Abstract
The introduction of two concepts, “local module” and “receptor heteromer”, facilitates the understanding of the role of interactions between different neurotransmitters in the brain. In artificial cell systems, cannabinoid CB1 receptors form receptor heteromers with dopamine D2, adenosine A2A and μ opioid receptors. There is indirect but compelling evidence for the existence of the same CB1 receptor heteromers in striatal local modules centered in the dendritic spines of striatal GABAergic efferent neurons, particularly at a postsynaptic location. Their analysis provides new clues for the role of endocannabinoids in striatal function, which cannot only be considered as retrograde signals that inhibit neurotransmitter release. Recent studies using a new method to detect heteromerization of more than two proteins, which consists of sequential BRET-FRET (SRET) analysis, has demonstrated that CB1, D2 and A2A receptors can form heterotrimers in transfected cells. It is likely that functional CB1-A2A-D2 receptor heteromers can be found where they are highly co-expressed, in the dendritic spines of GABAergic enkephalinergic neurons. The functional properties of these multiple receptor heteromers and their role in striatal function need to be determined.
Keywords: Local module, receptor heteromers, cannabinoid CB1 receptor, dopamine D2 receptor, adenosine A2A receptor, μ opioid receptor, striatum
1. Striatal spine modules and their diversity
The minimal portion of one or more neurons and/or one or more glial cells that operates as an independent integrative unit has been termed a “local module” (Ferré et al., 2007a). Conceptually, local module allows a better understanding of the functional relevance of extrasynaptic receptors, which are activated by volume transmission. Furthermore, the concept of local module provides a rationale for the functional relevance of neurotransmitter receptor heteromers, which can integrate signals that arise from the same or different elements that constitute the local module.
We recently analyzed the role of receptor heteromers in the “striatal spine module”, a common striatal local module which is centered in the dendritic spine of the GABAergic striatal efferent neurons (also called medium spiny neurons) (Ferré et al., 2007a). This type of neuron makes up more than 95% of the striatal neuronal population and receives two main extrinsic inputs that converge in the dendritic spine: mesencephalic dopaminergic inputs from the substantia nigra pars compacta and the ventral tegmental area and cortical, limbic and thalamic glutamatergic inputs (Gerfen, 2004). The main elements of the “striatal spine module” include the dendritic spine, glutamatergic and dopaminergic terminals that make synaptic contact with the head and neck of the dendritic spine, respectively, and astroglial processes that wrap the glutamatergic synapse (Ferré et al., 2007a). This arrangement allows dopaminergic neurotransmission to control glutamatergic neurotransmission. In our previous functional analysis of the striatal spine modules we also considered the role of acetylcholine, mostly released by volume transmission from asynaptic varicosities of cholinergic interneurons, and adenosine, mostly derived from ATP released from astroglia and glutamatergic terminals (Ferré et al., 2007a). We then analyzed the role of heteromers of dopamine, glutamate, adenosine and acetylcholine receptors in the processing of information by striatal spine modules (Ferré et al., 2007a,b). In our previous analysis we did not consider the roles endocannabinoid and endogenous opioid neurotransmission might play in striatal spines modules. We also did not consider the role of extensive intrinsic GABAergic input to striatal spine modules.
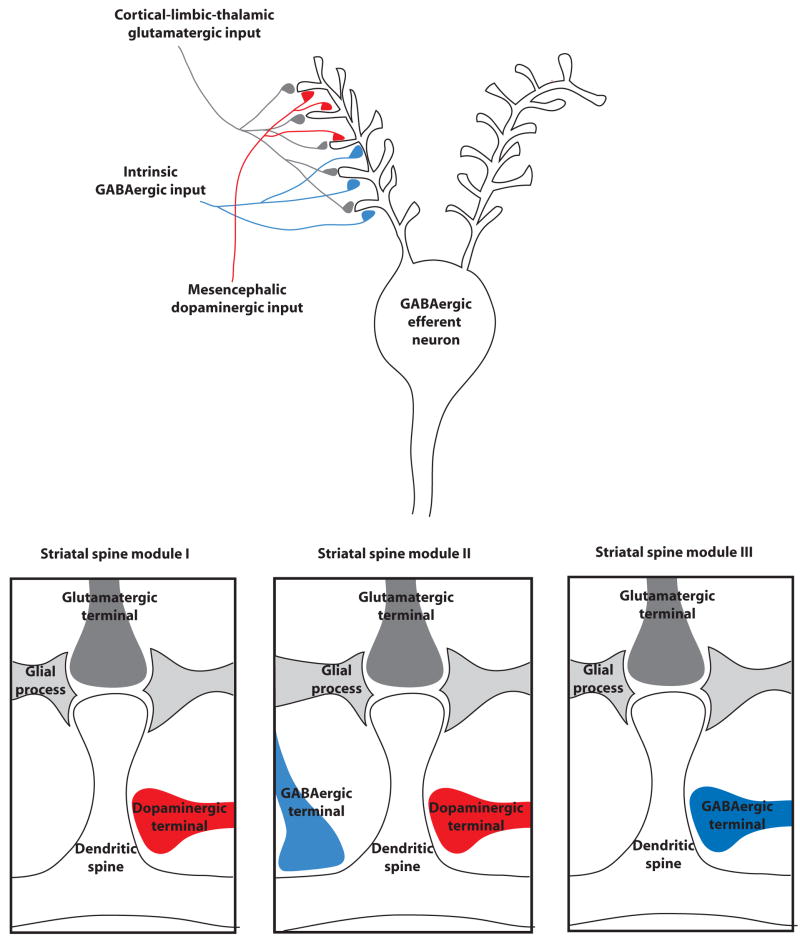
GABAergic innervation in the striatum predominantly originates from collaterals of projecting neurons and from interneurons (Yung et al., 1996; Kawaguchi et al., 1995; Kubota and Kawaguchi, 2000). Nerve terminals from pallidal neurons, conversely, represent only a minority of GABAergic synapses (Bevan et al., 1998). GABAergic interneurons, rather than axon collaterals of GABAergic striatal efferent neurons, provide the predominant inhibitory control of striatal neuron excitability (Koos and Tepper, 1999; Kubota and Kawaguchi, 2000). Like dopaminergic afferents, some GABAergic interneurons make synaptic contact with the neck of the dendritic spines at the same time that glutamatergic terminals make synaptic contact with the head of dendritic spines. In addition, many synapses of GABAergic interneurons make contact with the dendritic shafts but close to the base of the spine (Kubota and Kawaguchi, 2000). It is quite well established that there is a preponderance of dopaminergic inputs on distal portions of the dendrites, which also contain the greatest density of dopaminergic receptors in the dendritic tree (Smith and Bolam, 1990; Sesack et al., 1994). On the other hand, in the proximal portion of the dendrites there is a preponderance of GABAergic intrinsic inputs (and also cholinergic, although these mostly are found in asynaptic varicosities) (Smith and Bolam, 1990; Gerfen, 2004). Therefore, the previously described type of striatal spine module, which includes a glutamatergic and a dopaminergic synapse and we will call striatal spine module I (Figure 1), includes a glutamatergic and a dopaminergic synapse and is mostly localized in the distal portion of the dendritic tree. There are at least two additional types of modules localized in the middle and proximal portions of the dendritic field, which receive glutamatergic inputs. One type of striatal module receives both dopamine and GABAergic inputs (striatal spine module II; Figure 1), and the other type receives only GABAergic input (striatal spine modules III; Figure 1).
