Abstract
Background
Gut bifidobacteria are believed to influence immune-related diseases. The objective of this study was to assess the possible relationships between the gut bifidobacteria composition and coeliac disease (CD) in children.
A total of 48 faecal samples (30 and 18 samples from active and no active CD patients, respectively) and 33 duodenal biopsy specimens of CD patients (25 and 8 samples from active and non-active CD patients, respectively) were analysed. Samples (30 faecal samples and 8 biopsies) from a control age-matched group of children were also included for comparative purposes. Gut Bifidobacterium genus and species were analyzed by real-time PCR.
Results
Active and non-active CD patients showed lower numbers of total Bifidobacterium and B. longum species in faeces and duodenal biopsies than controls, and these differences were particularly remarkable between active CD patients and controls. B. catenulatum prevalence was higher in biopsies of controls than in those of active and non-active CD patients, whereas B. dentium prevalence was higher in faeces of non-active CD patients than in controls. Correlations between levels of Bifidobacterium and B. longum species in faecal and biopsy samples were detected in both CD patients and controls.
Conclusion
Reductions in total Bifidobacterium and B. longum populations were associated with both active and non-active CD when compared to controls. These bacterial groups could constitute novel targets for adjuvant dietary therapies although the confirmation of this hypothesis would require further investigations.
Background
Coeliac disease (CD) is a chronic inflammatory disorder of the small intestine that presents in genetically predisposed individuals following gluten consumption. Gluten removal from the diet is currently the only treatment available. This disease often presents in early childhood with small intestinal villous atrophy and signs of malabsorption [1]. Recently, other factors than gluten such as imbalances in the intestinal microbiota have been reported to be associated with CD [2-5]. Most of these studies have been focused on faecal microbiota composition but less information is available on mucosa-associated microbiota of CD patients [2,5]. Neither possible relation between faecal and duodenal bacterial populations has been reported in CD.
Bifidobacterium genus constitutes an important bacterial group in the human gut, where this is thought to be essential to maintain health via beneficial metabolic, trophic, and protective functions [6,7]. Bifidobacterium is the predominant intestinal bacterial genus during the first year of life, particularly in full-term breastfed infants, although becomes quantitatively less important in adult's microbiota [8,9]. Qualitative and quantitative differences in Bifidobacterium species composition have been related to the development of inflammatory diseases such as allergy, irritable bowel syndrome (IBS), inflammatory bowel diseases (IBD) and colorectal cancer compared to healthy controls [10-12]. In addition, different immunomodulatory properties have been attributed to different Bifidobacterium species and strains that in turn were related to different disease risks. Strains of B. adolescentis have been shown to be more proinfammatory or had non-effect on immunity, while strains of the species B. bifidum and B. longum were shown to have immunoregulatory properties [13-15]. In this context, it has been suggested that Bifidobacterium strains colonizing the human gut could contribute to regulate disturbances in the balance of T-helper 1 (Th1)/Th2 lymphocyte responses to exogenous antigens related to either allergic diseases (characterized by a Th2-phenotype polarization) or Crohn and CD (characterized by a Th1 phenotype polarization). As a consequence, Bifidobacterium species have been regarded as particularly attractive targets for dietary intervention within the gut ecosystem to maintain intestinal homeostasis and host health.
The objective of this study was to assess the Bifidobacterium species composition of duodenal biopsies and faecal samples from CD patients (with active and non-active disease) and age-matched controls by the use of quantitative real-time PCR technique to elucidate their possible role in this disorder.
Methods
Subjects
Three groups of children were included in this study: (1) active CD patients on a normal gluten-containing diet; (2) non-active CD patients after following a gluten-free diet for at least 2 years; and (3) control children without known gluten intolerance. Biopsy specimens of the control group were obtained from children who were investigated for weight loss, growth retardation or functional intestinal disorders of non-coeliac origin, confirmed by showing a normal villous structure after diagnosis by biopsy examination.
The following faecal samples and duodenal biopsy specimens from these group of subjects were included in the analyses: 30 faecal and 25 biopsy samples from active CD patients; 18 faecal and 8 biopsy samples from non-active CD patients and 30 faecal and 8 biopsy samples from control children.
None of the children included in the study was treated with antibiotics for at least 1 month before the sampling time and they were recommended not consuming probiotic-containing products for at least 15 days prior the sampling time to limit the detection of food-related bifidobacteria without delaying too much the diagnosis procedure. The adherence to this dietary recommendation was checked at the sampling time and children that did not comply with this recommendation were not included in the study. The study protocol was approved by the local committee on ethical practice from CSIC and Hospitals taking part in the study. Children were enrolled in the study after written informed consent was obtained from their parents.
Sampling preparation and DNA extraction
Samples were collected from every subject in sterile plastic recipients, frozen at -20°C immediately and kept at -80°C until further processing. Duodenal biopsy specimens were obtained by capsule and endoscopy after a 12-h fasting period. Faeces (1 g) and duodenal biopsy samples (10–15 mg) were weighted, diluted 1:10 (w/v) in PBS (pH 7.2) and homogenized by thorough agitation in a vortex. Aliquots of these dilutions were used for DNA extraction. DNA from both type of samples (faeces and biopsies) and from pure cultures of the different bacterial strains used as reference were extracted by using the QIAamp DNA stool Mini kit (Qiagen, Hilden, Germany) following the manufacturer's instructions.
