Abstract
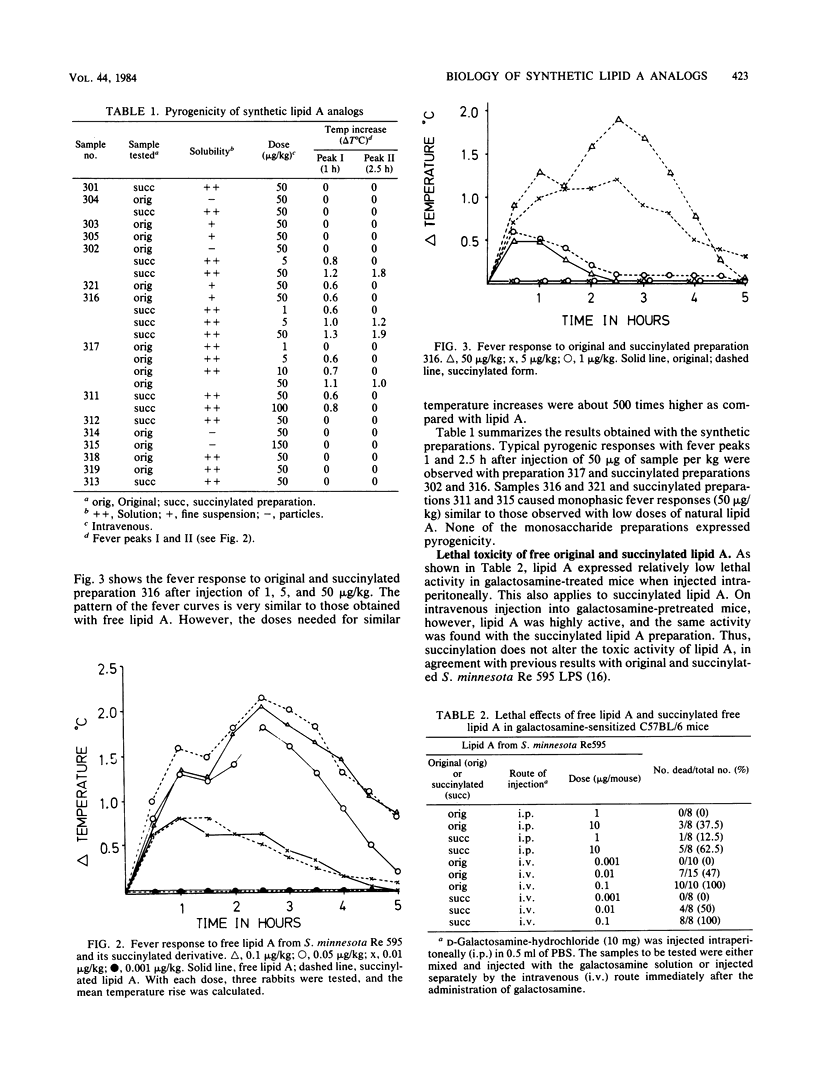
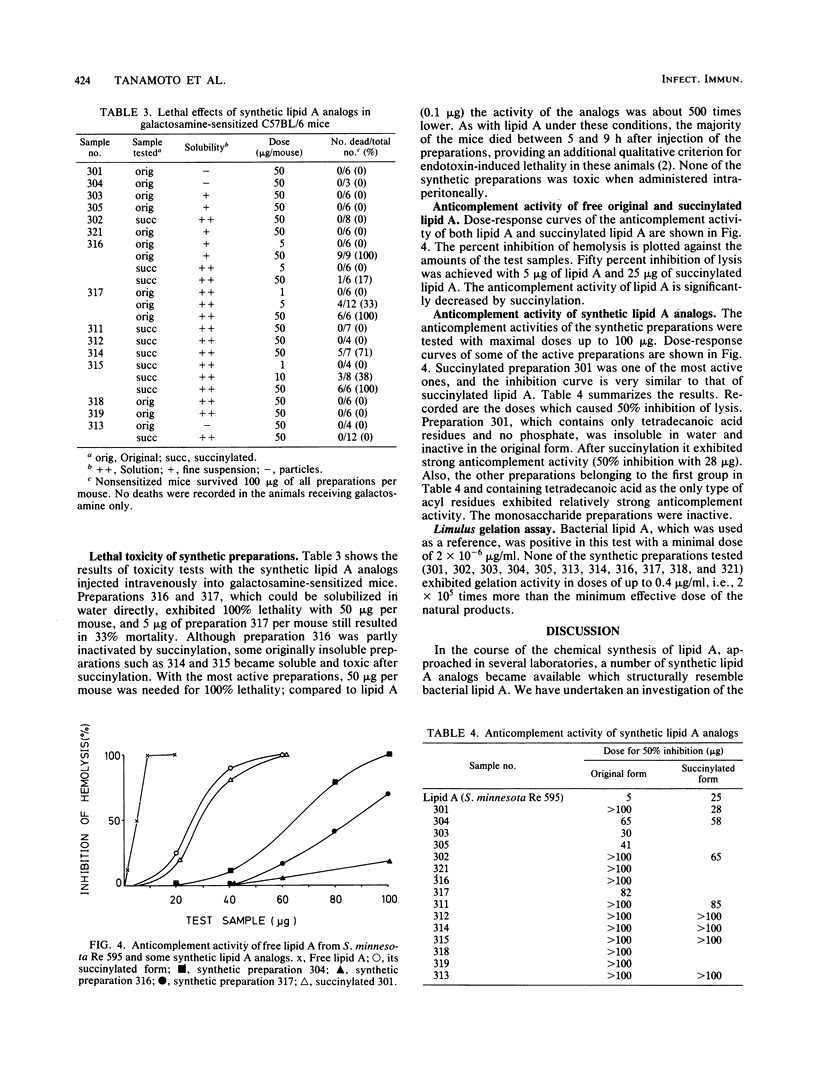
Chemically synthesized lipid A analogs were investigated for several endotoxic activities, including pyrogenicity, lethal toxicity, anticomplement activity, and the capacity to gelate Limulus amoebocyte lysate in comparison to natural lipid A. The synthetic preparations contained D-glucosamine or D-glucosamine-beta-1,6-D-glucosamine disaccharide substituted by ester- and amide-bound hydroxylated or non-hydroxylated fatty acids and by phosphate groups in different combinations. Some preparations which were insoluble in water were succinylated and thus rendered more soluble. Strong biphasic pyrogenic responses with a maximal increase in body temperature of 1 to 2 degrees C were obtained with 50 micrograms/kg doses of 3 disaccharide preparations of 15 tested. With two preparations (50 micrograms/kg) moderate pyrogenicity with monophasic fever curves and a maximal temperature increase of about 0.6 degrees C was obtained. Lethal toxicity tests were carried out in galactosamine-sensitized mice. Of 15 synthetic preparations, 4 exhibited lethal toxicity under these conditions. The effective doses of the lipid A analogs in both in vivo tests were, however, several hundred times higher than those of bacterial lipid A. For the activities in vivo, hydroxyacyl residues seemed to be important. Anticomplement activity was demonstrable in seven preparations, one of which expressed an activity comparable to that of lipid A. Preparations containing non-hydroxylated fatty acids seemed to be most active in this test. None of the synthetic preparations was found to exhibit gelation activity for Limulus amoebocyte lysate when tested in doses up to 0.4 micrograms, whereas bacterial free lipid A was active in doses of about 2 pg. None of the monosaccharide derivatives exhibited any of these activities.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cho Y., Tanamoto K., Oh Y., Homma J. Y. Differences of chemical structures of Pseudomonas aeruginosa lipopolysaccharide essential for adjuvanticity and antitumor and interferon-inducing activities. FEBS Lett. 1979 Sep 1;105(1):120–122. doi: 10.1016/0014-5793(79)80899-6. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Mori Y., Sakuta M., Kawasaki A., Inage M., Kusumoto S., Shiba T. Immunobiological activities of synthetic lipid A analogs and related compounds as compared with those of bacterial lipopolysaccharide, re-glycolipid, lipid A, and muramyl dipeptide. Infect Immun. 1983 Aug;41(2):758–773. doi: 10.1128/iai.41.2.758-773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Mayer H., Rietschel E. T., Weckesser J. Chemical structure and biological activities of lipid A's from various bacterial families. Naturwissenschaften. 1978 Nov;65(11):578–585. doi: 10.1007/BF00364907. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Galanos C. Lipid A antiserum-mediated protection against lipopolysaccharide- and lipid A-induced fever and skin necrosis. Infect Immun. 1977 Jan;15(1):34–49. doi: 10.1128/iai.15.1.34-49.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Galanos C., Tanaka A., Ruschmann E., Lüderitz O., Westphal O. Biological activities of chemically modified endotoxins. Eur J Biochem. 1971 Sep 24;22(2):218–224. doi: 10.1111/j.1432-1033.1971.tb01535.x. [DOI] [PubMed] [Google Scholar]
- Schenck J. R., Hargie M. P., Brown M. S., Ebert D. S., Yoo A. L., McIntire F. C. The enhancement of antibody formation by Escherichia coli lipopolysaccharide and detoxified derivatives. J Immunol. 1969 Jun;102(6):1411–1422. [PubMed] [Google Scholar]
- Tanamoto K., Abe C., Homma J. Y., Kojima Y. Regions of the lipopolysaccharide of Pseudomonas aeruginosa essential for antitumor and interferon-inducing activities. Eur J Biochem. 1979 Jul;97(2):623–629. doi: 10.1111/j.1432-1033.1979.tb13152.x. [DOI] [PubMed] [Google Scholar]
- Tanamoto K., Homma J. Y. Essential regions of the lipopolysaccharide of Pseudomonas aeruginosa responsible for pyrogenicity and activation of the proclotting enzyme of horseshoe crabs. Comparison with antitumor, interferon-inducing and adjuvant activities. J Biochem. 1982 Mar;91(3):741–746. doi: 10.1093/oxfordjournals.jbchem.a133760. [DOI] [PubMed] [Google Scholar]
- Yasuda T., Kanegasaki S., Tsumita T., Tadakuma T., Homma J. Y., Inage M., Kusumoto S., Shiba T. Biological activity of chemically synthesized analogues of lipid A. Demonstration of adjuvant effect in hapten-sensitized liposomal system. Eur J Biochem. 1982 May 17;124(2):405–407. [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]