Figure 1.
Scheme for the striatal spine modules of the GABAergic striatal efferent neuron. This is the most common neuron in the striatum, which receives two main extrinsic inputs: glutamatergic afferents from cortical, limbic and thalamic areas and dopaminergic afferents from the mesencephalon. Furthermore, it receives intrinsic inputs from GABAergic interneurons and from collaterals of other GABAergic efferent neurons. The glutamatergic terminals make synaptic contact with the head of the dendritic spine and glial processes wrap the glutamatergic synapse. The dopaminergic and GABAergic inputs contact preferentially the distal and proximal portions of the dendritic tree, respectively, and they make contact with the neck of the dendritic spine or the dendritic shaft close to the base of the dendritic spine. Three main types of striatal modules can be differentiated according to their localization in the dendritic tree (I, II and III; see text). The scheme is a simplification. The striatal GABAergic efferent neuron contains from 7–10 moderately branched dendrites that extend over an area of approximately 200 μm with a spine density of about 20–40 spines/μm (Wilson et al., 1983).
In addition to the differential localization of striatal spine modules in the dendritic tree, there are two other sources of variation that further subdivide the modules. First, two types of GABAergic efferent neurons, which are homogeneously distributed in the striatum, can be differentiated by their output connectivity and their expression of dopamine and adenosine receptors and neuropeptides. GABAergic enkephalinergic neurons connect the striatum with the globus pallidus (lateral globus pallidus) and express the peptide enkephalin and dopamine D2 and adenosine A2A receptors (Ferré et al., 1997; Gerfen, 2004). GABAergic dynorphinergic neurons connect the striatum with the substantia nigra and the entopeduncular nucleus (medial globus pallidus) and express the peptides dynorphin and substance P and dopamine D1 and adenosine A1 receptors (Ferré et al., 1997; Gerfen, 2004). Thus, there are at least two different types of striatal spine modules that should be considered, depending on these two types of GABAergic efferent neurons. Furthermore, a small percentage of GABAergic efferent neurons have a mixed phenotype and express both D1 and D2 receptors (Surmeier et al., 1996).
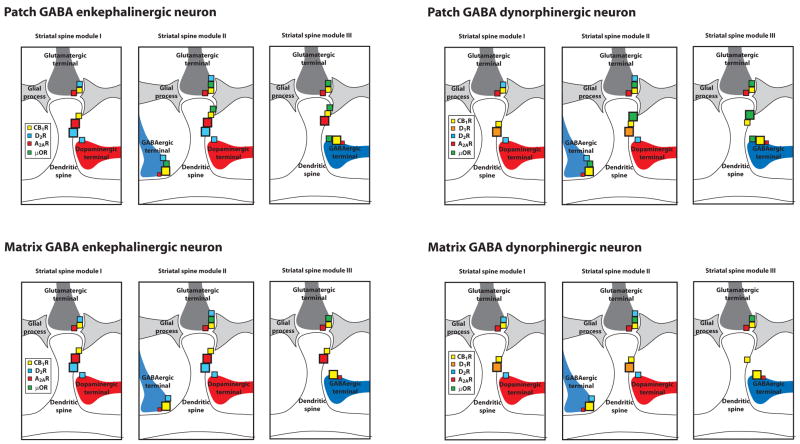
A third source of variation of the striatal spine modules depends on the subdivision of the striatum into patch and matrix compartments. There are two different phenotypes of GABAergic efferent neurons (for both enkephalinergic and dynorphinergic neurons) that are heterogeneously distributed in the patch (striosomes) or the matrix compartments. One of the main characteristics of the patch compartment is that it has a much higher expression of μ opioid receptors than the matrix compartment (Mansour et al., 1987; Gerfen, 2004). In addition to differential expression of other markers, there are clear differences in input and output connectivity, with patch-GABAergic efferent neurons receiving cortical input predominantly from periallocortical areas (such as the infralimbic and prelimbic cortices) and matrix-GABAergic efferent neurons receiving input from neocortical areas (Gerfen, 2004). Also, GABAergic dynorphinergic neurons that originate in the patch project predominantly to the substantia nigra pars compacta and those that originate in the matrix project predominantly to the pars reticulate (Gerfen 2004). In summary, there are at least 12 different types of striatal spine modules, based on variations in localization on the dendritic tree (I, II and III), enkephalinergic versus dynorphinergic phenotype, and localization in the patch or matrix compartment, and this should be considered when analyzing the role of a given neurotransmitter in the modulation of striatal function (Fig. 2).
Figure 2.
Relative expression of cannabinoid CB1, dopamine D2, adenosine A2A and μ opioid receptors in the different elements of the twelve subtypes of striatal spine modules that depend on their localization in the dendritic tree (I, II and III; see Fig. 1) and on the phenotype of the striatal GABAergic efferent neuron (enkephalinergic versus dynorphynergic and patch versus matrix). The squares sizes indicate the approximate relative level of expression in each element of the striatal module.
2. Endocannabinoid neurotransmission in the striatal spine modules
Endocannabinoids are membrane-derived signaling lipids which stimulate GPCRs receptors that are targeted by Δ9-tetrahydrocannabinol (THC), the addictive principle of marijuana. Two major endocannabinoids, anandamide and 2-arachidonylglycerol (2-AG), have been discovered. Like classical neurotransmitters they are released from neurons following neuronal depolarization and Ca2+ influx into the cell. Unlike classical neurotransmitters, they are not stored in vesicles, but are produced “on demand” from endocannabinoid precursors by the action of enzymes localized in the plasma membrane (Di Marzo et al., 1998; Freund et al., 2003; Piomelli, 2003). The enzymes necessary for the biosynthesis of anandamide are the Ca2+-dependent N-acyltransferase and N-acylphosphatidylethanolamine-phospholipase D (NAPE-PLD). For the biosynthesis of 2-AG, the main enzymes involved are the Ca2+-dependent and Gq-11-coupled receptor-activated phospholipase C and diacylglycerol lipase (DGL). The action of endocannabinoids is terminated by uptake or diffusion into cells followed by intracellular metabolism. Fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL) are the two primary enzymes involved in intracellular metabolism of anandamide and 2-AG, respectively (Di Marzo et al., 1998; Freund et al., 2003; Piomelli, 2003). Finally, the existence of an endocannabinoid membrane equilibrative transporter has been postulated to explain cellular uptake of both anandamide and 2-AG (Piomelli, 2003). Some studies suggest that this transporter, which has not yet been identified, plays a role in endocannabinoid release (Ronesi et al., 2004; Adermark and Lovinger, 2007).