Real-time PCR (qPCR) analysis
Quantitative real time PCR was used to characterize the faecal microbiota by using group and species-specific primers described previously [16,17]. Briefly, PCR amplification and detection were performed with an ABI PRISM 7000-PCR sequence detection system (Applied Biosystems, UK). Each reaction mixture of 25 μl was composed of SYBR® Green PCR Master Mix (SuperArray Bioscience Corporation, USA), 1 μl of each of the specific primers at a concentration of 0.25 μM, and 1 μl of template DNA. The fluorescent products were detected at the last step of each cycle. A melting curve analysis was made after amplification to distinguish the targeted PCR product from the non-targeted PCR product. Bacterial concentration from each sample was calculated by comparing the Ct values obtained from standard curves. A standard curve was made from serial dilutions of DNA isolated from each pure culture of the different reference strains. A linear relationship was observed between the cell number and CT values (r2 = 0.99–0.97) when the DNA was isolated from cultures containing between 102 and 109 log cells/ml, as determined by epifluorescence microscopy counts using DAPI. The following reference strains were used to construct the corresponding standard curves: Bifidobacterium longum subsp. longum CECT 4503, B. longum subsp. infantis CECT 4553, B. bifidum LMG 11041, B. breve LMG 11042, B. pseudocatenulatum CECT 5776, B. animalis subsp. lactis DSMZ 10140, B. adolescentis LMG 11037, and B. dentium CECT 687. The strains were obtained from the Spanish Collection of Type Cultures (CECT), the German Collection of Microorganisms and Cell Cultures (DSMZ) and the Belgian Coordinated Collections of Microorganisms (BCCM-LMG, University of Gent).
Statistical analyses
Statistical analyses were done using the SPSS 11.0 software (SPSS Inc, Chicago, IL, USA). Due to non-normal distribution, microbial data are expressed as medians with interquartile ranges (IQR). Comparisons among data of more than two groups of children were done by applying the Kruskal-Wallis test and comparisons between data of two groups of children were done by applying the Mann-Whitney U test. The possible correlation between variables was studied by applying the Spearman rank correlation coefficient and significance was established at 0.5%. The chi-square test was used to establish differences in species prevalence between the studied groups of children. A P < 0.050 was considered statistically significant. Bonferroni adjustment test was also applied to correct the significance for a multiple test comparisons among three groups (active and non-active CD patients and control groups), which has the advantage of reducing type I error and the disadvantage of increasing type II error.
Results
Subjects
Clinical characteristics of the children groups included in the study are shown in Table 1. Relative representation of males and females was almost equivalent in the study. Active CD patients were on a normal gluten-containing diet, showed clinical symptoms of the disease, positive CD serology markers (anti-gliadin antibodies AGA and anti-transglutaminase antibodies t-TG) and signs of severe enteropathy by duodenal biopsy examination classified as type 3 according to Marsh classification of CD [18]. Non-active CD patients, who had been on a gluten-free diet for at least 2 years, showed negative CD serology markers and normal mucosa or infiltrative lesion classified as type 0–1 according to Marsh classification. A total of 30 faecal samples from 56.4 months old children, and 25 biopsies from 60.6 months old children were included in the active CD patient group. A total of 18 faecal samples of 63.5 months old children and 8 biopsies of 57.8 months old children were included in the non-active CD patient group. Finally, a total of 30 faecal samples of 45.0 months old children and 8 biopsies of 49.2 months old children were included in the control group for comparative purposes.
Table 1.
Clinical characteristics of the studied subjects.
| Characteristics | Active CD | Non-active CD | Control |
| Number of patients | 30 | 18 | 30 |
| Age (average months and SD) | 56.4 (38.5) | 65.2 (37.7) | 45.0 (33.5) |
| Gender | |||
| - male | 12/30 (40.0%) | 8/18 (44.4%) | 13/30 (43.3%) |
| - female | 18/30 (60.0%) | 10/18 (55.6%) | 17/30 (56.7%) |
| Clinical | |||
| - Abdominal | 2/30 (6.6%) | - | - |
| - Diarrhoea | 28/30 (93.4%) | 2/18 (11.1%) | - |
| - Weight loss | 9/30 (30.0%) | - | - |
| - Anaemia | 14/30 (46.6%) | 8/18 (44.4%) | - |
| Biochemical | |||
| - Asymptomatic | 4/30 (13.3%) | 18/18 (100%) | - |
| - Iron deficiency | 10/30 (33.3%) | - | - |
| Serology | |||
| AGA (anti-gliadin antibodies) | AGA + (100%) | AGA + (0%) | AGA + (0%) |
| t-TG (anti-transglutaminase antibodies) | t-TG + (100%) | t-TG + (0%) | t-TG + (0%) |
| Duodenal Biopsy a | M3 (100%) | M0-1 (100%) | M0-1 (100%) |
a Modified Marsh Classification of CD[18]. M0: Normal mucosa; CD highly unlikely. M01: (Infiltrative lesion): Seen in patients on a gluten-free diet (suggesting minimal amounts of gliadin are being ingested); patients with dermatitis herpetiformis (DH); and family members of patients with CD. M2 (Hyperplasic type): seen occasionally in DH. M3: > 40 Intraepithelial Lymphocytes per 100 Enterocytes, crypts increased and villi with atrophy (partial or complete villous atrophy).
