INTRODUCTION
Proceeding in four steps, this review uses research on pediatric anxiety disorders to illustrate how fMRI studies might inform therapeutics. First, we describe how, in developmental psychopathology generally, basic and clinical fMRI research guide each other. Second, we discuss how investigators probe specific behaviors associated with anxiety. Herein we focus on one core feature of anxiety, the abnormal modulation of attention. Pictures of angry faces and other threatening stimuli capture attention to a greater degree in anxious than non-anxious individuals; in this way, such stimuli interact with and influence a cognitive process called “attention orienting”. Using threatening stimuli to manipulate the focus of attention, fMRI studies might eventually test whether interventions such as cognitive behavioral therapy (CBT) reduce anxiety by altering the abnormal orienting of attention.
Building on research in animal models, investigators have generated specific hypotheses about which brain regions contribute to abnormal attention orienting in pediatric anxiety disorders. Thus in our third section, we use examples from fMRI research in adolescents to describe how these hypotheses can now be tested. The paper concludes by describing how this general research approach generates novel insights that are potentially relevant to therapeutics.
THE INTERFACE OF BASIC AND CLINICAL RESEARCH
Perhaps the most exciting aspect of fMRI research derives from the opportunities it provides to bridge basic and clinical domains of psychiatric research. Such connections provide the possibility of grounding developmental psychopathology theory, as well as psychiatric practice, in neuroscience. The brain is the ultimate arbiter of inter-individual differences in cognitions, emotions, and behavior; therefore, the major goal of psychiatric treatment is to influence cognition, emotions, and behavior when they hamper function. fMRI provides the opportunity to observe these processes almost in real time.
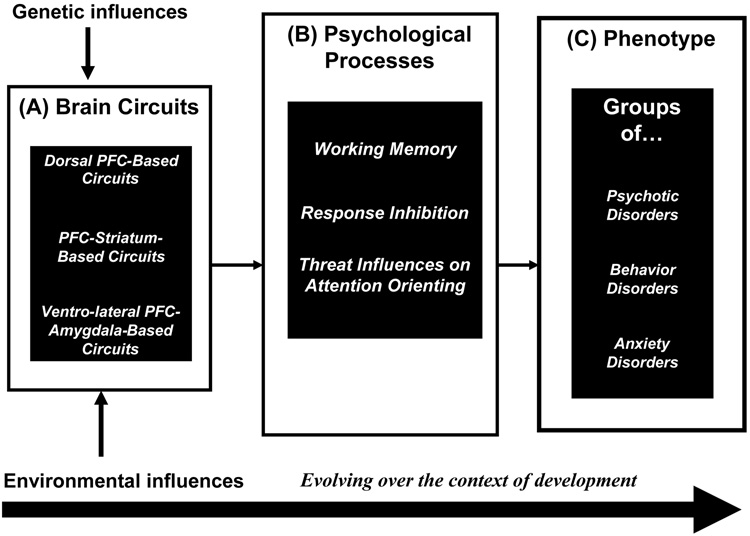
As shown in Figure 1 (Box A), genetic and environmental determinants influence the function of collections of brain structures operating within circuits. In turn, brain circuits mediate psychological processes (Box B), thus shaping behavior. Figure 1 highlights three broadly conceptualized circuits (Box A), which in turn influence correspondingly broad psychological processes (Box B) and groups of disorders, or phenotypes (Box C). These circuits, psychological processes, and phenotypes are all subject to change across development, and the pathways among them do not necessarily follow a one-to-one correspondence. Furthermore, the circuits and psychological processes are unlikely to map neatly onto DSM-based phenotypes. These caveats apply even to broadly conceptualized phenotypes, which remain descriptive and far removed from current understandings of brain-behavior associations. Indeed, as fMRI research continues to bridge neuroscience and clinical description, categories of psychiatric disorders are likely to change. Such changes will allow psychiatric diagnosis to evolve from resting primarily on clinical description to resting on clinical description integrated with knowledge of pathophysiology, as occurs throughout medicine.
Figure 1.
A schematic framework of mechanisms underlying associations among neural circuits (Box A), psychological processes (Box B), and clinical phenotypes (Box C), as influenced by genes, environments, and development. Pre-frontal cortex = PFC.
The framework in Figure 1 illustrates how studying individual differences in psychological processes (Box B) can bridge research on the brain (Box A) and clinical phenotypes (Box C). Thus, Figure 1 suggests that one key to linking clinical and neuroscience research rests on the careful identification of relevant, symptom-specific psychological processes (Box B) as the focus of research.