Of the two subtypes of cannabinoid receptors so far identified and characterized (CB1 and CB2 receptors) the CB1 subtype is the one predominantly expressed in the adult central nervous system. In fact, the CB1 receptor is considered the most abundant GPCR in the brain (Herkenham et al., 1990, 1991). CB1 receptors activate different signaling pathways through coupling to Gi proteins (Demuth and Molleman, 2006) and are often localized presynaptically where their stimulation usually inhibits neurotransmitter release (Di Marzo et al., 1998; Piomelli, 2003). There has been some debate about the cellular and subcellular localization of CB1 receptors in the striatum. Most studies would agree that CB1 receptors are localized presynaptically, predominantly in GABAergic terminals of interneurons or collaterals from GABAergic efferent neurons, and also in glutamatergic but not in dopaminergic terminals, (Pickel et al., 2004, 2006; Kofalvi et al., 2005; Matyas et al., 2006; Uchigashima et al., 2007). There is also general agreement that GABAergic enkephalinergic and dynorphynergic neurons express CB1 receptor mRNA and that CB1 receptors are highly expressed in the terminals of these neurons, in the globus pallidus (medial and lateral portions) and in the substantia nigra pars reticulata (Herkenham et al., 1991; Julian et al., 2003; Martin et al., 2008). However, there is disagreement about the existence of CB1 receptors in the somatodendritic area of the GABAergic striatal efferent neurons, with some electron microscopy studies being able (Rodriguez et al., 2001; Pickel et al., 2004, 2006) and others being unable (Matyas et al., 2006; Uchigashima et al., 2007) to recognize a postsynaptic localization of striatal CB1 receptors. These discrepancies may be due to the low resolution of electron microscopy (unable to detect low density receptors) and to problems of accessibility of antibodies to epitopes localized in the synapse (accessibility may be hindered by the dense protein matrix that keeps the synapse together). The application of fractionation methods that allow separation of presynaptic and postsynaptic components of striatal synapses has clearly shown that, in fact, CB1 receptors are significantly localized in a fraction enriched in the postsynaptic density (the postsynaptic side of the glutamatergic synapse in the dendritic spine) (Kofalvi et al., 2005).
One of the main functions of endocannabinoids is retrograde signaling with stimulation of presynaptic CB1 receptors and the consequent inhibition of neurotransmitter release. 2-AG, rather than anandamide, seems to be mainly responsible for endocannabinoid-mediated retrograde signaling in the striatum and, probably in most brain areas (Hashimotodani et al., 2007). A recent study has demonstrated that in the striatum DGL is mostly localized in the plasma membrane of the dendritic spines of GABAergic striatal efferent neurons (Uchigashima et al., 2007). The same study showed that, in GABAergic striatal efferent neurons, inhibition of DGL abolishes depolarization-induced suppression of inhibition (DSI) and the depolarization-induced suppression of excitation (DSE) (Uchigashima et al., 2007), which depend on activation of postsynaptic Gq-11-coupled receptors and on activation of presynaptic CB1 receptors with inhibition of GABA and glutamate release, respectively (Hashimotodani et al., 2007). The metabotropic glutamate receptor mGlu5 is a Gq-11-coupled receptor that is co-expressed with DGL in the dendritic spines of GABAergic striatal efferent neurons and it has been found to be particularly involved in endocannabinoid-dependent retrograde signaling (Uchigashima et al., 2007). Studies performed in the hippocampus suggest that, unlike 2-AG, anandamide may be preferentially involved in anterograde signaling, since NAPE-PLD was found to be concentrated presynaptically, where it was associated with intracellular calcium stores in several types of excitatory axon terminals (Nyilas et al., 2008). Furthermore, FAAH is mostly localized postsynaptically (Freund et al., 2003).
In addition to short-term plasticity mediated by endocannabinoids, which is represented by the short-term inhibition of neurotransmitter release induced by postsynaptic depolarization and activation of postsynaptic Gq-11-coupled receptors, endocannabinoid release is involved in long-term synaptic plasticity in several brain regions (for a recent review, see Hashimotodani et al., 2007). In the dorsal and ventral striatum (nucleus accumbens), stimulation of cortico-striatal glutamatergic inputs induces endocannabinoid-mediated long-term depression (LTD) (Gerdeman et al., 2002; Robbe et al., 2002). Although LTD induction requires postsynaptic depolarization and an increase in postsynaptic Ca2+ influx, LTD expression is associated with a decrease in the probability of glutamate release (Choi and Lovinger, 1997). It is believed that retrograde signaling is mostly involved in endocannabinoid-mediated LTD (Piomelli, 2003; Hashimotodani et al., 2007). Striatal long term potentiation (LTP) mediated by endocannabinoids also requires activation of D2 receptors (Kreitzer and Malenka, 2005). In fact, D2 receptor activation has been shown to enhance striatal endocannabinoid release by enhancing synthesis and inhibiting metabolism of endocannabinoids (Giuffrida et al., 1999; Centonze et al., 2004). Indeed, recent experiments by Kreitzer and Malenka (2007) indicate that only excitatory synapses of the GABAergic enkephalinergic neurons (which express D2 receptors), but not GABAergic dynorphinergic neurons (which do not express D2 receptors), show endocannabinoid-mediated LTP.
In summary, the whole machinery of endocannabinoid neurotransmission is highly represented in striatal spine modules and it seems to be designed to mostly play an inhibitory role on GABAergic and glutamatergic neurotransmission by means of both pre- and postsynaptic mechanisms. However, as we will see below, a more relevant fine-tuning of neurotransmission can potentially be achieved by the ability of CB1 receptors to heteromerize with other GPCRs, particularly, dopamine, adenosine and opioid receptors, which are differentially expressed in different types of striatal spine modules.
3. Oligomerization of GPCR
Oligomerization of GPCRs is a phenomenon that is becoming broadly accepted. When it comes to homomerization, at least two molecules of GPCR seem to be needed to interact with one heterotrimeric G protein complex (Baneres and Parello, 2003; Liang et al., 2003; Herrick-Davis et al., 2005). Strong support for homodimerization also comes from morphological evidence obtained with atomic force microscopy for rhodopsin (Fotiadis et al., 2003), protein crystallography for metabotropic glutamate receptors (Kunishima et al., 2000) and biophysical techniques for many transfected GPCRs (Bouvier, 2001; Gandia et al., 2007). Homomerization of CB1 receptors in the brain was demonstrated in immunoprecipitates from rat brain membrane preparations using an antibody that preferentially recognizes the dimerized form of the receptor (Wager-Miller et al., 2002, Mackie, 2005).
