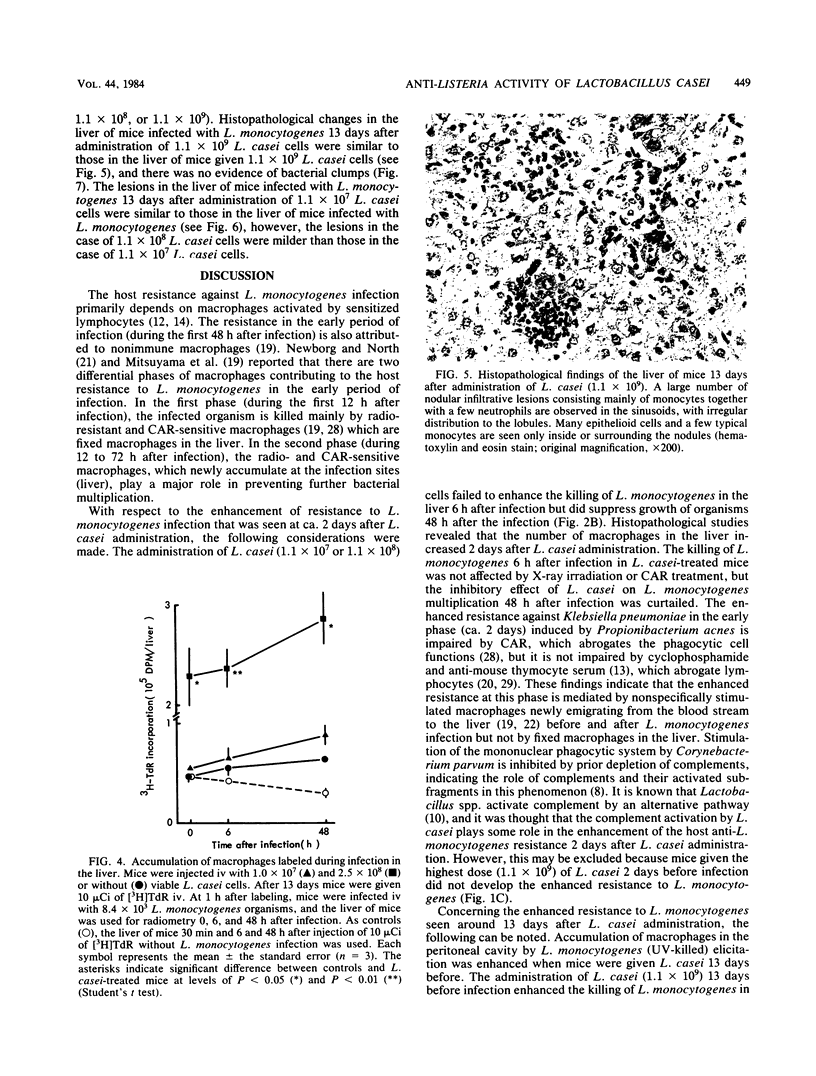
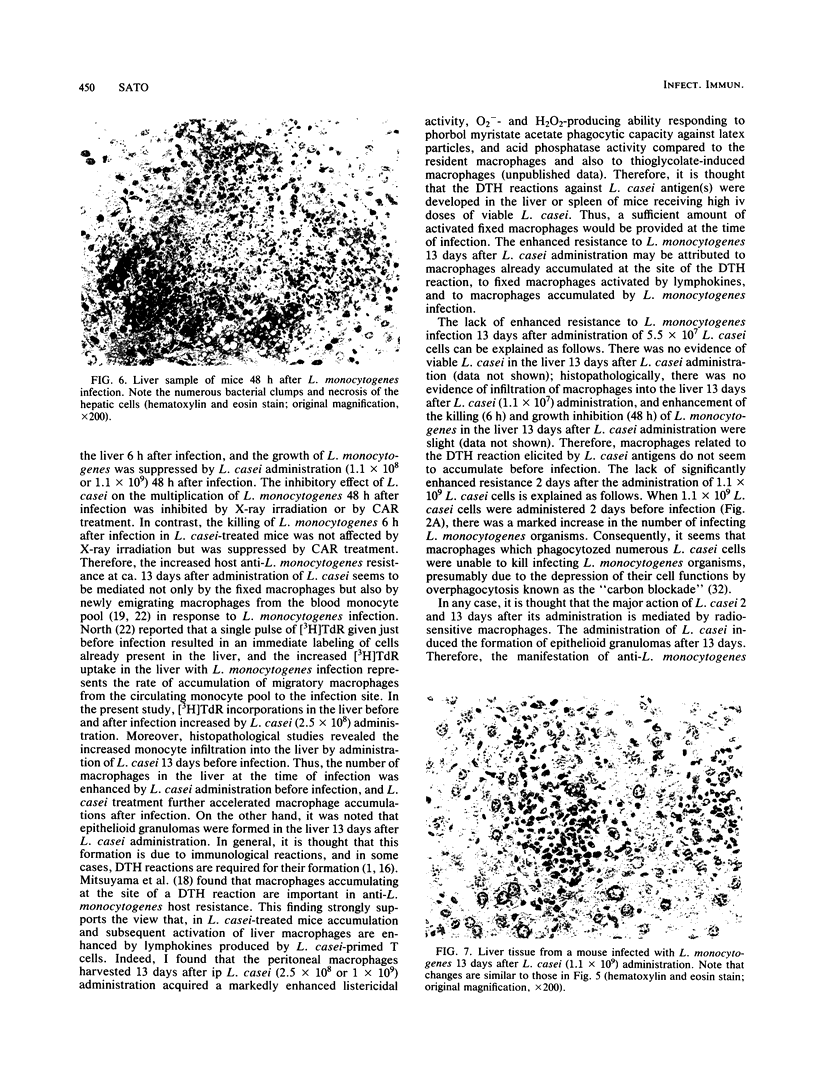
Abstract
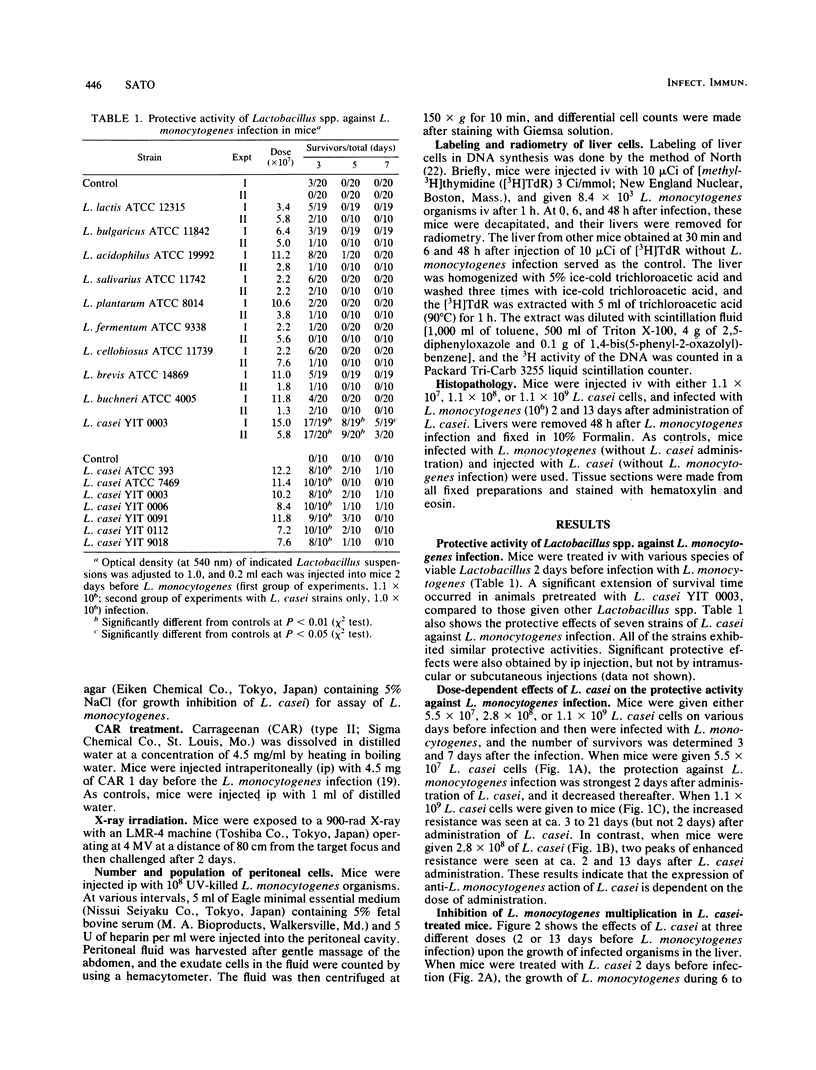
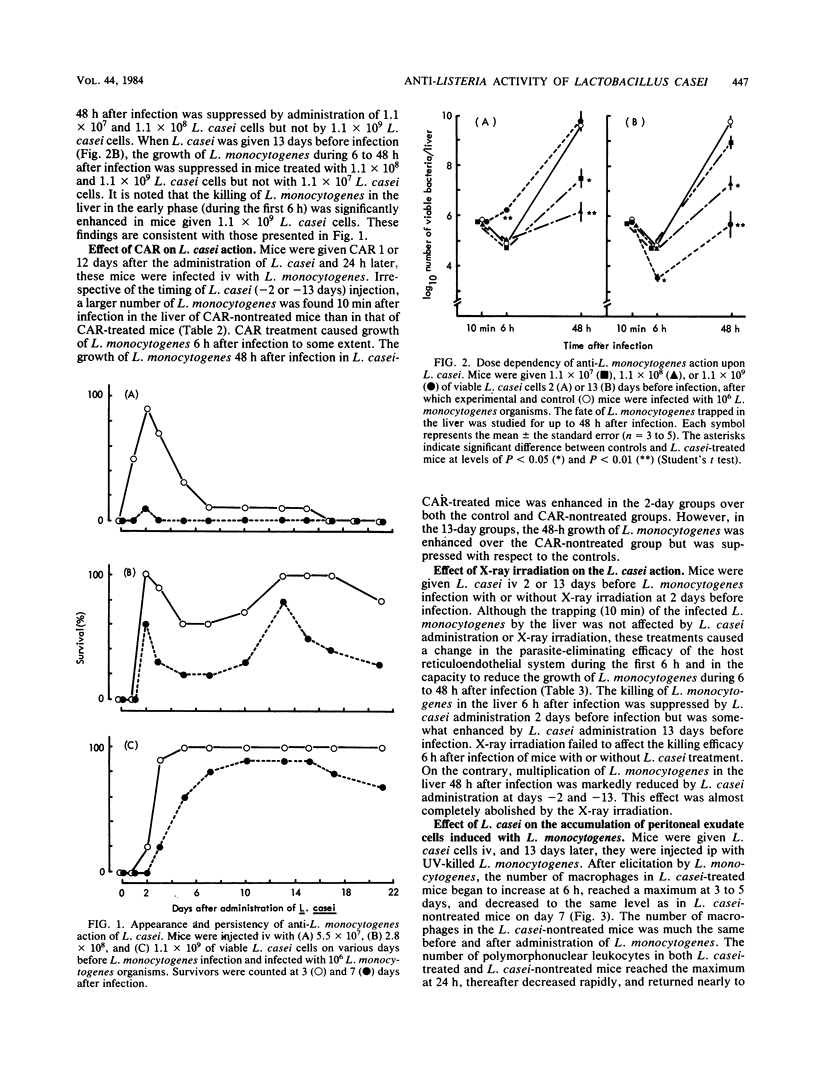
Among the 10 species of the genus Lactobacillus, L. casei showed the strongest protective action against Listeria monocytogenes infection in mice. The activity of L. casei differed with regard to the dose of administration. The anti-L. monocytogenes resistance in mice intravenously administered 5.5 X 10(7), 2.8 X 10(8), or 1.1 X 10(9) L. casei cells was most manifest at ca. 2, 2 and 13, and 3 to 21 days after its administration, respectively. The growth of L. monocytogenes in the liver of mice injected with L. casei (10(7), 10(8), or 10(9) cells) 48 h after infection was suppressed, particularly when 10(8) or 10(9) L. casei cells were given 2 or 13 days before the induced infection, respectively. This suppression of L. monocytogenes growth was overcome by carrageenan treatment or X-ray irradiation. [3H]thymidine incorporation into the liver DNA increased 13 days after administration of L. casei, and augmentation of [3H]thymidine incorporation during 6 to 48 h after infection was dependent on the dose of L. casei. Peritoneal macrophage accumulation observed 1 to 5 days after intraperitoneal injection of UV-killed L. monocytogenes was markedly enhanced when the mice were treated with L. casei cells 13 days before macrophage elicitation. Therefore, the enhanced host resistance by L. casei to L. monocytogenes infection may be mediated by macrophages migrating from the blood stream to the reticuloendothelial system in response to L. casei injection before or after L. monocytogenes infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A. Stimulation of lymphocyte proliferation by killed mycobacteria and other bacterial species. Infect Immun. 