Summary
Diurnal and circadian rhythms are prominent in nearly all bodily functions. Chronic disruptions in normal sleep wake and social schedules can lead to serious health problems such as those seen in shift worker’s syndrome. Moreover, genetic disruptions in normal circadian gene functions have recently been linked to a variety of psychiatric conditions including depression, bipolar disorder, seasonal affective disorder and alcoholism. Recent studies are beginning to determine how these circadian genes and rhythms are involved in the development of drug addiction. Several of these studies suggest an important role for these genes in limbic regions of the brain, outside of the central circadian pacemaker in the suprachiasmatic nucleus (SCN). This review summarizes some of the basic research into the importance of circadian genes in drug addiction.
Introduction
Drug addiction is a devastating disease that affects millions of people worldwide and contributes to the death of over 500,000 Americans per year (NIDA, 2007). The clinical picture of addiction is marked by compulsive drug use that the individual cannot fully control despite adverse consequences. Recent studies have revealed that this is likely a pathology of brain neuroplasticity (Kalivas and O’Brien, 2008). Repeated exposure to drugs of abuse leads to long-lasting changes that are not easily reversed in neuronal circuitry in specific brain regions. Some of these regions include the Nucleus Accumbens (NAc) and the Ventral Tegmental Area (VTA), both of which are part of the mesolimbic dopaminergic system and play a role in reward-related processes (Hyman et al., 2006). Although many aspects of drug addiction have been studied, there is still no truly effective treatment for this chronic disease. A high probability of relapse makes treatment and recovery very challenging. Understanding the molecular mechanisms that underlie the pathophysiological abnormalities that lead from drug use to addiction may help in designing new and more effective treatments. Interestingly, recent studies have suggested that the circadian clock, which controls the sleep/wake cycle and other physiological rhythms that cycle over twenty-four hours, plays an important role in drug addiction.
The Molecular Clock
Most living organisms exhibit daily cycles in behavior and physiology that enable them to adapt to their environment and react to a variety of stimuli known as Zeitgebers or “time-givers” (e.g. light, food, etc.). In mammals, the central pacemaker that controls most of these activity rhythms is located in the SCN of the anterior hypothalamus and is primarily entrained by light (Reppert and Weaver, 2001). In turn, the master clock in the SCN coordinates the timing and activity of other oscillators in other areas of the brain and in peripheral organs, like the kidney and liver (Reppert and Weaver, 2002). Thus, circadian clocks are present throughout the body and regulate a plethora of metabolic and behavioral rhythms. The molecular mechanisms that underlie the circadian clock have been conserved throughout evolution, from cyanobacteria and fungi to insects and mammals.