In addition to GPCR homomers, there is clear evidence for the existence of GPCR heteromers and we are beginning to understand their functional significance (Ferré et al., 2007b; Franco et al., 2007). Receptor heteromerization provides functional entities which have biochemical properties that are different than those of the individual components of the heteromer. A receptor unit in the heteromer can display several biochemical properties, which can be simply dependent on the presence of the other unit or on co-stimulation of the two (or more) receptor units in the heteromer. For instance, in the recently described D1-D2 receptor heteromer, ligands lose their D1 receptor selectivity and they can also activate the D2 receptor in the heteromer (Rashid et al., 2007). Another example is the significant decrease in affinity of the A2A receptor for caffeine when it heteromerizes with A1 receptor (Ciruela et al., 2006). This opens up the possibility that drugs can be developed that target a specific receptor heteromer (George et al., 2002; Franco et al., 2007). Very often, activation of one of the receptor units in the heteromer induces changes in the binding properties of the other unit. This implies a processing of information at the membrane level of signals impinging on the heteromer (Ferré et al., 2007b). This intermolecular cross-talk is also known as “intramembrane receptor-receptor interaction” (Agnati et al., 2003) and is a common property of GPCR heteromers and constitutes a “biochemical fingerprint” that allows their identification in brain tissue (Ferré et al., 2007b, Franco et al., 2007). As an example, stimulation of the A2A receptor decreases the affinity of the D2 receptor in the A2A-D2 receptor heteromer, which provides the basis for the successful clinical application of A2A receptor antagonists with L-DOPA in the treatment of Parkinson’s disease (Muller and Ferré, 2007). Finally, another common property of GPCR heteromers is their switch to a new type of G protein coupling. For instance, D2 normally couples to Gi-o proteins, while D1 and A2A receptors are prototypical Gs-olf ptotein-coupled receptors. However, in D1-D2 receptor heteromers, and probably in A2A-D2 receptor heteromers, the D2 receptor switches its coupling to Gq/11 protein, resulting in an ability to activate PLC signaling (Ferré et al., 2007c; Rashid et al., 2007). In artificial cell systems, CB1 receptors have recently been shown to form receptor heteromers with D2, A2A and μ receptors (Kearn et al., 2005; Rios et al., 2006; Carriba et al., 2007; Marcellino et al., 2008). We will now analyze the possible functional significance of CB1 receptor heteromers in striatal function.
4. Cannabinoid CB1-dopamine D2 receptor heteromers
CB1-D2 receptor heteromerization has been demonstrated in co-transfected cells by co-immunoprecipitation and Fluorescence Resonance Energy Transfer (FRET) techniques (Kearn et al., 2005; Marcellino et al., 2008). The CB1-D2 receptor heteromer provides a nice example of G protein switching in the heteromer. Thus, both CB1 and D2 receptors are usually coupled to Gi/o proteins and their individual activation in co-transfected cells or primary striatal neurons in culture leads to inhibition of forskolin-induced adenylyl-cyclase activation. On the other hand, in these cellular models, co-stimulation, but not individual receptor stimulation of CB1 and D2 receptors, results in a Gs protein-dependent (pertussis toxin-insensitive) adenylyl-cyclase activation (Glass and Felder, 1997; Kearn et al., 2005). Another study suggested that simply co-expressing CB1 and D2 receptors is sufficient to induce stimulation of adenylyl cyclase in response to CB1 receptor activation (Jarrahian et al., 2004). The reasons for differences between these studies remain to be resolved, but all of these studies demonstrate that activation of CB1-D2 receptor heteromer can have completely opposite effects than activation of the individual receptors.
CB1 and D2 receptors are co-localized in different elements of striatal spine modules. As mentioned above, D2 receptors are highly expressed in the dendritic spines of GABAergic enkephalinergic neurons, particularly those striatal spine modules localized in the distal portion of the dendritic tree (striatal modules I and II in the GABAergic enkephalinergic neurons; Figure 2). Furthermore, in the striatal spine modules, D2 receptors are also known to be localized in dopaminergic, glutamatergic and GABAergic terminals, where their stimulation inhibits neurotransmitter release (Usiello et al., 2000; Bamford et al., 2004; Centonze et al., 2004). There are two isoforms of D2 receptors, D2L and D2S (long and short isoforms, respectively), which differ by the presence of 29 additional amino acids within the third intracellular loop of D2L (Giros et al., 1989). Studies using specific antibodies indicate that the predominant isoform for the postsynaptic D2 receptor (in the dendritic spine) is D2L and the predominant isoform for the D2 autoreceptor (localized in dopaminergic terminals) is D2S (Khan et al., 1998). More recent studies using gene inactivation technology suggest that both isoforms participate in presynaptic inhibition of GABA transmission in the striatum, while modulation of glutamate release depends mostly on the D2S isoform. In summary, when considering the above mentioned striatal distribution of CB1 receptors, CB1-D2 colocalization and, therefore, the potential localization of CB1-D2 receptor heteromers can take place both postsynaptically in the dendritic spines of GABAergic enkephalinergic neuron and presynaptically, in GABAergic and glutamatergic terminals of both enkephalinergic and dynorphinergic neurons. In fact, a recent electron microscopy analysis with double labeling in the ventral striatum has confirmed the existence of overlapping subcellular distributions of CB1 and D2 receptor immunoreactivities both at the pre- and postsynaptic levels (Pickel et al., 2006), providing important support for the existence of CB1-D2 receptor heteromers in the striatum. Additional compelling evidence has recently been provided by experiments in rat striatal membrane preparations, with the demonstration of an intramembrane receptor-receptor interaction. In these preparations, stimulation of CB1 receptors decreased the affinity of D2 receptors for dopamine (Marcelino et al., 2008). As mentioned above, intramembrane receptor-receptor interactions constitute a common property of GPCR heteromers (Agnati et al., 2003; Ferré et al., 2007b; Franco et al., 2007).
Both properties of the CB1-D2 receptor heteromer, G protein switching and the antagonistic intramembrane interaction, would predict that in functional experiments CB1 receptor activation should counteract the effects of striatal D2 receptor-mediated neurotransmission. In fact, it is well known that CB1 receptor agonists and antagonists counteract and potentiate, respectively, D2 receptor agonist-mediated motor effects (Maneuf et al., 1997; Rodriguez De Fonseca et al., 1998; Giuffrida et al., 1999; Andersson et al., 2005; Martin et al., 2008; Marcelino et al., 2008). It has, therefore, been suggested that CB1 receptor agonist-mediated motor depressant effects depend on their ability to counteract dopamine neurotransmission (De Fonseca et al., 1998). Since motor activation induced by D2 receptor agonists is mostly dependent on postsynaptic D2 receptors, these behavioral studies strongly suggest that striatal CB1-D2 heteromers are localized in the dendritic spines of GABAergic enkephalinergic neurons. Also, as indicated by Maneuf et al. (1997), another locus where CB1-D2 heteromerization could partially explain CB1-D2 receptor interactions at the behavioral level are the terminals of the GABAergic enkephalinergic neurons in the lateral globus pallidus (known to have a very high expression of CB1 receptors and a moderate expression of D2 receptors; see above). In fact, the same authors also reported that CB1 receptor activation in slices of rat lateral globus pallidus produces a paradoxical increase in cAMP (Maneuf and Brotchie, 1997). Indirectly this supports the existence of CB1-D2 receptor heteromers in the striatal collaterals of GABAegic enkephalinergic neurons. On the other hand, although CB1 and D2 receptors are colocalized in striatal glutamatergic terminals, recent studies by Kreitzer and Malenka (2007) do not support that they form functional heteromers in this location. As mentioned before, D2 receptor stimulation favors CB1 receptor-mediated inhibition of presynaptic glutamatergic neurotransmission, most probably by a D2 receptor-mediated stimulation of endocannabinoid release (Kreitzer and Malenka, 2007). In view of the differential distribution of D2 receptor isoforms, it is tempting to speculate that in the striatum CB1 receptors form heteromers with D2L but not D2S receptors.