Duodenal Bifidobacterium species composition
B. longum was one of the most frequently detected species in biopsy samples followed by B. breve, B. bifidum, B. catenulatum and B. lactis (Table 2). Currently, the species B. longum included B. longum subsp. longum, B. longum subsp. infantis and B. longum subsp. suis, which were quantified with the set of primers for the quoted species. B. breve was significantly more prevalent in active CD patients than in non-active CD patients and controls although the differences were not significant (P > 0.05). B. catenulatum group was detected more frequently in controls than in active CD (P = 0.050) and non-active CD patients (P = 0.038). In addition, B. lactis group was detected more frequently in active CD patients (P = 0.003) and in controls (P = 0.012) than in non-active CD patients. B. dentium was found in active CD and non-active CD patients but not in controls. The prevalence of B. lactis group was also significantly different in active CD patients and controls as compared to that of non-active CD patients by applying the Bonferroni adjustment, but this was not the case for the rest of bacterial groups.
Table 2.
Prevalence of Bifidobacterium group and species in faeces and duodenal biopsies of children
| Microbial group in biopsy samples | Prevalence (%)a | P-value Chi-square test Bonferroni adjustment | ||||
| Active CD (n = 25) | Non-active CD (n = 8) | Control (n = 8) | Active- non-active CD | Control- active CD | Control- non-active CD | |
| Bifidobacterium group | 100.0 (25/25) | 100.0 (8/8) | 100.0 (8/8) | - | - | - |
| B. longum | 100.0 (25/25) | 100.0 (8/8) | 100.0 (8/8) | - | - | - |
| B. breve | 64.0 (16/25) | 37.5 (3/8) | 37.5 (3/8) | 0.181 | 0.181 | 0.695 |
| B. bifidum | 52.0 (13/25) | 25.0 (2/8) | 37.5 (3/8) | 0.180 | 0.380 | 0.500 |
| B. adolescentis | 36.0 (9/25) | 12.5 (1/8) | 25.0 (2/8) | 0.380 | 0.669 | 0.500 |
| B. catenulatum group | 52.0 (13/25) | 37.5 (3/8) | 87.5 (7/8) | 0.381 | 0.050* | 0.038* |
| B. angulatum | 32.0 (8/25) | 12.5 (1/8) | 50.0 (4/8) | 0.277 | 0.419 | 0.282 |
| B. lactis | 60.0 (15/25) | 0.0 (0/8) | 62.5 (5/8) | 0.003*, i | 0.618 | 0.012*, i |
| B. longum subsp. infantis | 0.0 (0/25) | 0.0 (0/8) | 0.0 (0/8) | - | - | - |
| B. dentium | 8.0 (2/25) | 12.5 (1/8) | 0.0 (0/8) | 0.578 | 0.568 | 0.500 |
| Microbial group in faecal samples | Prevalence (%)a | P-value Chi-square test Bonferroni adjustment | ||||
| Active CD (n = 30) | Non-active CD (n = 18) | Control (n = 30) | Active- non-active CD | Control- active CD | Control- non-active CD | |
| Bifidobacterium group | 100.0 (30/30) | 100.0 (18/18) | 100.0 (30/30) | - | - | - |
| B. longum | 100.0 (30/30) | 100.0 (18/18) | 100.0 (30/30) | - | - | - |
| B. breve | 80.0 (24/30) | 66.7 (12/18) | 66.7 (20/30) | 0.325 | 0.500 | 0.751 |
| B. bifidum | 100.0 (30/30) | 100.0 (18/18) | 100.0 (30/30) | - | - | - |
| B. adolescentis | 50.0 (15/30) | 83.3 (15/18) | 40.0 (12/30) | 0.016*, i | 0.452 | 0.045* |
| B. catenulatum group | 100.0 (30/30) | 100.0 (18/18) | 100.0 (30/30) | - | - | - |
| B. angulatum | 20.0 (6/30) | 16.7 (3/18) | 23.0 (7/30) | 0.546 | 0.601 | 0.521 |
| B. lactis | 56.7 (17/30) | 61.1 (11/18) | 63.3 (19/30) | 0.502 | 0.975 | 0.775 |
| B. longum subsp. infantis | 36.7 (11/30) | 22.2 (4/18) | 36.7 (11/30) | 0.351 | 0.795 | 0.532 |
| B. dentium | 13.3 (4/30) | 27.7 (5/18) | 6.6 (2/30) | 0.265 | 0.407 | 0.040* |
a Prevalence (Pr) reflects the number of positive amplifications from total samples analysed by PCR (n = number of samples analysed)
* Statistical differences were calculated by using Chi-square test 2 × 2. Significantly difference between groups was consider at P < 0.050
i Statistical differences were corrected for a multiple comparison test (3 variable) by using Bonferroni adjustment. Significantly difference between groups was considered at P < 0.017.