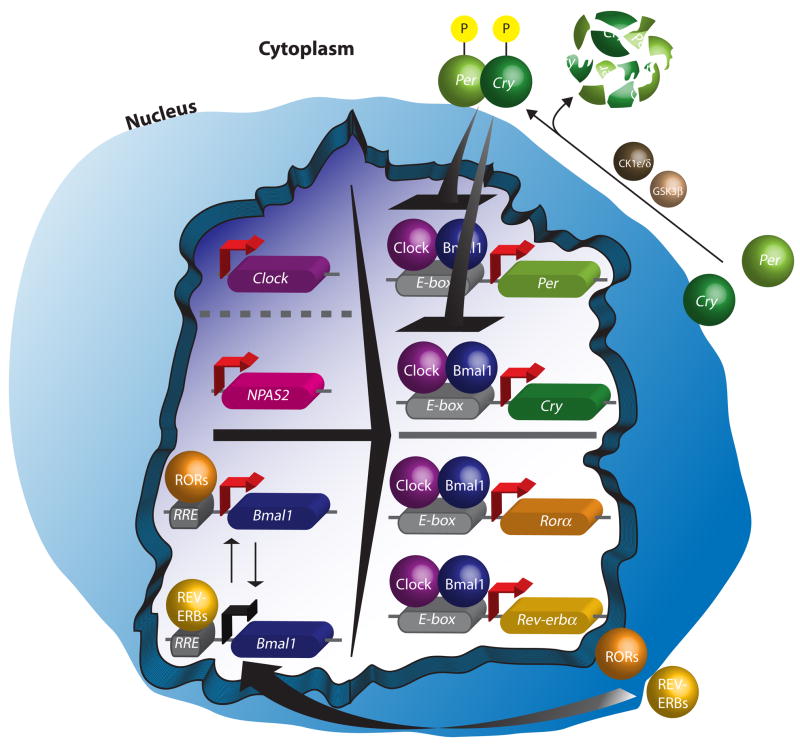
The circadian clock (Fig. 1) is based on a series of interconnected transcriptional positive-negative feedback loops that are regulated over the course of twenty-four hours in the absence of environmental input (Reppert and Weaver, 2001; Ko and Takahashi, 2006). In mammals, the circadian locomotor cycles kaput (CLOCK) and brain and muscle Arnt-like protein-1 (BMAL-1) proteins act as major transcriptional activators by forming a heterodimer that promotes transcription of the Period genes (Per1, Per2, and Per3), the Cryptochrome genes (Cry1 and Cry2), as well as many other genes by binding to E-box elements (CANNTG) in their promoters (Reppert and Weaver, 2001). Following translation of the PER and CRY proteins, they are phosphorylated by casein kinase 1 (CK1) ε and δ, and glycogen synthase kinase 3β (GSK3β). These phosphorylation events can alter PER and CRY stability, dimerization, and nuclear entry (Harms et al., 2003). The PER and CRY proteins dimerize and enter the nucleus to inhibit CLOCK-BMAL1 mediated transcription, hence creating a negative feedback loop. An adjoining oscillatory feedback loop that regulates the expression of Bmal1 by binding to RORE elements in its promoter is composed of the nuclear receptor REV-ERBα and the transcriptional regulator RORA (Reppert and Weaver, 2001). In forebrain regions or in conditions where CLOCK is nonfunctional, Neuronal PAS domain protein 2 (NPAS2), a protein similar in structure and function to CLOCK, can induce expression of the Per and Cry genes (Reick et al., 2001; Debruyne et al., 2006). Interestingly, NPAS2, which has high expression in striatal regions, has been linked to the formation of emotional memory, sleep, and food entrainment (Garcia et al., 2000; Dudley et al., 2003; Franken et al., 2006). A major target of the master circadian clock is the pineal gland, which results in periodic discharge of the hormone melatonin. This hormone is exclusively released at night, even in nocturnal animals, and has been found to promote and regulate sleep and other rhythmic physiological events including seasonal adaptations (Pandi-Perumal et al., 2006).
Figure 1.
Cartoon depicting the molecular clock. CLOCK and BMAL1 (or NPAS2 and BMAL1) regulate the expression of the Period and Cryptochrome genes. These are translated in the cytoplasm and are phosphorylated. They enter the nucleus and inhibit the activity of CLOCK:BMAL1. A separate loop depicted at the bottom of the nucleus shows the regulation of Bmal1 by Rorα and Rev-erbα.