1976 Jul;14(1):28–32. doi: 10.1128/iai.14.1.28-32.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloksma N., de Heer E., van Dijk H., Willers J. M. Adjuvanticity of lactobacilli. I. Differential effects of viable and killed bacteria. Clin Exp Immunol. 1979 Aug;37(2):367–375. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- EFTHYMIOU C., HANSEN P. A. An antigenic analysis of Lactobacillus acidophilus. J Infect Dis. 1962 May-Jun;110:258–267. doi: 10.1093/infdis/110.3.258. [DOI] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Hof H. Cell-mediated resistance to infection with Listeria monocytogenes in nude mice. Infect Immun. 1977 Feb;15(2):382–385. doi: 10.1128/iai.15.2.382-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galleli A., Le Garrec Y., Chedid L. Increased resistance and depressed delayed-type hypersensitivity to Listeria monocytogenes induced by pretreatment with lipopolysaccharide. Infect Immun. 1981 Jan;31(1):88–94. doi: 10.1128/iai.31.1.88-94.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffar A. The activation of macrophages by Corynebacterium parvum: effect of anti-complementary agents cobra venom factor and sodium cyanate. J Reticuloendothel Soc. 1980 Mar;27(3):327–335. [PubMed] [Google Scholar]
- Glasgow L. A., Fischbach J., Bryant S. M., Kern E. R. Immunomodulation of host resistance to experimental viral infections in mice: effects of Corynebacterium acnes, Corynebacterium parvum, and Bacille calmette-guérin. J Infect Dis. 1977 May;135(5):763–770. doi: 10.1093/infdis/135.5.763. [DOI] [PubMed] [Google Scholar]