STUDYING PSYCHOLOGICAL PROCESSES MEDIATING ILLNESS: THE EXAMPLE OF THE DOT-PROBE TASK
Research on anxiety provides a particularly rich avenue for integrating basic and clinical data, since brain-behavior correlates of anxiety identified in rodents and non-human primates also exist in humans.1 By mapping relationships among brain function, psychological processes, and clinical features, 2 one can see how abnormal response to threats, and particularly the abnormal modulation of attention by threats, underlies or produces the symptoms of anxiety.
The neural circuitry engaged by threats have striking cross-species parallels. Across species, threats have a unique ability to capture attention: when mammals process complex stimulus arrays, threats compete successfully with other stimuli for limited neural and cognitive resources. Studies in rodents map precisely the timing of brain activity during the processing of threatening stimuli.1–3 Similarly, in humans, specific paradigms exist for assessing the timing of threat-attention interactions. This allows researchers to extend work in rodents while studying individual differences in humans, as those differences relate to psychopathology and brain function.4
Paradigms used successfully in humans engage relatively specific behaviors associated with the capture of attention. The “dot-probe” paradigm arguably provides the most consistent evidence linking individual differences in anxiety to individual differences in attention-orienting.5–7
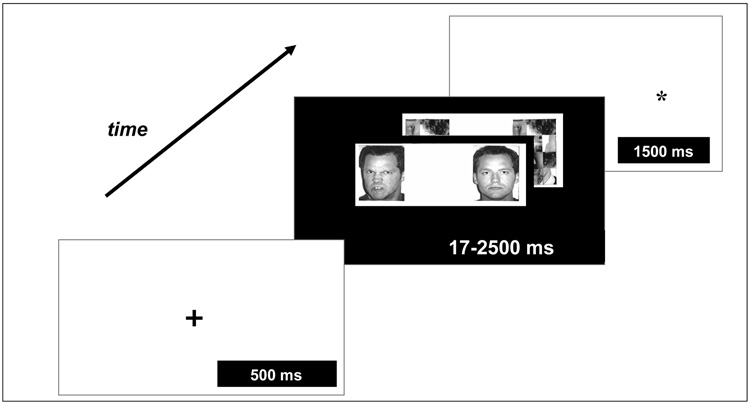
Figure 2 depicts one version of the dot-probe paradigm. In this task, participants first see a “warning” cue. Next, a picture of an angry-threat or neutral face appears on the left or right side of the screen. Finally, a “dot-probe” cue appears on the left or right, and participants must indicate on which side of the screen this dot-probe cue has appeared.
Figure 2.
An example of the “dot-probe” paradigm used to assess attention orientation and its modulation by threats. A cue is presented, which is followed by the presentation of pairs of stimuli depicting threat or non-threat cues, which can be presented for varying time durations across trials (e.g. 17 milliseconds [msec]; 500 msec). Next, a dot-probe appears to which participants respond via a button press. Attention bias to threat can be assessed through the time it takes to respond to the probe and associated changes in neural response.
In this paradigm, angry faces represent threats, whereas neutral faces are control stimuli. The orienting of attention to threatening stimuli can be measured using reaction time (RT) to the dot-probe on trials displaying angry faces. For example, researchers compare RTs on trials in which the dot-probe cue and angry face are on the same side of the screen, to RT on trials in which the dot-probe cue and angry face are on opposite sides. In the latter case, attention might be captured initially by the angry face, but then must be re-allocated to the opposite side when the dot-probe appears. The longer an individual takes to shift attention from the angry face to the dot-probe, the greater the bias of attention to threats. A greater attentional bias in anxious, relative to non-anxious, individuals represents the best-replicated finding on this task.5
Basic research delineates the timing with which specific neural systems are engaged during attention orienting.1–3 Modifications of the dot-probe paradigm extend these findings to humans. For example, the duration of angry-face displays can be varied to manipulate the length of the exposure to threat (Figure 2). Such variations in the task isolate immediate from later, more elaborated behavioral responses to threatening stimuli. Moreover, performance on the dot-probe task can be monitored over hours or, with repeated training, over days, whereupon individuals, including adolescents, learn to change their focus of attention.8, 9 Studies charting such gradually-evolving behavioral plasticity might inform work on the emergence and maintenance of anxiety across development.8–10
Use of the dot-probe task in research on healthy and anxious youth illustrates how investigators can use behavioral tasks to study psychological processes in clinical phenotypes, thus linking Boxes B and C in Fig. 1. In fMRI studies, data are acquired regarding the functional characteristics of neural systems that mediate these connections (Box A, Fig. 1). Thus, in fMRI, selection of a clinically-relevant behavioral task for participants to perform during scanning represents one of the most important aspects of study design. For this reason, data gathered outside the scanner on tasks such as the dot-probe are a crucial prelude to fMRI studies.