The composition of biopsy-associated bifidobacteria assessed by qPCR is shown in Table 3. The most predominant bifidobacterial species detected in biopsy samples were B. longum, followed by B. breve, B. lactis, B. bifidum and B. catenulatum, whereas B. longum subsp. infantis and B. dentium were less prevalent.
Table 3.
Bifidobacterium group and species of faeces and duodenal biopsies from children quantified by qPCR.
| Microbial group in biopsy samples | Bacterial countsa (Log cells/g) | P-value Mann-Whitney/Test Bonferroni adjustment | |||||||
| Active CD (n = 25) | Non-active CD (n = 8) | Control (n = 8) | Active- non-active CD | Control- active CD | Control- non-active CD | ||||
| Median | IQR | Median | IQR | Median | IQR | ||||
| Bifidobacterium group | 5.95 | 5.55–6.21 | 6.15 | 4.97–6.28 | 6.27 | 6.03–6.80 | 0.604 | 0.009*, i | 0.461 |
| B. longum | 4.66 | 4.36–5.37 | 4.95 | 4.90–5.60 | 5.60 | 5.33–5.73 | 0.310 | 0.004*, i | 0.368 |
| B. breve | 5.14 | 4.59–5.46 | 3.05 | 3.02–3.50 | 5.21 | 5.00–5.80 | 0.020* | 0.630 | 0.100 |
| B. bifidum | 4.35 | 3.40–4.75 | 3.98 | 2.15–4.44 | 4.17 | 3.48–4.66 | 0.800 | 0.700 | 0.950 |
| B. adolescentis | 3.22 | 2.86–3.74 | 3.06 | - | 3.87 | 1.80–3.30 | 0.600 | 0.327 | 0.667 |
| B. catenulatum group | 4.08 | 3.16–4.60 | 4.12 | 4.04–4.53 | 4.10 | 3.76–4.46 | 0.736 | 0.757 | 0.660 |
| B. angulatum | 2.95 | 1.54–3.80 | 4.10 | - | 3.55 | 1.58–4.44 | 0.275 | 0.933 | 0.900 |
| B. lactis | 6.33 | 5.50–6.18 | - | - | 5.28 | 4.59–5.70 | - | 0.033* | - |
| B. longum subsp. infantis | - | - | - | - | - | - | - | - | - |
| B. dentium | 4.23 | 3.45–5.23 | 4.00 | - | - | - | 0.627 | - | - |
| Microbial group in faecal samples | Bacterial countsa (Log cells/g) | P-value Mann-Whitney test Bonferroni adjustment | |||||||
| Active CD (n = 30) | Non-active CD (n = 18) | Control (n = 30) | Active- non-active CD | Control- active CD | Control- non-active CD | ||||
| Median | IQR | Median | IQR | Median | IQR | ||||
| Bifidobacterium group | 9.67 | 8.68–9.90 | 8.77 | 8.58–9.60 | 9.80 | 9.23–10.33 | 0.183 | 0.014*, i | 0.002*, i |
| B. longum | 8.90 | 8.56–9.40 | 8.30 | 7.78–9.00 | 9.28 | 8.88–10.10 | 0.030* | 0.038* | < 0.001*, i |
| B. breve | 6.97 | 5.56–7.82 | 4.02 | 3.08–5.15 | 6.94 | 6.18–8.02 | < 0.001*, i | 0.860 | < 0.001*, i |
| B. bifidum | 7.64 | 6.42–8.16 | 6.74 | 6.40–6.87 | 6.96 | 6.67–7.93 | 0.030* | 0.577 | 0.050* |
| B. adolescentis | 6.95 | 5.55–7.92 | 5.40 | 4.93–7.76 | 5.97 | 5.37–6.60 | 0.112 | 0.050* | 0.633 |
| B. catenulatum group | 7.16 | 6.50–8.68 | 7.84 | 7.07–8.50 | 7.65 | 7.56–8.42 | 0.425 | 0.106 | 0.758 |
| B. angulatum | 4.96 | 4.64–7.20 | 4.68 | 4.24–5.07 | 4.65 | 4.12–5.00 | 0.548 | 0.153 | 0.569 |
| B. lactis | 7.12 | 5.30–7.45 | 5.17 | 4.66–7.20 | 5.45 | 4.66–7.07 | 0.175 | 0.081 | 0.780 |
| B. longum subsp. infantis | 6.57 | 5.80–7.76 | 7.47 | 6.83–7.82 | 6.68 | 6.45–7.06 | 0.192 | 0.341 | 0.117 |
| B. dentium | 6.28 | 6.10–6.30 | 5.24 | 4.66–5.82 | 5.20 | 3.86–5.30 | 0.111 | 0.133 | 0.571 |
a Data are shown as medians and interquartile range (IQR) of cell number per gram of faecal or duodenal biopsy sample.