Diurnal and Circadian Rhythms in Drug Addiction
Drug addiction has long been linked to disruptions in diurnal rhythms. For example, drug addicts generally have severe disruptions in their sleep/wake cycle, activity cycles, eating habits, as well as, abnormal rhythms in body temperature, hormone levels, and blood pressure (Wasielewsky et al., 2001; Jones et al., 2003). The disruptions in sleep following drug use are highly problematic, persist long after drug use has ceased, and very often lead to relapse (Jones et al., 2003). Many of these disruptions were originally thought to arise as an indirect result of chronic exposure to drugs of abuse, however, studies have shown that repeated drug use can directly affect ongoing diurnal rhythms. For example, cocaine exposure was found to alter the rhythms of autonomic, immune and sleep mechanisms (Irwin et al., 2007; Morgan et al., 2006). There is also a diurnal variation in the sensitivity to almost all drugs of abuse. Indeed, retrospective studies analyzing the admission of drug overdose patients in the emergency room of urban hospitals revealed that the majority of patients presented at around 6:30 pm compared to other times of day, suggesting a diurnal effect (Raymond et al., 1992), though there may be environmental and societal factors that influence this time of day effect as well. Additionally, addiction may be more prevalent in individuals with a compromised circadian clock, or with mood disorders which may have a circadian basis, such as Major Depressive Disorder, Bipolar Disorder, and Seasonal Affective Disorder, among others (Kandel et al., 2001; Grandin et al., 2006; McClung, 2007). The use of addictive drugs has been found to follow seasonal patterns, with an increase in alcohol use predominantly during the winter, when individuals are more susceptible to depression (McGrath and Yahia, 1993). In addition, people with genetic sleep disorders and insomnia are more prone to addiction (Shibley et al., 2008).
Drug sensitivity is associated with rhythm abnormalities in animal models as well. Rats that were selectively bred based on a high preference for ethanol versus a low preference for ethanol have a shorter free-running period when animals are housed in constant light. One of the lines (the HAD line) also display a “splitting” of circadian activity in that they show two distinct bouts of activity in constant light which is not seen in the low ethanol preferring lines (Rosenwasser et al., 2005). A modest shortening of the free-running period was also found in ethanol-preferring mice compared to those selectively bred for low ethanol preference (Hofstetter et al., 2003). These results suggest that genetic ethanol preference is associated with abnormal circadian rhythms.
Drugs of Abuse Entrain Molecular and Behavioral Rhythms
Even though the master pacemaker is located in the SCN, circadian genes and proteins are widely expressed throughout the brain, thereby forming SCN-independent pacemakers that entrain to other non-photic stimuli such as food (Iijima et al., 2002; Stephan, 1984). Drugs of abuse can also serve as powerful Zeitgebers for some of these clocks outside of the SCN. Several studies have shown that drugs of abuse, like cocaine, methamphetamine, nicotine and alcohol, can entrain locomotor activity rhythms (Kosobud et al., 2007). Furthermore, in rodents with a lesioned SCN, methamphetamine in the drinking water restores their activity rhythms in a robust manner, and animals can be entrained to daily methamphetamine injections (Iijima et al., 2002; Masubuchi et al., 2000). Interestingly, methamphetamine treatment shifts the expression of the Per genes in striatal regions in a manner that matches the shifts in activity rhythms and is independent from the SCN rhythms (Iijima et al., 2002). Furthermore, acute methamphetamine treatment leads to a rapid induction of mPer1, but not mPer2 or mPer3 expression in the dorsal striatum (Nikaido et al., 2001). This suggests that the induction of circadian genes in these regions is specific, or that mPer1 responds to cocaine as an immediate early gene. Indeed, rapid induction of mPer1 is seen in the SCN in response to light (Crosio et al., 2000). Circulating melatonin rhythms remained unaffected by these treatments however; melatonin receptors are differentially regulated in the striatum following chronic cocaine treatment (Imbesi et al., 2006). There are no known direct projections from the SCN to the striatal regions, so melatonin could be a factor which synchronizes these regions. Interestingly, pinealectomy abolishes Per1 rhythms in striatal regions, but has no effect on rhythms in other limbic regions of the brain including the oval nucleus of the bed nucleus of the stria terminalis, the central nucleus of the amygdala, and the hippocampus (Amir et al., 2006). However, it is still unclear what the role for melatonin is in mediating drug-induced responses. It is possible that changes in melatonin signaling in striatal regions could lead to alterations in mood, motivation, or other processes related to addiction.
