubMed] [Google Scholar]
- Kano M, Gyoba J, Kamachi M, Mochizuki H, Hongo M, Yanai K. Low doses of alcohol have a selective effect on the recognition of happy facial expressions. Hum Psychopharmacol. 2002;18:131–139. doi: 10.1002/hup.440. [DOI] [PubMed] [Google Scholar]
- Kemmis L, Hall JK, Kingston R, Morgan MJ. Impaired fear recognition in regular recreational cocaine users. Psychopharmacology (Berl) 2007;194:151–159. doi: 10.1007/s00213-007-0829-5. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kilts C. Imaging the roles of the amygdala in drug addiction. Psychopharmacol Bull. 2001;35:84–94. [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KPG. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KPG. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Kalasinsky KS, Derkach P, Schmunk GA, Guttman M, Ang L, Adams V, Furukawa Y, Haycock JW. Striatal dopaminergic and serotonergic markers in human heroin users. Neuropsychopharmacology. 2001;24:561–567. doi: 10.1016/S0893-133X(00)00209-8. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Dan B, Foisy M, Hess U, Le Bon O, Pelc I, Verbank P. Impaired emotional facial expression recognition in alcoholism compared with obsessive-compulsive disorders and normal controls. Psychiatry Res. 2001;102:235–248. doi: 10.1016/s0165-1781(01)00261-x. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Foisy M, Philippot P, Dan P, Tecco J, Noël X, Hees U, Pelec I, Verbank P. Impaired emotional facial expression recognition in alcoholic, opiate dependent subjects, methadone maintenance subjects, and mixed alcohol-opiate antecedent subjects compared with normal controls. Psychiatry Res. 2003;119:251–260. doi: 10.1016/s0165-1781(03)00130-6. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Evans AH, Lees AJ. Compulsive use of dopamine replacement therapy in Parkinson’s disease: reward systems gone awry? Lancet Neurol. 2003;2:595–204. doi: 10.1016/s1474-4422(03)00529-5. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Streeter CC, Ahn KH, Lee HK, Pollack MH, Silveri MM, Nassar L, Levin JM, Sarid-Segal O, Ciraulo DA, Renshaw PF, Kaufman MJ. White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Res. 2004;131:135–145. doi: 10.1016/j.pscychresns.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropsychopharmacology. 2004;47:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS, Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Martin CO, Denburg NL, Tranel D, Granner MA, Bechara A. The effects of vagus nerve stimulation on decision-making. Cortex. 2004;40:605–612. doi: 10.1016/s0010-9452(08)70156-4. [DOI] [PubMed] [Google Scholar]
- Martin L, Clair J, Davis P, O’Ryan D, Hoshi R, Curran HV. Enhanced recognition of facial expressions of disgust in opiate users receiving maintenance treatment. Addiction. 2006;101:1598–1605. doi: 10.1111/j.1360-0443.2006.01574.x. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Chevalley AF, Kunig G, Missimer J, Magyar S, Mino A, Schultz W, Leenders KL. Changes in reward-induced brain activation in opiate addicts. Eur J Neurosci. 2001;18:2605–2610. doi: 10.1046/j.0953-816x.2001.01753.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Mathews SC, Simmons AN, Lane SD, Paulus MP. Selective activation of the nucleus accumbens during risk-taking and decision-making. Neuroreport. 2004;15:2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet J-L, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early onset alcoholism. Alcohol Clin Exp Res. 2000;24:1036–1040. [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Valdes I, Swann AC, Barrat ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier K, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct. Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, Coles MG. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neurosci Biobehav Rev. 2004;28:441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, Papps BP. Decision making in humans: the effect of manipulating the central noradrenergic system. J Neurol Neurosurg Psychiatry. 2003;74:376–378. doi: 10.1136/jnnp.74.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrocki J, Schmoldt A, Buchert R, Andresen B, Petersen K, Thomasius R. Specific neurotoxicity of chronic use of ecstasy. Toxicol Lett. 2002;127:285–297. doi: 10.1016/s0378-4274(01)00511-2. [DOI] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new self report animal model. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Clark L, Mehta MA, Joyce E, King M. Neuropsychological predictors of clinical outcome in opiate addiction. Drug Alcohol Depend. 2008;94:82–91. doi: 10.1016/j.drugalcdep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GB, Schuckit MA. Decision-making by methamphetamine-dependent subjects is associated with error-rate independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Zauscher BE, Frank L, Brown GB, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Lovero KL, Wittmann M, Leland DS. Reduced Behavioral and Neural Activation in Stimulant Users to Different Error Rates during Decision Making. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.09.007. Epug ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Perretta JG, Pari G, Peninger RJ. Effects of Parkinson disease on two putative nondeclarative learning tasks: probabilistic classification and gambling. Cogn Behav Neurol. 2005;18:185–192. doi: 10.1097/01.wnn.0000187939.81541.1d. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93:729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Pirastu R, Fais R, Messina M, Bini V, Spiga S, Falconieri D, Diana M. Impaired decision-making in opiate-dependent subjects: effect of pharmacological therapies. Drug Alcohol Depend. 2006;83:163–168. doi: 10.1016/j.drugalcdep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kuhn KU, Hoppe C, Westheide J, Maier W, Daum I, Wagner M. Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA (“Ecstasy”) Psychopharmacology (Berl) 2007;189:517–530. doi: 10.1007/s00213-005-0256-4. [DOI] [PubMed] [Google Scholar]