* Statistical differences were calculated by using Mann-Whitney U test comparing two variables. Significantly difference between groups was considered at P < 0.050.
i Statistical differences were corrected for a multiple comparison test (3 variable) by using Bonferroni adjustment. Significantly difference between groups was considered at P < 0.017.
Significant differences were detected by using the Kruskal-Wallis test among active and non-active CD patient and control groups for total Bifidobacterium (P = 0.040), B. longum (P = 0.017), B. breve (P = 0.018) and B. bifidum (P = 0.022). No differences were found for the other analysed species. Comparisons of bifidobacterial levels between groups by using the Mann-Whitney U test allowed the detection of significantly higher levels of total Bifidobacterium in controls than in active CD patients (P = 0.009), although no significant differences were found between non-active CD patients and controls (P = 0.461). B. longum levels were also significantly higher in controls than in active CD patients (P = 0.004) and slightly higher than in non-active CD patients although not significantly (P = 0.368). Total Bifidobacterium and B. longum group levels were also significantly different between active CD and control children by applying the Bonferroni adjustment. B. breve levels were significantly lower in non-active CD patients than in active CD patients (P = 0.020) and also slightly lower (P = 0.100) than in control children as it was the case for faeces. A similar trend was found for B. bifidum but none of the differences reached statistical significance. B. lactis levels were higher in active CD patients than in controls (P = 0.033), while this species was not detected in non-active CD patients.
Faecal Bifidobacterium species composition
The number of positive samples for Bifidobacterium group and species detected by PCR (prevalence) compared to the total number of samples tested in the study are shown in Table 2. B. longum, B. bifidum and B. catenulatum groups were detected in all faecal samples, whereas the other Bifidobacterium species analysed were not detected in every sample (Table 2). B. breve was detected more frequently in active CD patients than non-active CD patients and controls, although the differences were not significant (P > 0.05). B. adolescentis was detected more frequently in non-active CD patients than in active CD patients (P = 0.016) and controls (P = 0.045). B. dentium was significantly more prevalent in non-active CD patients than in controls (P = 0.040), and the same trend was detected between active CD patients and controls but the differences were not statistically significant. B. adolescentis prevalence was also significantly different between non-active CD patients and active CD patients (P = 0.016) by applying the Bonferroni adjustment test. No significant differences were found for the other Bifidobacterium groups or species.
The bacterial composition of faecal samples from the three groups of children under study assessed by qPCR is shown in Table 3. The most predominant Bifidobacterium groups present in faecal samples were B. longum, B. catenulatum group and B. bifidum. Significant differences were obtained by using the Kruskal-Wallis test among active and non-active CD patient and control groups for total Bifidobacterium (P = 0.002), B. longum (P < 0.001), B. breve (P < 0.001), B. bifidum (P = 0.030) and B. adolescentis (P = 0.020). No differences were found for the other studied species. The comparison of bifidobacterial levels between groups by using the Mann-Whitney U test allowed the detection of significant differences in several cases. Total Bifidobacterium levels were significantly higher in control samples than in those of active CD (P = 0.014) and non-active CD patients (P = 0.002). No differences were found between active and non-active CD patients (P = 0.183). B. longum levels were significantly higher in controls than in active CD (P = 0.038) and non-active CD patients (P < 0.001); moreover, B. longum levels were significantly higher in non-active CD patients than in active CD patients (P = 0.030). Most of these differences were also statistically significant by applying the Bonferroni adjustment at P < 0.017 (Table 3). B. breve levels were significantly higher in active CD (P = 0.001) and control children (P < 0.001) than in non-active CD patients, which showed the lowest counts of this species, but differences between active CD patients and controls (P = 0.860) were not found by either Mann Whitney or Bonferroni adjustment test. Similarly, B. bifidum levels were higher in active CD patients (P = 0.030) and controls (P = 0.050) than in non-active CD patients, whereas significant differences were not found between active CD and control children (P = 0.577). B. adolescentis levels were significantly higher (P = 0.050) in active CD patients than in controls while differences were not found neither between active and non-active CD patients nor between non-active CD patients and controls. However, differences in B. bifidum and B. adolescentis were not statistically significant when applying the Bonferroni adjustment test. No differences were found for any other Bifidobacterium species analysed between the three groups of children under study (Table 3).