The Response to Drug Exposure is Different over the Light/Dark Cycle
Several animal studies of addiction have shown that there are diurnal differences in drug-induced behavioral responses, specifically locomotor activity, drug sensitivity, sensitization, conditioned place preference (CPP), and self-administration. A study by Baird and Gauvin found that rats display an increase in the sensitivity to the reinforcing properties of cocaine at 1:00am and 1:00pm compared to rats tested at 7:00am and 7:00pm, indicated by self-administration at lower doses and decreased drug intake (Baird and Gauvin, 2000). However, in general rats show a striking diurnal pattern of self-administration with a greater intake during the active dark phase than during the light phase (Lynch et al., 2008; Roberts et al., 2002). Interestingly, cocaine intake is significantly increased and the diurnal pattern of intake is nearly abolished when animals are given high doses of cocaine (2.5 mg/kg) or access to more trials (Roberts et al., 2002). This loss of diurnal intake rhythms may be very important in the development of addiction in which there is a loss of control and escalation of drug intake that interferes with normal activity (Ahmed and Koob, 1998). In contrast to the self-administration studies, mice treated for several days with cocaine during the day show a greater level of sensitization than those treated at night (Akhirasoglu et al., 2004; Abarca et al., 2002). Moreover, conditioned place preference for cocaine also displays a diurnal rhythm, with greater effects seen when drug is given during the day than during the night (Kurtuncu et al., 2004; Abarca et al., 2002). Intriguingly, studies performed in rats revealed that in opposition to short-term sensitization, long-term sensitization (2 weeks after last injection) was greater when the drug was given at the onset of the dark phase (Sleipness et al., 2005). These studies suggest that there is a change in the reward value for the drug and locomotor sensitivity to the drug over the light/dark cycle that is still not well understood.
A recent study by Sleipness et al. (2007) found that the SCN plays a role in the diurnal regulation of cocaine reward-related behavior. In this study, the authors found that acquisition of CPP behavior was tonically influenced by the SCN, as extinction of CPP behavior was SCN-dependent and reinstatement of CPP behavior was SCN-independent, suggesting an extra-SCN oscillator at work in mediating this behavior (Sleipness et al., 2007a). Many of these diurnal differences in models of addiction may be due to diurnal regulation of dopaminergic transmission in the mesolimbic pathway. In fact, rhythms of cocaine sensitivity correlate with rhythms in postsynaptic levels of dopamine and the activity of the dopaminergic receptors in striatal regions (Naber et al., 1980). Interestingly, studies in Drosophila found that dopamine receptor responsiveness displays a diurnal modulation (Andretic and Hirsh, 2000). Additionally, in mammals the expression of nearly all of the elements involved in dopaminergic transmission have a diurnal rhythm, including the dopamine receptors, the dopamine transporter, and tyrosine hydroxylase (Weber et al., 2004; Schade et al., 1995; Shieh et al., 1997). These diurnal differences in dopamine transporter and tyrosine hydroxylase expression levels are somewhat SCN-dependent, since SCN-lesioned animals have dampened rhythms in comparison to sham controls (Sleipness et al., 2007b). Moreover, a recent study by Hampp et al. (2008) found that the monoamine oxidase A (MAOA) gene, which metabolizes dopamine, is a transcriptional target of BMAL1 and the PER2 protein. PER2 positively regulates its expression and mice with a mutation in Per2 (Per2Brdm1) have a decrease in Maoa expression in the NAc and VTA. These mice also have an increase in midbrain dopamine levels and release and an increase in the sensitization to cocaine (Hampp et al., 2008; Abarca et al., 2002).