5. Cannabinoid CB1-adenosine A2A receptor heteromers
CB1-A2A receptor heteromerization has been demonstrated by Bioluminescence Resonance Energy Transfer (BRET) in cotransfected cells (Carriba et al., 2007). In a human neuroblastoma cell line, Gi-dependent CB1 receptor signaling (inhibition of adenylyl cyclase activity) was found to be completely dependent on A2A receptor co-activation. Thus, A2A receptor blockade or incubation with adenosine deaminase counteracted the ability of a CB1 agonist to inhibit forskolin-induced cAMP accumulation (Carriba et al., 2007). Consistent with these results in artificial cell lines, some biochemical effects of CB1 receptor agonists in primary striatal cell cultures and striatal slices have been reported to depend on A2A receptor function. Those include cAMP-PKA signaling involving AGS3, an activator of G protein signaling, as well as βγ subunits and types II–IV adenylyl cyclase (Yao et al., 2003, 2006) and DARPP-32 phosphorylation (Andersson et al., 2005).
In the striatal spine modules, A2A receptors are preferentially expressed in dendritic spines of GABAergic enkephalinergic neurons (Rosin et al., 2003), where they are co-localized and form functional heteromers with D2 receptors (reviewed in Ferré et al., 2007a,b,c). Furthermore, A2A receptors are localized in a high proportion of glutamatergic terminals, where they form heteromers with A1 receptors that control glutamate release (Ciruela et al., 2006; Ferré et al., 2007a,b,c). On the other hand, A2A receptors show very little expression in the dopaminergic and GABAergic terminals (Rosin et al., 2003). However, there is clear functional evidence for the existence of A2A receptors localized in the lateral globus pallidus and their stimulation facilitates GABA release (Mayfield et al., 1993; Shindou et al., 2002; Simola et al., 2006). Recent studies suggest A2A receptors are also present in the striatal collaterals of the GABAergic enkephalinergic neurons (Shindou et al., 2008). Less clear is the significance of some results that suggest the existence of striatal A2A receptors localized in GABA terminals, which inhibit GABA release when activated (Mori and Shindou, 2003). Striatal A2A receptors are, therefore, mostly co-localized with CB1 receptors in the dendritic spines of GABAergic enkephalinergic neuron and in glutamatergic nerve terminals (Figure 2). A recent double immunohistochemical confocal analysis in rat striatal sections demonstrated a strong colocalization of CB1 and A2A receptors in fibrilar structures, compatible with dendritic processes or nerve terminals (Carriba et al., 2007). Furthermore striatal CB1-A2A receptor complexes were obtained by co-immunoprecipitation (Carriba et al., 2007).
The biochemical properties of the CB1-A2A receptor heteromer, with its dependence on A2A receptor activation, would predict that A2A receptor antagonists would produce effects similar to CB1 receptor antagonists. Consistent with this prediction, motor depression induced by the bilateral striatal infusion of a CB1 receptor agonist in rats was completely counteracted by systemic administration of either CB1 or A2A receptor antagonists (Carriba et al., 2007). Similarly, genetic inactivation of A2A receptors in mice significantly decreased the cataleptic and rewarding effects (in a conditioned place preference paradigm) of systemically administered CB1 receptor agonists (Andersson et al., 2005; Soria et al., 2006). As mentioned before, the motor depressant effects of CB1 receptor agonists seem to depend on postsynaptic D2 receptor-mediated dopaminergic neurotransmission and some of the biochemical CB1-A2A receptor interactions described above also seem to depend on D2 receptor function (Yao et al., 2003; Andersson et al., 2005). Since strong physical and functional interdependence of striatal A2A and D2 receptors has also been demonstrated (Ferré et al., 2007a,b,c), it becomes an obvious possibility that CB1, A2A and D2 receptors form part of the same macromolecular complexes and that the three receptors can heteromerize in dendritic spines of GABAergic enkephalinergic neurons.
We recently introduced a new method to detect heteromerization of more than two proteins in transfected cells, which consists of a Sequential BRET-FRET (SRET) analysis (Carriba et al., 2008). In SRET experiments, emission of light by a bioluminescent donor triggers excitation of an acceptor fluorophor by BRET and this fluorophor emits light on a wavelength that excites a second acceptor fluorophor by FRET. Thus, SRET requires co-expression of three fusion proteins, one fused to the BRET donor (Renilla luciferase; Rluc), another fused to the BRET acceptor, which is also a FRET donor (p.e., GFP2), and the third coupled to the FRET acceptor (p.e., YFP). If the proteins under study form heteromers, the addition of an Rluc substrate should elicit a sequence with the final emission of light by the FRET acceptor (Carriba et al., 2008). A very significant SRET was demonstrated when mammalian cells were co-transfected with Rluc-A2A, D2-GFP2 and CB1-YFP receptors (Carriba et al., 2008). Although it has not yet been demonstrated, it is likely that functional CB1-A2A-D2 receptor heteromers can be found where they are highly co-expressed in the striatum, particularly in the dendritic spines of GABAergic enkephalinergic neurons, the preferential localization of CB1-D2, CB1-A2A and A2A-D2 receptor heteromers. The functional properties of these multiple heteromers and their role in striatal function needs to be determined.
6. Cannabinoid CB1-μ opioid receptor heteromers
Heteromerization of CB1 and μ receptors was recently demonstrated in co-transfected cells with BRET (Rios et al., 2006), but direct functional interactions between these receptors was demonstrated much earlier by investigators looking for endogenous targets of THC, well before the discovery of endocannabinoids and cannabinoid receptors. It was found that THC allosterically modulates the binding of μ and δ (but not κ) opioid receptor ligands in membrane preparations from rat brain (Vaysse et al., 1987). These results were recently confirmed and extended by another research group (Kathmann et al., 2006) that found a specific increase in the dissociation of μ and δ receptor ligands with THC in rat cortical membrane preparations (although a similar effect was observed with cannabidiol, another constituent of marijuana that has low affinity for CB1 receptors; Kathmann et al., 2006). That this allosteric modulation involves a CB1-μ intramembrane receptor interaction and receptor heteromerization is supported by a recent study that shows the absence of direct effects of CB1 receptor ligands on μ receptors expressed in Xenopus oocytes (Kracke et al., 2007). Biochemical experiments in co-transfected cells suggest that occupancy of CB1 receptors in CB1-μ receptor heteromer has an antagonistic effect on μ receptor-mediated G protein activation (Rios et al., 2006), which would agree with the antagonistic intramembrane interaction.