- Huis in 't Veld J. H., Berrens L. Inactivation of hemolytic complement in human serum by an acylated polysaccharide from a gram-positive rod: possible significance in pigeon-breeder's disease. Infect Immun. 1976 Jun;13(6):1619–1625. doi: 10.1128/iai.13.6.1619-1625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Kobayashi S., Yokokura T., Mutai M. Antitumor activity of Lactobacillus casei in mice. Gan. 1981 Aug;72(4):517–523. [PubMed] [Google Scholar]
- Kaufmann S. H. Effective antibacterial protection induced by a Listeria monocytogenes-specific T cell clone and its lymphokines. Infect Immun. 1983 Mar;39(3):1265–1270. doi: 10.1128/iai.39.3.1265-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi F., Nagoya T., Koshi T., Saino Y. Biphasic protection against bacterial infection in mice induced by vaccination of Propionibacterium acnes. Infect Immun. 1980 Feb;27(2):391–396. doi: 10.1128/iai.27.2.391-396.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiba H., Gojobori M., Matsunaga K. Antitumor effect of combined use of OK-432 and yeast cell wall with mitomycin-C in mice. Gan. 1977 Oct;68(5):703–708. [PubMed] [Google Scholar]
- McGee M. P., Myrvik Q. N., Leake E. S. Organization of allergic granulomas and dependence on insoluble antigen. J Reticuloendothel Soc. 1978 Sep;24(3):253–262. [PubMed] [Google Scholar]
- Milas L., Hunter N., Withers H. R. Corynebacterium-induced protection against artificial pulmonary metastases of a syngeneic fibrosarcoma in mice. Cancer Res. 1974 Mar;34(3):613–620. [PubMed] [Google Scholar]