In response to drugs of abuse, mesocorticolimbic dopaminergic activity leads to long lasting plasticity in the glutamatergic projections from the prefrontal cortex to the primarily GABAergic NAc (Kalivas, 2007). This altered plasticity is thought to be very important in the development of addiction (Kalivas, 2007). Extracellular levels of glutamate (Glu) and gamma-aminobutyric acid (GABA) in the dorsal striatum and NAc have both a diurnal pattern in light/dark conditions and a circadian rhythm in constant conditions with highest levels at night (Castaneda et al., 2004). Perfusion with melatonin prevents the daytime decrease in both Glu and GABA levels thereby dampening the rhythm (de Prado et al., 2000). This suggests that melatonin regulates striatal rhythms in Glu and GABA transmission. Moreover, expression of the vesicular glutamate transporter 1 (VGlut1) protein in synaptic vesicles has a diurnal rhythm with high levels at the start of the light period which decline by noon, rise again at the start of the dark period and fall again at midnight (Yelamanchili et al., 2006). Mice lacking Per2 do not have any rhythm in VGlut1 expression, suggesting that components of the circadian clock regulate glutamatergic vesicular sorting. Furthermore, mice with a mutation in Per2 also show an increase in glutamate levels on the extracellular space of the NAc, due in part to a reduction in levels of the glutamate transporter Eaat1, and this increase in glutamate is involved in the increased alcohol intake measured in these mice (Spanagel et al., 2005). Thus Per2 plays an important role in regulating the expression of key genes involved in glutamatergic transmission in the striatum.
Circadian Genes in Animal Models of Addiction
Animal studies of drug responsiveness, sensitization, and reward have found that the circadian genes are important regulators of the behavioral responses to drugs of abuse. The first studies that revealed this relationship were done in Drosophila, and found that flies with mutations in the circadian genes Per, Clock, Cycle, or Doubletime all fail to sensitize to cocaine following repeated exposure, while those that had a mutation in the Timeless gene showed normal cocaine responses (Andretic et al., 1999). These pioneering studies provided evidence for the impact of specific circadian genes on addictive processes, which might be conserved throughout evolution. Following these studies, several studies found that cocaine is able to induce or repress specific circadian gene expression in various regions of the mammalian brain. Yuferov et al. found that rPer1 was induced in the dorsal striatum following acute cocaine, while rPer2 was only induced following a chronic “binge” pattern of cocaine (Yuferov et al., 2003). Furthermore, Uz et al., found that chronic cocaine treatment (rather than acute in most cases) resulted in the up or downregulation of several circadian genes in both the striatum and hippocampus (Uz et al., 2005a). These changes were distinct from those observed after chronic treatment with the antidepressant, fluoxetine. Our group has also found that the Period genes as well as Npas2 are regulated in striatal regions in response to chronic cocaine (Peevey et al., submitted; McClung and Nestler, 2003). Moreover, a recent microarray study in animals self-administering cocaine found that with one day of withdrawal, 29 genes were differentially regulated in the striatum that are known to have a circadian function or be associated with the circadian system (Lynch et al., 2008). Using pathway analysis software, Lynch et al. found that indeed changes to the circadian system represented the most significantly altered pathway following cocaine self-administration in the striatum (Lynch et al., 2008). These results suggest that alterations in the molecular clock in the striatum are important in a relevant model of addiction.
The importance of the circadian genes in cocaine preference was first shown by Abarca et al., who found that mice that lack a functional mPer1 gene failed to sensitize to cocaine and show a complete lack of cocaine reward as measured by CPP. In contrast, mice that lack mPer2 exhibited a hypersensitized response after repeated drug exposure with no change in cocaine-induced place preference (Abarca et al., 2002). In addition, mPer1 may partially regulate morphine dependence, since mice treated with a DNAzyme towards this gene and morphine simultaneously show a reduction in the conditioned preference for the drug, while those that were treated with the DNAzyme after the morphine treatment did not show a difference when compared to the control group (Liu et al., 2005). This regulation of morphine reward by mPer1 could be through its regulation of extracellular signal-regulated kinase (ERK) signaling since targeted disruption of mPer1 by DNAzyme prevents the increase in ERK expression that is seen following morphine treatment (Liu et al., 2007). Further studies have found that mPer2 is involved in influencing alcohol consumption. Spanagel and colleagues found that mice carrying a mutation in the PAS domain of Per2 have an increase in alcohol intake that is linked to changes in glutamatergic transmission (Spanagel et al., 2005). The authors also found that variations in the Per2 gene in humans are linked to modulation of alcohol intake, making these variations functionally relevant to human addiction.