The μ receptor, the main target for morphine, is heavily expressed in the striatum, particularly in the patch compartment (Mansour et al., 1987; Gerfen 2004), where it seems to be preferentially expressed in GABAergic dynorphinergic neurons (Guttenberg et al., 1996). Ultrastructural analysis with electron microscopy has shown that μ receptors in the patch compartment are preferentially localized in the same elements of striatal spine modules as CB1 receptors, i.e., in the dendritic spines and in the glutamatergic and GABAergic nerve terminals (Pickel et al., 2004; Rodriguez et al., 2001) (Figure 2), where they exert an inhibitory role on neurotransmitter release (Schoffelmeer et al., 2006; Miura et al., 2007). Recent electrophysiological experiments indicate that there is a similar μ receptor-dependent modulation of glutamate release in both patch and matrix compartments, while μ receptor-dependent modulation of GABA release only takes place in the patch compartment (Miura et al., 2007).
The pentapeptide enkephalins are the main endogenous opioids involved in stimulation of striatal μ receptors. Enkephalins (Met-enkephalin and Leu-enkephalin, from the precursor proenkephalin) are released by GABAergic enkephalinergic neurons and are also endogenous ligands for δ, but not κ receptors (Mansour et al., 1995; Akil et al., 1998). On the other hand, dynorphins (A and B), are released by GABAergic dynorphinergic neurons and are the main endogenous ligands for κ receptors, with very low affinities for μ and δ receptors (Mansour et al., 1995; Akil et al., 1998). Nevertheless, the precursor of dynorphins, prodynorphin, can potentially generate Leu-enkephalin (as well as Leu-enkephalin-Arg, which has similar affinities for the three opioid receptors; Mansour et al., 1995). In fact, the GABAergic dynorphinergic neurons have been shown to be an important source of Leu-enkephalin in the substantia nigra (Zamir et al., 1984). In the striatum, however, total and extracellular levels of Met-enkephalin are significantly higher than those of Leu-enkephalin (Nylander et al., 1995; Baseski et al., 2005). Endomorphins (1 and 2) are endogenous tetrapeptides which also bind to μ receptors with high affinity and have a low selectivity for δ and κ receptors (Fichna et al., 2007). However, the density of endomorphin-like immunoreactivity is low in the striatum (Martin-Schild et al., 1999) and it is not yet known if they are functional significance in striatal spine modules. Also, the precursor molecules for endomorphins, as well as the striatal neurons that release endomorphins, still need to be determined (Fichna et al., 2007; Martin-Schild et al., 1999). In summary, the main source of endogenous agonist for μ receptors seems to be the collaterals of GABAergic enkephalinergic neurons, which establish synaptic contact with the dendritic shafts and spines of GABAergic striatal efferent neurons (Yung et al., 1996).
Electron microscopy studies with double-labeling have shown CB1-μ receptor colocalization in both pre- and postsynaptic elements (Rodriguez et al., 2001; Pickel et al., 2004). Clearer evidence for the existence of functional CB1-μ receptor heteromers in the striatum comes from biochemical experiments, such as the above mentioned intramembrane interactions, as well as from experiments in rat striatal slices analyzing CB1-μ receptor interactions in the modulation of neurotransmitter release (Schoffelmeer et al., 2006). In these studies, a μ receptor antagonist prevented a CB1 receptor antagonist from blocking the inhibition of glutamate and GABA release mediated by a CB1 receptor agonist. Conversely, a CB1 receptor antagonist prevented a μ receptor antagonist from blocking the inhibition of glutamate and GABA release mediated by a μ receptor agonist (Schoffelmeer et al., 2006). These results suggest additional intramembrane crosstalk between CB1 and μ receptors, since ligand binding to one of the receptors changes the binding characteristics of the other receptor. Also, in the same study, evidence was obtained for additive and synergistic CB1-μ receptor interactions in the modulation of striatal glutamate and GABA release by studying the effect of CB1 and μ receptor agonists, alone and in combination (Schoffelmeer et al., 2006). This is not consistent with the antagonistic intramembrane CB1-μ receptor interactions (Vaysse et al., 1987; Kathmann et al., 2006) and with interactions at the signaling level obtained in co-transfected cells (Rios et al., 2006). Nevertheless, there are also results of synergistic CB1-μ receptor interactions on cAMP-PKA signaling in striatal neurons in culture, which seem to depend on A2A receptor activation (Yao et al., 2006), suggesting that, in vivo, we might be dealing with A2A-CB1-μ receptor heteromerization. Furthermore, an important amount of data from in vivo and ex vivo biochemical and behavioral experiments indicates that there is a strong dependence of μ receptor co-activation on CB1 receptor activation and vice versa (Gardner et al., 1988; Tanda et al., 1997; Ledent et al., 1999; Manzanares et al., 1999; Berrendero et al., 2003; Justinova et al., 2004; Solinas and Goldberg, 2005). Probably, striatal CB1-μ receptor heteromers (and maybe A2A-CB1-μ receptor heteromers) contribute to the behavioral effects of CB1 and μ receptor ligands. This should not underestimate the contribution of other loci of interactions in the brain and the reported stimulation of the release of endogenous opioids by CB1 receptor activation (reviewed in Vigano et al., 2005). For instance, THC induces an increase in the extracellular concentration of enkephalin in the striatum (Valverde et al., 2001).
7. Concluding remarks
The introduction of the two concepts, “local module” and “receptor heteromer”, facilitates the understanding of the role of interactions between different neurotransmitters in the brain (Ferré et al., 2007a). Local module also provides the best framework for understanding the role of “volume transmission” (Zoli et al., 1999). In the local module, the overflow of synaptically released and non-synaptically (neuronal or glial in origin) released neurotransmitters can converge and activate extrasynaptically located receptor heteromers, which act as processors of computations that modulate cell signaling (Ferré et al., 2007b,c). Furthermore, receptor heteromers can be localized intrasynaptically (pre or postsynaptically), where they can act as concentration-dependent switches (in case of heteromers of receptors for the same neurotransmitter) or as processors of information of different neurotransmitters released in the synapse (Ferré et al., 2007c).
The study of CB1 receptor heteromers in the striatal spine modules provides new clues for the role of endocannabinoids in neuronal function, which cannot only be considered as retrograde signals that inhibit neurotransmitter release. There is strong evidence for tight morphological and functional postsynaptic interactions between CB1 receptors and D2, A2A and μ receptors which play important roles in striatal function. Furthermore, the differential expression of CB1, D2, A2A and μ receptors in the various types of striatal spine modules allows a significant plasticity in the modulatory role of endocannabinoids in the striatum.
Although the field of receptor heteromers is just beginning, we can foresee that it will have a huge impact on the fields of receptor physiology and pharmacology. Many questions remain to be answered and new ways of understanding receptor function will certainly emerge. For instance, there is already overwhelming experimental evidence that homodimers are the minimal functional unit of GPCRs. This suggests that receptor heteromers are probably heteromers of different homodimers. At the pharmacological level, the unique binding characteristics of receptor heteromers open the possibility of selectively targeting not only specific neurons in a specific brain area, but even specific local modules or specific elements of specific local modules.