In sum, the work described above illustrates how, by building on basic research, investigators can design behavioral tasks that assess psychological processes relevant to psychiatric illness, thus setting the stage for fMRI studies linking circuitry function, psychological processes, and clinical phenotypes (Fig. 1).
NEURAL CIRCUITRY AND ADOLESCENT ANXIETY
As illustrated by a recent study using the dot-probe task,11 fMRI research in adolescents has successfully linked the function of neural circuits with psychological processes and clinical anxiety. Drawing on basic research, this study hypothesized that individual differences in pediatric anxiety were linked to individual differences in amygdala function. Rodent studies suggest that activation of the amygdala is particularly important for the rapid processing of threatening stimuli. Thus, individual differences in function of the amygdala in adolescents were expected to be evident specifically during the rapid processing of threatening stimuli. To test this hypothesis, the study used threat exposures lasting only 17 milliseconds (msec). As predicted, anxious adolescents showed more activation of the amygdala than did their non-anxious peers. Moreover, amygdala activation correlated positively both with attention bias, as measured with RTs, and anxiety severity, as measured clinically. This finding suggests that, in anxious relative to non-anxious youth, attention is more readily influenced by threatening stimuli, and that this influence is associated with or mediated by excessive activity of the amygdala.
A second fMRI dot-probe study also compared anxious and healthy adolescents, but used 500 msec, as opposed to 17 msec, durations of exposure to threatening stimuli.12 By examining results across the two studies, scientist can learn how the duration of threat exposure modulates brain activity in regions implicated in anxiety. With longer, 500 msec threats, anxious and non-anxious adolescents had similar amygdala responses. However, relative to non-anxious adolescents, anxious adolescents showed hyperactivity in the ventrolateral prefrontal cortex (vlPFC). Compared to the amygdala, the vlPFC mediates more comprehensive and extended processing of threatening stimuli.13 During long exposures, activation of the vlPFC correlated negatively with the severity of anxiety, contrasting with the positive correlation between amygdala activation and the severity of anxiety during short threat exposures. Together, these findings suggest that activation of the amygdala may reflect processes associated with anxiety itself, whereas activation of the vlPFC may reflect processes associated with modulation of both amygdala activity and the associated emotional response.
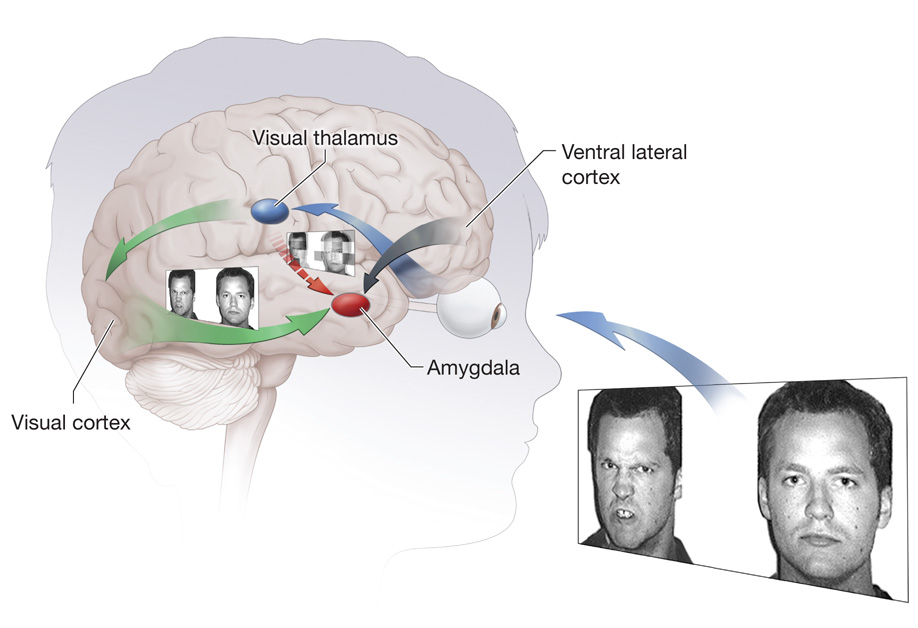
Figure 3 illustrates how associations among anxiety, brain function, and attention might unfold when adolescents encounter threats. As represented by blue and red arrows, detail-poor visual information about threats is processed immediately in a circuit connecting the thalamus and amygdala. Individual differences in anxiety could relate to hypersensitivity of this circuit. A second circuit component, depicted in green, mediates cortically-based, gradual, elaborative processing of threats, thus providing conceptually rich information to the amygdala. The implementation of specific behaviors, such as indicating where on the screen a dot-probe appears, occurs after vlPFC integration of signals from the amygdala, which represent the salience of stimuli, with goal-relevant information. The vlPFC-amygdala circuits become active when goals require attentional shifts either toward or away from locations associated with threat.13
Figure 3.