Studies from our lab have also identified a role for the Clock gene in cocaine reward and dopaminergic transmission. Mice with a mutation in the Clock gene (Δ19) show a robust sensitization to cocaine, an increase in cocaine preference, and an increase in the reward value for cocaine as measured by intracranial self-stimulation following cocaine treatment (McClung et al., 2005; Roybal et al., 2007). These mice also have an increase in dopaminergic activity in the VTA which may be responsible for the increase in reward value for cocaine (McClung et al., 2005). This includes an increase in dopamine cell firing, bursting, and levels of TH and phosphor-TH (McClung et al., 2005). In further studies it was found that these mice display a complete behavioral profile that is very similar to human bipolar patients in the manic state (Roybal et al., 2007). This is interesting since mania is very often associated with an increase in psychostimulant use (Brown, 2005). Intriguingly, in a separate group of studies, we found that in contrast to the Clock mutant mice, Npas2 mutant mice show a decrease in cocaine preference (Peevey et al., submitted). Additionally, Npas2 expression and activity is enhanced in striatal regions following chronic cocaine treatment while Clock levels remain unchanged. (Peevey et al., submitted). Thus, NPAS2 and CLOCK might have distinct functions in limbic regions in the modulation of cocaine reward.
Conclusions
More and more studies are identifying an important role for circadian rhythms and the genes that make up the circadian clock in the development of drug addiction. Drug addicts generally have very disrupted diurnal rhythms and this suggests that abnormal clock function may lead to an increased vulnerability for addiction. This may be particularly true for people whose drug abuse coincides with periods of depression or mania. Furthermore, drugs of abuse are able to entrain both molecular and behavioral rhythms by altering clocks in limbic brain regions that are able to function in a manner independent of the SCN. Moreover, drugs of abuse alter the expression of circadian genes in reward-related regions of the brain, and the removal of specific circadian genes affects the sensitivity and reward value for several different drugs of abuse. Since the removal of specific genes leads to different responses, it is possible that these circadian genes are serving independent functions in regulating drug responses that are unrelated to their roles in circadian rhythms. It is also possible that these differences are due to the different functions of specific genes in the negative feedback loop that controls rhythms. Moreover, if drugs of abuse are entraining reward-related clocks in brain regions outside of the SCN, this might lead to an increase in drug seeking and craving at the time of day when drugs are anticipated. Future studies will help elucidate the important functions of these genes in the development of addiction.
Acknowledgments
We would like to thank Laurent Coque for his work on the circadian clock diagram and Rachel Arey for helpful comments on the manuscript. Work from our group was funded by NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Akhisaroglu M, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in quinpirole-induced locomotor behaviors and striatal D2/D3 receptor levels in mice. Pharmacol Biochem Behav. 2005;80:371–377. doi: 10.1016/j.pbb.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Amir S, Harbour VL, Robinson B. Pinealectomy does not affect diurnal PER expression in the rat limbic forebrain. Neurosci Lett. 2006;399:147–150. doi: 10.1016/j.neulet.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Andretic R, Hirsh J. Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:1873–1878. doi: 10.1073/pnas.97.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TJ, Gauvin D. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol Biochem Behav. 2000;65:289–299. doi: 10.1016/s0091-3057(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Brown ES. Bipolar disorder and substance abuse. Psychiatr Clin North Am. 2005;28:415–425. doi: 10.1016/j.psc.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36:177–185. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- Crosio C, Cermakian N, Allis CD, Sassone-Corsi P. Light induces chromatin modifications in cells of the mammalian circadian clock. Nat Neurosci. 2000;3:1241–1247. doi: 10.1038/81767. [DOI] [PubMed] [Google Scholar]
- Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Franken P, Dudley CA, Estill SJ, Barakat M, Thomason R, O'Hara BF, McKnight SL. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc Natl Acad Sci U S A. 2006;103:7118–7123. doi: 10.1073/pnas.0602006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JA, Zhang D, Estill SJ, Michnoff C, Rutter J, Reick M, Scott K, Diaz-Arrastia R, McKnight SL. Impaired cued and contextual memory in NPAS2-deficient mice. Science. 2000;288:2226–2230. doi: 10.1126/science.288.5474.2226. [DOI] [PubMed] [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin Psychol Rev. 2006;26:679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Current Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Harms E, Young MW, Saez L. CK1 and GSK3 in the Drosophila and mammalian circadian clock. Novartis Found Symp. 2003;253:267–77. discussion 102–109, 277–284. [PubMed] [Google Scholar]
- Hofstetter JR, Grahame NJ, Mayeda AR. Circadian activity rhythms in high-alcohol-preferring and low-alcohol-preferring mice. Alcoho. 2003;30:81–85. doi: 10.1016/s0741-8329(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur J Neurosci. 2002;16:921–929. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- Imbesi M, Uz T, Yildiz S, Arslan AD, Manev H. Drug- and region-specific effects of protracted antidepressant and cocaine treatment on the content of melatonin MT(1) and MT(2) receptor mRNA in the mouse brain. Int J Neuroprot Neurogener. 2006;2:185–189. [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmos L, Wang M, Valladares EM, Motivala SJ, Fong T, Newton T, Butch A, Olmstead R, Cole SW. Cocaine dependence and acute cocaine induce decreases of monocyte proinflammatory cytokine expression across the diurnal period: autonomic mechanisms. J Pharmacol Exp Ther. 2007;320:507–515. doi: 10.1124/jpet.106.112797. [DOI] [PubMed] [Google Scholar]
- Jones EM, Knutson D, Haines D. Common problems in patients recovering from chemical dependency. Am Fam Physician. 2003;68:1971–1978. [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialoques Clin Neurosci. 2007;9:389–397. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Huang FY, Davies M. Comorbidity between patterns of substance use dependence and psychiatric syndromes. Drug Alcohol Depend. 2001;64:233–241. doi: 10.1016/s0376-8716(01)00126-0. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Suppl 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kosobud AEK, Gillman AG, Leffel JK, II, Pecoraro NC, Rebec GV, Timberlake W. Drugs of abuse entrain circadian rhythms. The Scientific World Journal. 2007;7(S2):203–212. doi: 10.1100/tsw.2007.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol. 2004;489:203–205. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Jiang Z, Wan C, Zhou W, Wang Z. The extracellular signal-regulated kinase signaling pathway is involved in the modulation of morphine-induced reward by mPer1. Neuroscience. 2007;146:265–271. doi: 10.1016/j.neuroscience.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Wan C, Zhou W, Peng T, Wang Z, Li G, Cornelisson G, Halberg F. The role of mPer1 in morphine dependence in mice. Neuroscience. 2005;130:383–388. doi: 10.1016/j.neuroscience.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Girgenti MJ, Breslin FJ, Newton SS, Taylor JR. Gene profiling the resonse to repeated cocaine self-administration in dorsal striatum: A focus on circadian genes. Brain Res. 2008;1213:166–177. doi: 10.1016/j.brainres.2008.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez de Prado B, Castaneda TR, Galindo A, del Arco A, Segovia G, Reiter RJ, Mora F. Melatonin disrupts circadian rhythms of glutamate and GABA in the neostriatum of the aware rat: a microdialysis study. J Pineal Res. 2000;29:209–216. doi: 10.1034/j.1600-0633.2002.290403.x. [DOI] [PubMed] [Google Scholar]
- Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, Honma K. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci. 2000;12:4206–4214. [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath RE, Yahia M. Preliminary data on seasonally related alcohol dependence. J Clin Psychiatry. 1993;54:260–262. [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep dependent procedural learning and vigilance in chronic cocaine users: evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Naber D, Wirz-Justice A, Kafka MS, Wehr TA. Dopamine receptor binding in rat striatum: ultradian rhythm and its modification by chronic imipramine. Psychopharmacology (Berl) 1980;68:1–5. doi: 10.1007/BF00426642. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abbuse. Drugs, brains and behavior: the science of drug addiction. NIH Pub No. 07-5605. 2007. [Google Scholar]