Acknowledgments
Supported by the National Institute on Drug Abuse (Intramural Research funds), National Institutes of Health, Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adermark L, Lovinger DM. Retrograde endocannabinoid signaling at striatal synapses requires a regulated postsynaptic release step. Proceedings of the National Academy of USA. 2007;104:20564–20569. doi: 10.1073/pnas.0706873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacological Reviews. 2003;55:509–550. doi: 10.1124/pr.55.3.2. [DOI] [PubMed] [Google Scholar]
- Akil H, Owens C, Gutstein H, Taylor L, Curran E, Watson S. Endogenous opioids: overview and current issues. Drug and Alcohol Dependence. 1998;51:127–140. doi: 10.1016/s0376-8716(98)00071-4. [DOI] [PubMed] [Google Scholar]
- Andersson M, Usiello A, Borgkvist A, Pozzi L, Dominguez C, Fienberg AA, Svenningsson P, Fredholm BB, Borrelli E, Greengard P, Fisone G. Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. Journal of Neuroscience. 2005;25:8432–8438. doi: 10.1523/JNEUROSCI.1289-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. Journal of Neuroscience. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneres JL, Parello J. Structure-based analysis of GPCR function: evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. Journal of Molecular Biology. 2003;329:815–829. doi: 10.1016/s0022-2836(03)00439-x. [DOI] [PubMed] [Google Scholar]
- Baseski HM, Watson CJ, Cellar NA, Shackman JG, Kennedy RT. Capillary liquid chromatography with MS3 for the determination of enkephalins in microdialysis samples from the striatum of anesthetized and freely-moving rats. Journal of Mass Spectrometry. 2005;40:146–153. doi: 10.1002/jms.733. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Mendizábal V, Murtra P, Kieffer BL, Maldonado R. Cannabinoid receptor and WIN 55 212–2-stimulated [35S]-GTPgammaS binding in the brain of mu-, delta- and kappa-opioid receptor knockout mice. European Journal of Neuroscience. 2003;18:2197–2202. doi: 10.1046/j.1460-9568.2003.02951.x. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. Journal of Neuroscience. 1998;18:9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nature Reviews Neuroscience. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Muller C, Woods AS, Hope BT, Ciruela F, Casado V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferré S. Striatal Adenosine A(2A) and Cannabinoid CB(1) Receptors Form Functional Heteromeric Complexes that Mediate the Motor Effects of Cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- Carriba P, Navarro G, Ciruela F, Ferré S, Casado V, Agnati LF, Cortes A, Mallol J, Fuxe K, Canela EI, Lluis Franco R. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nature Methods. 2008 doi: 10.1038/nmeth.1229. (in press) [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agrò A, Bernardi G, Calabresi P, Maccarrone M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proceedings of the National Academy of Sciences USA. 1997;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. Journal of Neuroscience. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signaling. Life Sciences. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends in Neurosciences. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends in Neurosciences. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Agnati LF, Ciruela F, Lluis C, Woods AS, Fuxe K, Franco R. Neurotransmitter receptor heteromers and their integrative role in ‘local modules’: The striatal spine module. Brain Research Reviews. 2007a;55:55–67. doi: 10.1016/j.brainresrev.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Woods AS, Lluis C, Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends in Neurosciences. 2007b;30:440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Quiroz C, Lujan R, Popoli P, Cunha RA, Agnati LF, Fuxe K, Woods AS, Lluis C, Franco R. Adenosine receptor heteromers and their integrative role in striatal function. TheScientificWorldJOURNAL. 2007c;7:74–85. doi: 10.1100/tsw.2007.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichna J, Janecka A, Costentin J, Do Rego JC. The endomorphin system and its evolving neurophysiological role. Pharmacological Reviews. 2007;59:88–123. doi: 10.1124/pr.59.1.3. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- Franco R, Casado V, Cortes A, Ferrada C, Mallol J, Woods A, Lluis C, Canela EI, Ferré S. Basic concepts in G-protein-coupled receptor homo- and heteromerization. TheScientificWorldJOURNAL. 2007;7:48–57. doi: 10.1100/tsw.2007.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiolgical Reviews. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D. Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology. 1988;96:142–144. doi: 10.1007/BF02431546. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal Ganglia. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; Amsterdam: 2004. pp. 445–508. [Google Scholar]
- George SR, O’Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nature Reviews in Drug Discovery. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nature Neuroscience. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Giros B, Sokoloff P, Martres MP, Riou JF, Emorine LJ, Schwartz JC. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;342:923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nature Neuroscience. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. Journal of Neuroscience. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenberg ND, Klop H, Minami M, Satoh M, Voorn P. Co-localization of mu opioid receptor is greater with dynorphin than enkephalin in rat striatum. NeuroReport. 1996;7:2119–2124. doi: 10.1097/00001756-199609020-00011. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Harrigan TJ, Mazurkiewicz JE. Inhibition of serotonin 5-hydroxytryptamine2c receptor function through heterodimerization: receptor dimers bind two molecules of ligand and one G-protein. Journal of Biological Chemistry. 2005;280:401–451. doi: 10.1074/jbc.M507396200. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrahian A, Watts VJ, Barker EL. D2 dopamine receptors modulate Galpha-subunit coupling of the CB1 cannabinoid receptor. Journal of Pharmacology and Experimental Therapeutics. 2004;308:880–886. doi: 10.1124/jpet.103.057620. [DOI] [PubMed] [Google Scholar]
- Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–318. doi: 10.1016/s0306-4522(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology. 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedeberg’s Archives of Pharmacology. 2006;372:354–361. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends in Neurosciences. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Molecular Pharmacology. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proceedings of the National Academy of Sciences USA. 1998;95:7731–7736. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlágh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. Journal of Neuroscience. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nature Neuroscience. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Kracke GR, Stoneking SP, Ball JM, Tilghman BM, Washington CC, Hotaling KA, Johnson JO, Tobias JD. The cannabinoid receptor agonists, anandamide and WIN 55,212–2, do not directly affect mu opioid receptors expressed in Xenopus oocytes. Naunyn Schmiedeberg’s Archives of Pharmacology. 2007;376:285–293. doi: 10.1007/s00210-007-0201-7. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. Journal of Neuroscience. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. Journal of Neuroscience. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. Journal of Biological Chemistry. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptor homo- and heterodimerization. Life Sciences. 2005;77:1667–1673. doi: 10.1016/j.lfs.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Brotchie JM. Paradoxical action of the cannabinoid WIN 55,212–2 in stimulated and basal cyclic AMP accumulation in rat globus pallidus slices. British Journal of Pharmacology. 