Relationships between duodenal and faecal Bifidobacterium species composition
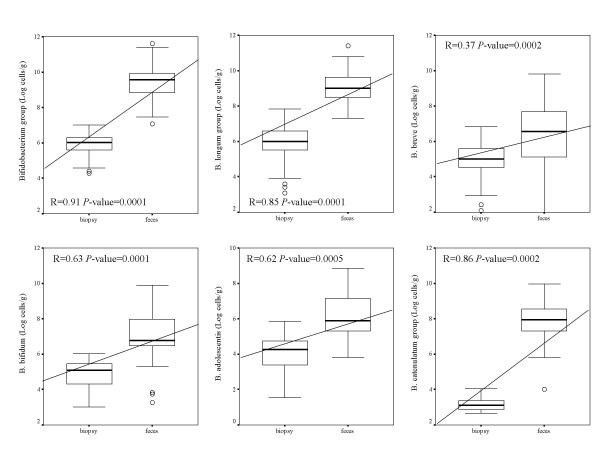
Faecal samples showed higher numbers (P < 0.050) of bifidobacteria than duodenal biopsies samples for every analyzed group, as anticipated (Fig. 1). Correlations were generally found between levels of each bifidobacterial group detected in faecal and biopsy samples within the same individual. Thus, low faecal bifidobacterial levels corresponded to low biopsy bifidobacterial levels in the same subjects and vice versa. Correlations between total Bifidobacterium levels in faecal and biopsy samples were significant in active CD patients (R = 0.84, P < 0.001), non-active CD patients (R = 0.67, P = 0.001) and controls (R = 0.68, P < 0.001). Similarly, correlations between B. longum levels in faecal and biopsy samples were significant in active CD patients (R = 0.80, P < 0.001), non-active CD patients (R = 0.79, P < 0.001) and controls (R = 0.53, P = 0.001). In active CD patients, correlations were also found for B. breve (R = 0.44, P = 0.001), B. bifidum (R = 0.52, P = 0.001), B. adolescentis (R = 0.57, P = 0.002), B. catenulatum (R = 0.70, P < 0.001) and B. lactis (R = 0.70, P < 0.001), whereas no correlations were found for B. angulatum, B. longum subsp.infantis and B. dentium. In controls, significant correlations between bifidobacterial levels in faeces and biopsy samples were also found for B. catenulatum group (R = 0.40, P = 0.017) and B. longum subsp.infantis (R = 0.54, P = 0.030) and in non-active CD patients for B. bifidum (R = 0.54, P = 0.012) and B. catenulatum group (R = 0.70, P = 0.001).
Figure 1.
Correlations of bifidobacterial groups of faecal and duodenal biopsy samples from all children (active and non-active CD patients and controls) under study. Data represent the positive samples. The line in the box is the median (50% percentile), with the lower line the lower 25% border (25% percentile) and the upper line the 75% (75% percentile) border. The end of the upper vertical line is the maximum data value, outliers not considered. The end of the lower vertical line is the lowest value, outliers not considered. The separate dots or asterisks indicate outliers. Significant differences (P < 0.050) were found between faeces and biopsy levels of every bifidobacterial group analysed when considering all subjects.
Discussion
Bifidobacterium species are regarded as key biological markers of a healthy gut. Herein, features of the composition of this bacterial genus in the gut ecosystem of CD patients, with active and non-active disease, and control children have been reported for the first time, and the existing correlations between faecal and duodenal biopsy-associated bifidobacteria. Active and non-active CD patients showed lower numbers of total Bifidobacterium in both types of samples analysed, faeces and duodenal biopsy specimens. These differences were significant in every case except for biopsy samples of non-active CD patients and controls. A similar trend was obtained by using the Bonferroni adjustment test although differences in faecal B. longum levels between control children and active CD patients were not significant. Faecal imbalances in total Bifidobacterium levels were detected in both active and non-active CD patients as compared to controls and therefore they seemed to be irrespectively of disease activity. However, duodenal Bifidobacterium levels seemed to be partially restored after the gluten-free diet since significant differences were not found between non-active CD patients and control children. These different trends could be a consequence of the limited number of biopsy samples available when compared with faecal samples. Bifidobacterium numbers of the mucosa of IBD patients and allergic infants were also found to be reduced compared to controls [10,19,20]. Some reports also showed that allergic infants were colonized by bifidobacteria less often and at lower concentrations than controls [9,10,21,22]. A significant reduction of gut bifidobacterial levels was also reported to precede the development of atopic diseases, indicating a relation between relative abundance of this bacterial genus and the development of immune-related disorders [10]. A recent report also indicated that high numbers of bifidobacteria may correlate positively with the normalization of inflammatory status and improved glucose tolerance and glucose-induced insulin secretion in an obesity animal model induced by a high-fat containing diet [23]. These findings, together with the present results, suggest that lower numbers of total bifidobacteria may be associated with inflammatory processes, supporting the hypothesis that bifidobacteria are required to maintain intestinal homeostasis.
The most predominant bifidobacterial groups detected in both, biopsies and faeces, were B. longum, B. bifidum and B. catenulatum followed by B. breve and B. lactis. In agreement with previous studies, B. longum was the species most commonly found in the faecal and duodenal mucosa-associated microbiota [9,12]. The levels of B. longum were markedly lower in active CD patients and to a lesser extent in non-active CD patients than in controls in both faecal and biopsy samples according with the data obtained for total Bifidobacterium; the differences were significant in every case except for biopsy samples of non-active CD patients and controls presumably due to their limited number. Imbalances in B. longum levels were found irrespectively of the phase of the disease (active or non-active) particularly in faeces; however, the gluten-free diet could also influence the levels of this species since differences were found between active and non-active CD patients. Duodenal B. longum levels seemed to be partially restored after the gluten-free diet, following the same trend as that detected for total Bifidobacterium levels.