An illustration of two core neural circuits engaged during threat processing. The amygdala-thalamus circuitry immediately processes simple, low-level information about threats. The visual cortex and amygdala-ventrolateral-prefrontal-cortex circuitry processes complex information about threats and integrates this information with representations of goals that guide behavior.
Together, Figure 3 and the two associated fMRI-dot-probe studies in adolescents extend to humans a model of interaction between threat and attention that is derived from rodent research and that generates therapeutically relevant insights. For example, anxiety-related attention orienting engages subcortical and cortical brain regions that operate on different time scales. In CBT, explicit instructions to patients may induce plasticity in cortical systems that mediate slowly emerging processes. However, perturbed attention orienting in states of anxiety also occurs rapidly, suggesting that computer-based attention re-training in anxious youths might be implemented through repeated exposures to threatening stimuli in the context of a dot-probe paradigm. Such training might target rapidly-deployed attention-related processes that are perturbed in anxiety, in ways not targeted by CBT.9, 10, 14 Indeed, such training regimens have been shown to alter the reactivity to stress.8, 10, 14
Finally, these clinical studies suggest that experiments could be conducted in animals that involve the manipulation of specific brain regions that activate during attention orienting to threatening stimuli. For example, researchers might manipulate activity in the vlPFC to determine its influence on amygdala function, anxiety, and attention. These circuitry-based manipulations might produce in animal models clinically-relevant changes in both attention-orienting and fear responses. Once tight, causal relationships have been demonstrated between vlPFC activity, attention orienting, and anxiety, animal models might further serve to delineate specific training regimens that produce robust changes in vlPFC activity and that have reverberating influences on attention-orienting and anxiety. Such work then could generate hypotheses regarding the timing of stimulus delivery and the number of repetitions that should be used in attention retraining paradigms for anxious adolescents. Thus, studies in animals could generate insights about the specific form that attention-retraining should take to produce robust changes in neural circuits that subserve anxiety, thus illustrating how the interactions of basic and clinical research paradigms (Fig. 1) builds bridges from bench-to-bedside and back again.
CONCLUSIONS
By summarizing data from dot-probe studies in anxious youth, this review demonstrates how fMRI methodology can provide a bridge to basic research. The core tenets of this framework rest on using fMRI to study the neural correlates of psychological processes in clinical samples. Specifically, fMRI can be used to demonstrate parallels between the neural architecture of attention-orienting behavior in rodents or non-human primates, on the one hand, and the neural architecture of individual differences in human anxiety, on the other. Ongoing work suggests that experimental manipulations of attention may provide novel therapeutic approaches that target functions within a specific neural circuit. Thus, this clinical-to-basic-to-clinical pathway might guide the development of novel treatments by integrating research on clinical phenotypes with research on brain circuits and their influence by genes, environments, and development.
Footnotes
Disclosures: The authors report no conflicts of interest.
References
- 1.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 2.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 3.LeDoux J. Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 1998;44(12):1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- 4.Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 5.Bar-Haim Y, et al. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behav Res Ther. 1998;36(9):809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 7.Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. J Abnorm Psychol. 2000;109(4):695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- 8.Eldar S, Ricon T, Bar-Haim Y. Plasticity in attention: implications for stress response in children. Behav Res Ther. 2008 doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Monk CS, et al. Experience-dependent plasticity for attention to threat: Behavioral and neurophysiological evidence in humans. Biol Psychiatry. 2004;56(8):607–610. doi: 10.1016/j.biopsych.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 10.MacLeod C, et al. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol. 2002;111(1):107–123. [PubMed] [Google Scholar]
- 11.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortext activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008 doi: 10.1001/archpsyc.65.5.568. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monk CS, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 13.Blair RJ, et al. The development of psychopathy. J Child Psychol Psychiatry. 2006;47(3–4):262–276. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- 14.Dandeneau SD, et al. Cutting stress off at the pass: reducing vigilance and responsiveness to social threat by manipulating attention. J Pers Soc Psychol. 2007;93(4):651–666. doi: 10.1037/0022-3514.93.4.651. [DOI] [PubMed] [Google Scholar]