1997;120:1397–1398. doi: 10.1038/sj.bjp.0701101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, Crossman AR, Brotchie JM. The cannabinoid receptor agonist WIN 55,212–2 reduces D2, but not D1, dopamine receptor-mediated alleviation of akinesia in the reserpine-treated rat model of Parkinson’s disease. Experimental Neurology. 1997;148:265–270. doi: 10.1006/exnr.1997.6645. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. Journal of Neuroscience. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Research. 1995;700:89–98. doi: 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernández-Ruiz JJ, Ramos JA, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends in Pharmacological Sciences. 1999;20:287–294. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, Tanganelli S, Müller CE, Fisone G, Lluis C, Agnati LF, Franco R, Fuxe K. Antagonistic cannabinoid CB(1)/dopamine D(2) receptor interactions in striatal CB(1)/D(2) heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Martín AB, Fernandez-Espejo E, Ferrer B, Gorriti MA, Bilbao A, Navarro M, Rodriguez de Fonseca F, Moratalla R. Expression and Function of CB(1) Receptor in the Rat Striatum: Localization and Effects on D(1) and D(2) Dopamine Receptor-Mediated Motor Behaviors. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301558. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. Journal of Comparative Neurology. 1999;405:450–471. [PubMed] [Google Scholar]
- Mátyás F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Suzuki F, Zahniser NR. Adenosine A2a receptor modulation of electrically evoked endogenous GABA release from slices of rat globus pallidus. Journal of Neurochemistry. 1993;60:2334–2337. doi: 10.1111/j.1471-4159.1993.tb03526.x. [DOI] [PubMed] [Google Scholar]
- Miura M, Saino-Saito S, Masuda M, Kobayashi K, Aosaki T. Compartment-specific modulation of GABAergic synaptic transmission by mu-opioid receptor in the mouse striatum with green fluorescent protein-expressing dopamine islands. Journal of Neuroscience. 2007;27:9721–9728. doi: 10.1523/JNEUROSCI.2993-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A, Shindou T. Modulation of GABAergic transmission in the striatopallidal system by adenosine A2A receptors: a potential mechanism for the antiparkinsonian effects of A2A antagonists. Neurology. 2003;61:S44–S48. doi: 10.1212/01.wnl.0000095211.71092.a0. [DOI] [PubMed] [Google Scholar]
- Muller CE, Ferré S. Blocking striatal adenosine A2A receptors: A new strategy for basal ganglia disorders. Recent Patents in CNS Drug Discovery. 2007;2:1–21. doi: 10.2174/157488907779561772. [DOI] [PubMed] [Google Scholar]
- Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: effects of morphine tolerance and withdrawal. Brain Research. 1995;683:25–35. doi: 10.1016/0006-8993(95)00279-y. [DOI] [PubMed] [Google Scholar]
- Nyilas R, Dudok B, Urbán GM, Mackie K, Watanabe M, Cravatt BF, Freund TF, Katona I. Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. Journal of Neuroscience. 2008;28:1058–1063. doi: 10.1523/JNEUROSCI.5102-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodríguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. Journal of Comparative Neurology. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nature Reviews in Neurosciences. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proceedings of the National Academy of Sciences USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. British Journal of Pharmacology. 2006;148:385–386. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proceedings of the National Academy of Sciences USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. Journal of Neuroscience. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Del Arco I, Martín-Calderón JL, Gorriti MA, Navarro M. Role of the endogenous cannabinoid system in the regulation of motor activity. Neurobiology of Disease. 1998;5:483–501. doi: 10.1006/nbdi.1998.0217. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. Journal of Neuroscience. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–S8. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. Journal of Neuroscience. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola N, Fenu S, Baraldi PG, Tabrizi MA, Morelli M. Involvement of globus pallidus in the antiparkinsonian effects of adenosine A(2A) receptor antagonists. Experimental Neurology. 2006;202:255–257. doi: 10.1016/j.expneurol.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Shindou T, Nonaka H, Richardson PJ, Mori A, Kase H, Ichimura M. Presynaptic adenosine A(2A) receptors enhance GABAergic synaptic transmission via a cyclic AMP dependent mechanism in the rat globus pallidus. British Journal of Pharmacology. 2002;136:296–302. doi: 10.1038/sj.bjp.0704702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindou T, Arbuthnott GW, Wickens JR. Actions of adenosine A2A receptors on synaptic connections of spiny projection neurons in the neostriatal inhibitory network. Journal of Neurophysiology. 2008 doi: 10.1152/jn.01259.2007. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, Hogenboom F, Wardeh G, De Vries TJ. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology. 2006;51:773–781. doi: 10.1016/j.neuropharm.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends in Neurosciences. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Soria G, Castañé A, Ledent C, Parmentier M, Maldonado R, Valverde O. The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2006;31:978–987. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. Journal of Neuroscience. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. Journal of Neuroscience. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Valverde O, Noble F, Beslot F, Daugé V, Fournié-Zaluski MC, Roques BP. Delta9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. European Journal of Neuroscience. 2001;12:533–539. doi: 10.1046/j.0953-816x.2001.01558.x. [DOI] [PubMed] [Google Scholar]
- Vaysse PJ, Gardner EL, Zukin RS. Modulation of rat brain opioid receptors by cannabinoids. Journal of Pharmacology and Experimental Therapeutics. 1987;241:534–539. [PubMed] [Google Scholar]
- Vigano D, Rubino T, Vaccani A, Bianchessi S, Marmorato P, Castiglioni C, Parolaro D. Molecular mechanisms involved in the asymmetric interaction between cannabinoid and opioid systems. Psychopharmacology. 2005;182:527–536. doi: 10.1007/s00213-005-0114-4. [DOI] [PubMed] [Google Scholar]
- Wager-Miller J, Westenbroek R, Mackie K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chemistry and Physics of Lipids. 2002;121:83–89. doi: 10.1016/s0009-3084(02)00151-2. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM, Kitai ST, Linder JC. Three-dimensional structure of dendritic spines in the rat neostriatum. Journal of Neuroscience. 1983;3:383–388. doi: 10.1523/JNEUROSCI.03-02-00383.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Mailliard WS, Gordon AS, Diamond I. Addicting drugs utilize a synergistic molecular mechanism in common requiring adenosine and Gi-beta gamma dimers. Proceedings of the National Academy of Sciences USA. 2003;100:14379–14384. doi: 10.1073/pnas.2336093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Ueda T, Diamond I. Adenosine A2a blockade prevents synergy between mu-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proceedings of the National Academy of Sciences USA. 2006;103:7877–7882. doi: 10.1073/pnas.0602661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung KK, Smith AD, Levey AI, Bolam JP. Synaptic connections between spiny neurons of the direct and indirect pathways in the neostriatum of the rat: evidence from dopamine receptor and neuropeptide immunostaining. European Journal of Neuroscience. 1996;8:861–869. doi: 10.1111/j.1460-9568.1996.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Zamir N, Palkovits M, Weber E, Mezey E, Brownstein MJ. A dynorphinergic pathway of Leu-enkephalin production in rat substantia nigra. Nature. 1980;307:643–645. doi: 10.1038/307643a0. [DOI] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Syková E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends in Pharmacological Sciences. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]