- Rainville P, Bechara A, Naqvi N, Damasio AR. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int J Psychophysiol. 2006;61:5–18. doi: 10.1016/j.ijpsycho.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulated but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Lancaster M, Wakeley J, Bhagwagar Z. Effects of beta-adrenoceptor blockade on components of human decision-making. Psychopharmacology (Berl) 2004;172:157–164. doi: 10.1007/s00213-003-1641-5. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28:153–162. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Sahakian BJ. Relationship between ecstasy use and depression: a study controlling for poly-drug use. Psychopharmacology. 2004;173:411–417. doi: 10.1007/s00213-003-1705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Pavlakis S, Bitsios P. Planning, decision-making and the COMT rs4818 polymorphism in healthy males. Neuropsychologia. 2008;46:757–763. doi: 10.1016/j.neuropsychologia.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Salloum JB, Ramchandani VA, Bodurka J, Rawlings R, Momenan R, George D, Hommer DW. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res. 2007;31:1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Pearlson GD, Wong DF, Marenco S, Dannals RF. PET study of competition between intravenous cocaine and [11C]Raclopride at dopamine receptors in human subjects. Am J Psychiatry. 1997;154:1209–1213. doi: 10.1176/ajp.154.9.1209. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Gouzolis-Mayfrank E, Sabri O, Arning C, Zimny M, Zeggel T, Wagenknetch G, Kaiser HJ, Sass H, Buell U. Ecstasy”-induced changes of cerebral glucose metabolism and their correlation to acute psychopathology. Eur J Nucl Med. 1999;26:1573–1579. doi: 10.1007/s002590050497. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takey N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, Delman H, Malhotra A. Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology (Berl) 2006;188:228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao LY, Copersino ML, Fang YX, Chen Y, Tian J, Deng Y, Shuai Y, Jin J, Lu L. PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol. 2008;579:160–166. doi: 10.1016/j.ejphar.2007.09.042. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RSJ, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev. 2004;4:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rock SL, Campbell MC, Busemeyer JR, Finn PR. Psychological Processes Underlying Risky Decisions in Drug Abusers. Psychol Addict Behav. 2005;19:148–157. doi: 10.1037/0893-164X.19.2.148. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Watson DR, Barrett SL, Cooper SJ. Rapid tryptophan depletion improves decision-making cognition in healthy humans without affecting reversal learning or set shifting. Neuropsychopharmacology. 2006;31:1519–1525. doi: 10.1038/sj.npp.1300980. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Hum Brain Mapp. 2007;28:1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. Cognitive concepts of craving. Alcohol Res Health. 1999;23:215–224. [PMC free article] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Mixed emotions: alcoholics’ impairment in the recognition of specific facial expressions. Neuropsychologia. 2003;41:773–782. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR. the covert learning of affective valence does not require structures in hippocampal system or amygdala. J Cogn Neurosci. 1993;5:79–88. doi: 10.1162/jocn.1993.5.1.79. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I, Schlebusch P, Trenckmann U. Processing of affective stimuli in alcoholism. Cortex. 2005;41:189–194. doi: 10.1016/s0010-9452(08)70893-1. [DOI] [PubMed] [Google Scholar]
- van Honk J, Hermans EJ, Putman P, Montagne B, Schutter DJ. Defective somatic-markers in subclinical psychopathy. Neuroreport. 2002;13:1025–1027. doi: 10.1097/00001756-200206120-00009. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology. 2007a;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Ecological assessment of executive functions in substance dependent individuals. Drug Alcohol Depend. 2007b;90:48–55. doi: 10.1016/j.drugalcdep.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Substance abusers’ self-awareness of the neurobehavioral consequences of addiction. Psychiatry Res. 2008;158:172–180. doi: 10.1016/j.psychres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Rivas-Perez C, Vilar-Lopez R, Perez-Garcia M. Strategic self-regulation, decision-making and emotion processing in poly-substance abusers in their first year of abstinence. Drug Alcohol Depend. 2007;86:139–146. doi: 10.1016/j.drugalcdep.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Verney SP, Brown GG, Frank L, Paulus MP. Error-rate related caudate and parietal cortex activation during decision-making. Neuroreport. 2003;14:923–928. doi: 10.1097/01.wnr.0000072842.93264.b6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding Y-S, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann RJ, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann RJ, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley J, Logan SJ, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan SJ, Franceschi D, Maynard L, Ding YS, Gatley SJ, Gifford A, Zhu W, Swanson JM. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43:181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan SJ, Gatley J, Hitzemann RJ, Chen AD, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos P, Logan SJ, Gatley J, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann RJ, Zhu W, Logan J, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, White Addictive drugs as reinforcers: Multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wilkinson L, Jahanshahi M. The striatum and probabilistic implicit sequence learning. Brain Res. 2007;1137:117–30. doi: 10.1016/j.brainres.2006.12.051. [DOI] [PubMed] [Google Scholar]