- Nikaido T, Akiyama M, Moriya T, Shibata S. Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol. 2001;59:894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: Nature's most versatile biological signal? FEBS J. 2006;273:2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- Peevey J, Falcón E, Roybal K, Phillips C, Sasaki T, Kumar A, Barrot M, McClung CA. Differential regulation of circadian genes in striatal regions by cocaine, and their role in drug reward. Submitted. [Google Scholar]
- Raymond RC, Warren M, Morris RW, Leikin JB. Periodicity of presentations of drugs of abuse and overdose in an emergency department. J Toxicol Clin Toxicol. 1992;30:467–478. doi: 10.3109/15563659209021561. [DOI] [PubMed] [Google Scholar]
- Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug and Alcohol Dep. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW, Reed JD, Cotter SJ, Seggio JA. Circadian activity rhythms in selectively bred ethanol-preferring and nonpreferring rats. Alcohol. 2005;36:69–81. doi: 10.1016/j.alcohol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, McClung CA. From the cover: mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade R, Vick K, Ott T, Sohr R, Pfister C, Bellach J, Golor G, Lemmer B. Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats–influence on dopaminergic stimulation. Chronobiol Int. 1995;12:87–99. doi: 10.3109/07420529509064504. [DOI] [PubMed] [Google Scholar]
- Shibley HL, Malcolm RJ, Veatch LM. Adolescents with insomnia and substance abuse: consequences and comorbidities. J Psychiatr Pract. 2008;14:146–153. doi: 10.1097/01.pra.0000320113.30811.46. [DOI] [PubMed] [Google Scholar]
- Shieh KR, Chu YS, Pan JT. Circadian change of dopaminergic neuron activity: effects of constant light and melatonin. Neuroreport. 1997;8:2283–2287. doi: 10.1097/00001756-199707070-00037. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Time of day alters long-term sensitization to cocaine in rats. Brain Res. 2005;1065:132–137. doi: 10.1016/j.brainres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Contribution of the suprachiasmatic nucleus to day:night variation in cocaine-seeking behavior. Physiology and Behavior. 2007a;91:523–530. doi: 10.1016/j.physbeh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res. 2007b;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Stephan FK. Phase shifts of circadian rhythms in activity entrained to food access. Physiol Behav. 1984;32:663–671. doi: 10.1016/0031-9384(84)90323-8. [DOI] [PubMed] [Google Scholar]
- Uz T, Ahmed R, Akisaroglu M, Kurtuncu M, Imbesi M, Dirim Arslan A, Manev H. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neurosci. 2005;134:1309–1326. doi: 10.1016/j.neuroscience.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Uz T, Arslan AD, Kurtuncu M, Imbesi M, Akhisaroglu M, Dwivedi Y, Pandey GN, Manev H. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res. 2005;136:45–53. doi: 10.1016/j.molbrainres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Wasielewski JA, Holloway FA. Alcohol's interactions with circadian rhythms. A focus on body temperature. Alcohol Res Health. 2001;25:94–100. [PMC free article] [PubMed] [Google Scholar]
- Weber M, Lauterburg T, Tobler I, Burgunder JM. Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neurosci Lett. 2004;358:17–20. doi: 10.1016/j.neulet.2003.12.053. [DOI] [PubMed] [Google Scholar]
- Yelamanchili SV, Pendyala G, Brunk I, Darna M, Albrecht U, Ahnert-Hilger G. Differential sorting of the vesicular glutamate transporter 1 into a defined vesicular pool is regulated by light signaling involving the clock gene Period2. J Biol Chem. 2006;281:15671–15679. doi: 10.1074/jbc.M600378200. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Kroslak T, Laforge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after "binge" cocaine administration: advantage of triplicate microarray analysis. Synapse. 2003;48:157–69. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]