- Mitsuyama M., Nomoto K., Takeya K. Direct correlation between delayed footpad reaction and resistance to local bacterial infection. Infect Immun. 1982 Apr;36(1):72–79. doi: 10.1128/iai.36.1.72-79.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Möller G., Zukoski C. Differential effect of heterologous anti-lymphocyte serum on antibody-producing cells and antigen-sensitive cells. J Immunol. 1968 Aug;101(2):325–332. [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada Y., Mitsuyama M., Matsumoto K., Une T., Otani T., Ogawa H., Nomoto K. Stimulation of resistance of immunocompromised mice by a muramyl dipeptide analog. Infect Immun. 1982 Sep;37(3):1285–1288. doi: 10.1128/iai.37.3.1285-1288.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Sato K. Effect of streptococcal preparation, OK-432, on experimental infection due to Mycobacterium lepraemurium in mice of C3H/Jms strain. Hiroshima J Med Sci. 1983 Jun;32(2):231–234. [PubMed] [Google Scholar]
- Schwartz H. J., Leskowitz S. The effect of carrageenan on delayed hypersensitivity reactions. J Immunol. 1969 Jul;103(1):87–91. [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Swartzberg J. E., Krahenbuhl J. L., Remington J. S. Dichotomy between macrophage activation and degree of protection against Listeria monocytogenes and Toxoplasma gondii in mice stimulated with Corynebacterium parvum. Infect Immun. 1975 Nov;12(5):1037–1043. doi: 10.1128/iai.12.5.1037-1043.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Shimotori S., Taniguchi T., Nomoto K. Cellular mechanisms in the protection against infection by Listeria monocytogenes in mice. J Gen Microbiol. 1977 Jun;100(2):373–379. doi: 10.1099/00221287-100-2-373. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Matsumoto T., Nomoto K., Takeya K. Relationship between non-specific activity of macrophages and immune responses to Listeria monocytogenes. Immunology. 1980 Jul;40(3):295–301. [PMC free article] [PubMed] [Google Scholar]