Bifidobacteria have been demonstrated to have a species and strain-specific influence on immunity [14,15,24]. Strains of the genus Bifidobacterium have been shown to polarized Th2/Th1 responses in a specific manner, thereby modulating unbalanced cytokine production characteristic of either Th2-type (e.g. allergy) or Th1-type diseases (e.g. Crohn disease and CD) and overall inflammation [13,25]. It has been speculated that typical adult-type bifidobacterial species such as B. adolescentis and B. catenulatum group could favour Th2-biased immune responses characteristic of allergy inflammation [14]. In contrast, anti-inflammatory properties have been generally attributed to strains of the species B. longum mainly related to their ability to stimulate regulatory cytokine production (e.g. IL-10) [25,26]. In this study, the higher levels of B. longum detected in control samples (biopsies and faeces) compared to those found in active and non-active CD patient samples suggest that this bifidobacterial group could exert a protective role in CD. Otherwise, changes in the intestinal environment of CD patients, such as the mucus layer composition, could secondarily lead to changes in gut bacterial populations. In this context, lower levels of B. longum have also been reported in IBD and colorectal cancer patients [12].
B. breve and B. bifidum numbers were particularly reduced in non-active CD patients when compared with active CD-patients and controls in both biopsy specimens, and especially in faecal samples, indicating that these reductions could be due to the gluten-free diet rather than to the disease.
B. adolescentis were detected in slightly higher numbers in faecal samples of active CD patients than in controls and its prevalence was also higher in faeces of non-active CD patients than in those of controls. However, this loosely association between B. adolescentis and CD was neither confirmed in biopsies nor in a preliminary study carried out previously in a few faecal samples of active CD patients and controls by PCR-DGGE [7].
In general, this study confirms that the dominant bifidobacterial species detected in faeces represented those found in duodenal biopsies although in different quantities (Fig. 1), supporting previous reports on adults and infants [27,28]. Significant correlations were detected between levels of total Bifidobacterium and the species B. longum in faecal and biopsy samples, which were the bacterial groups most clearly related to CD. Therefore, faecal alterations of Bifidobacterium and B. longum levels reflect those occurring in the duodenum. These could be used as indexes of CD in faeces without the use of invasive biopsy techniques, although further studies should be carried out in other population groups to confirm such hypothesis.
Conclusion
Active and non-active CD is associated with changes in number, composition and prevalence of Bifidobacterium populations. The microbiota of CD patients is characterised by reductions in total Bifidobacterium and B. longum numbers. These microbial deviations are not completely restored after treatment with a gluten-free diet. Thus, the results suggest that total and specific Bifidobacterium species could be possible protective factors for CD. Therefore, the administration of specific probiotics and prebiotics to increase their intestinal levels could constitute a possible adjuvant therapeutic strategy for this disorder. Confirmation of such hypothesis would require further investigations.
Authors' contributions
The author's responsibilities were as follows: YS. made the microbiological study concept and design, MCC acquired microbiology data and made the statistical analyses. MC, CR-K and ED acquired clinical data. All authors participated in preparation of the manuscript and approved the final version. None of the authors has conflict of interests.
Acknowledgments
Acknowledgements
This work was supported by grant AGL2005-05788-C02-01 and Consolider Fun-C-Food CSD2007-00063 from the Spanish Ministry of Science and Innovation. The I3P-CSIC Postdoctoral Contract from the European Social Fund to MC. Collado is fully acknowledged.
Contributor Information
Maria Carmen Collado, Email: ciecol@iata.csic.es.
Ester Donat, Email: donat_est@gva.es.
Carmen Ribes-Koninckx, Email: ribes_car@gva.es.
Miguel Calabuig, Email: mcalabuig@comv.es.
Yolanda Sanz, Email: yolsanz@iata.csic.es.
References
- Fasano A, Catassi C. Coeliac disease in children. Best Pract Res Clin Gastroenterol. 2005;19:467–478. doi: 10.1016/j.bpg.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Forsberg G, Fahlgren A, Horstedt P, Hammarström S, Hernell O, Hammarström ML. Presence of bacteria and innate immunity of intestinal epithelium in childhood coeliac disease. Am J Gastroenterol. 2004;99:894–904. doi: 10.1111/j.1572-0241.2004.04157.x. [DOI] [PubMed] [Google Scholar]
- Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, Taki I, Norris JM, Erlich HA, Eisenbarth GS, Rewers M. Rotavirus infection frequency and risk of coeliac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101:2333–2340. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- Collado MC, Calabuig M, Sanz Y. Differences between the faecal microbiota of coeliac children and healthy controls. Curr Issues Intest Microbiol. 2007;8:9–14. [PubMed] [Google Scholar]
- Nadal I, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol. 2007;56:1669–74. doi: 10.1099/jmm.0.47410-0. [DOI] [PubMed] [Google Scholar]
- Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- Sanz Y, Sánchez E, Marzotto M, Calabuig M, Torriani S, Dellaglio F. Differences in faecal bacterial communities in coeliac and healthy children as detected by PCR and denaturing gradient gel electrophoresis. FEMS Immunol Med Microbiol. 2007;51:562–8. doi: 10.1111/j.1574-695X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- Favier CF, Vaughan EE, De Vos WM, Akkermans ADL. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68:219–26. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönlund MM, Gueimonde M, Laitinen K, Kociubinski G, Grönroos T, Salminen S, Isolauri E. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin Exp Allergy. 2007;37:1764–72. doi: 10.1111/j.1365-2222.2007.02849.x. [DOI] [PubMed] [Google Scholar]
- Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- Ouwehand AC, Isolauri E, He F, Hashimoto H, Benno Y, Salminen S. Differences in Bifidobacterium flora composition in allergic and healthy infants. J Allergy Clin Immunol. 2001;108:144–5. doi: 10.1067/mai.2001.115754. [DOI] [PubMed] [Google Scholar]
- Gueimonde M, Ouwehand A, Huhtinen H, Salminen E, Salminen S. Qualitative and quantitative analyses of the bifidobacterial microbiota in the colonic mucosa of patients with colorectal cancer, diverticulitis and inflammatory bowel disease. World J Gastroenterol. 2007;13:3985–9. doi: 10.3748/wjg.v13.i29.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73:444S–450S. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- Young SL, Simon MA, Baird MA, Tannock GW, Bibiloni R, Spencely K, Lane JM, Fitzharris P, Crane J, Town I, Addo-Yobo E, Murray CS, Woodcock A. Bifidobacterial species differentially affect expression of cell surface markers and cytokines of dendritic cells harvested. Clin Diagn Lab Immunol. 2004;11:686–90. doi: 10.1128/CDLI.11.4.686-690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina C, Santana A, Llopis M, Paz-Cabrera MC, Antolín M, Mourelle M, Guarner F, Vilaseca J, Gonzalez C, Salas A, Quintero E, Malagelada JR. Induction of colonic transmural inflammation by Bacteroides fragilis : implication of matrix metalloproteinases. Inflamm Bowel Dis. 2005;11:99–105. doi: 10.1097/00054725-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Ventura M, Reniero R, Zink R. Specific identification and targeted characterization of Bifidobacterium lactis from different environmental isolates by a combined multiplex-PCR approach. Appl Environ Microbiol. 2001;67:2760–5. doi: 10.1128/AEM.67.6.2760-2765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki T, Watanabe K, Fujimoto J, Kado Y, Takada T, Matsumoto K, Tanaka R. Quantitative PCR with 16S rRNA gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl Environ Microbiol. 2004;70:167–173. doi: 10.1128/AEM.70.1.167-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BC, Strentker CJ, Chetty R. Coeliac disease: an update for pathologists. J Clin Pathol. 2006;59:1008–1016. doi: 10.1136/jcp.2005.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S, Furrie E, Kennedy A, Cummings JH, Macfarlane GT. Mucosal bacteria in ulcerative colitis. Br J Nutr. 2005;93:S67–S72. doi: 10.1079/BJN20041347. [DOI] [PubMed] [Google Scholar]
- Mylonaki M, Rayment NB, Rampton DS, Hudspith BN, Brostoff J. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:481–487. doi: 10.1097/01.MIB.0000159663.62651.4f. [DOI] [PubMed] [Google Scholar]
- Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–20. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Narisawa Y, Arase S, Okamatsu H, Ikenaga T, Tajiri Y, Kumemura M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. 2003;111:587–91. doi: 10.1067/mai.2003.105. [DOI] [PubMed] [Google Scholar]
- Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetología. 2007;50:2374–83. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- He F, Morita H, Ouwehand AC, Hosoda M, Hiramatsu M, Kurisaki J, Isolauri E, Benno Y, Salminen S. Stimulation of the secretion of pro-inflammatory cytokines by Bifidobacterium strains. Microbiol Immunol. 2002;46:781–5. doi: 10.1111/j.1348-0421.2002.tb02765.x. [DOI] [PubMed] [Google Scholar]
- Medina M, Izquierdo E, Ennahar S, Sanz Y. Differential immunomodulatory properties of Bifidobacterium longum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol. 2007;150:531–8. doi: 10.1111/j.1365-2249.2007.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolauri E, Kirjavainen PV, Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut. 2002;50:III54–9. doi: 10.1136/gut.50.suppl_3.iii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. 2002;68:3401–7. doi: 10.1128/AEM.68.7.3401-3407.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwehand AC, Salminen S, Arvola T, Ruuska T, Isolauri E. Microbiota composition of the intestinal mucosa: association with fecal microbiota? Microbiol Immunol. 2004;48:497–500. doi: 10.1111/j.1348-0421.2004.tb03544.x. [DOI] [PubMed] [Google Scholar]