This study suggests that cytochrome-1 is a valuable predictor of disease progression in early-stage chronic lymphocytic leukemia.
Keywords: Cryptochrome-1, prognostic marker, chronic lymphocytic leukemia, ZAP70, LPL, IgVH
Abstract
Chronic lymphocytic leukemia is an adult-onset leukemia with a heterogeneous clinical behavior. When chronic lymphocytic leukemia cases were divided on the basis of IgVH mutational status, widely differing clinical courses were revealed. Since IgVH sequencing is difficult to perform in a routine diagnostic laboratory, finding a surrogate for IgVH mutational status seems an important priority. In the present study, we proposed the use of Cryptochrome-1 as a new prognostic marker in early-stage chronic lymphocytic leukemia. Seventy patients (Binet stage A, without treatment) were included in the study. We correlated Cryptochrome-1 mRNA with well established prognostic markers such as IgVH mutations, ZAP70, LPL or CD38 expression and chromosomal abnormalities. High Cryptochrome-1 expression correlated with IgVH unmutated samples. In addition, Cryptochrome-1 was a valuable predictor of disease progression in early-stage chronic lymphocytic leukemia, therefore it can be introduced in clinical practice with the advantage of a simplified method of quantification.
Introduction
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease with a variable clinical course and overall survival times ranging from months to decades.1 The staging systems developed by Rai2 and Binet3 are the standard methods of assessing survival and treatment requirements in CLL patients, however, they are insufficient for the identification of patients at risk of progression, especially in the early-stages of CLL. There is a need to investigate reliable prognostic factors for predicting stable or progressive forms of CLL that facilitate risk-adapted treatment strategies.4 Mutational status of immunoglobulin heavy chain variable region (IgVH) correlates with clinical behavior and is a powerful prognostic factor in CLL. Unmutated IgVH patients have a reduced survival and responsiveness to chemotherapy.5,6
However, IgVH sequencing is difficult to perform in a routine laboratory, thus, finding a surrogate for IgVH mutations seems to be an important priority. One of the proposed markers identified through transcriptional studies is the quantification of ZAP70 expression,7,8 but there are still problems related to the technical validation and standardization of the flow cytometric (FC) method used for its determination, as well as around 20% of discordant results between ZAP70 and IgVH mutations.9 Another prognostic marker in CLL is lipoprotein lipase (LPL), which shows itself to be as effective as IgVH mutational status and more reliable than ZAP70 when tested in unpurified CLL samples.10,11
In a previous gene profiling study, we identified a limited set of genes which were expressed differentially between unmutated and mutated CLLs.12 Cryptochrome-1 (CRY1) emerged as one of the genes that provides a good segregation of samples according to IgVH mutational status, in concordance with previous reports.13,14 CRY1 is one of the key components involved in circadian clock control but its role in CLL is still little understood.
The objective of this study was to evaluate the prognostic significance of CRY1 expression in early-stage CLL, and its relationship with other well established prognostic markers in CLL.
Design and Methods
Chronic lymphocytic leukemia patients and samples
Seventy samples from previously untreated CLL patients (Binet stage A) were included in this study after informed consent was obtained. Clinical diagnosis was based on standard morphological and immunophenotypic criteria. Definition of progressive disease was based on the NCI guidelines.15
Mononuclear cells (PBMC) were isolated from peripheral blood by Ficoll-Hypaque (Lymphoprep™) density centrifugation. Samples were then processed for CD19+ selection, FC analysis and DNA/RNA isolation.
Analysis of IgVH somatic mutation status
DNA was prepared using a standard protocol and IgVH gene rearrangements were performed using BIOMED-2 primers.16 We considered unmutated those samples with ≥98% homology with the closest germinal line.
RTqPCR assays
cDNA was synthesized from 1.5 μg of total RNA using random hexanucleotides and SuperScriptII (Invitrogen) following the manufacturer’s instructions. RTqPCR assays were carried out using “Hs00172734-m1 (CRY1), Hs00277148-m1 (ZAP70) and Hs00173425-m1 (LPL) primers and probe sets (TaqMan® Gene Expression Assays, Applied Biosystems). Amplification of GUS gene was performed in all cases to normalize gene expression.
Interphase fluorescence in situ hybridization (FISH)
A panel of commercially available probes (Vysis) was used to target chromosomes 11q22.3 (ATM), 13q14 (D13S25 and D13S319), 17p13 (TP53) deletions and trisomy 12 on blood smears according to a standard protocol.
ZAP70 flow cytometry assay
Intracellular ZAP-70 expression was analyzed in 69 samples by FC according to Crespo et al.17 with some modifications. We use an isotype control as negative control. Results ≥20% were considered positive.
Statistical analysis
Significant differences between groups were assessed by the Mann-Whitney test and considered significant when p values <0.05. All statistical calculations were performed using the SPSS 13.0 software. Progression free survival (PFS) was calculated from the date of diagnosis to disease progression or last follow-up. For more detailed information see the Online Supplementary Appendix.
Results and Discussion
CRY1 expression and IgVH mutational status
Out of the 70 Binet’s stage A patients tested for IgVH mutational status, 50 were mutated in IgVH genes (71.4%). These percentages are slightly different from those reported in previous studies6,18,19 because in most of them, patients with different stages of the disease were included.
To assess the usefulness of CRY1 expression as surrogate marker for IgVH mutational status, we determined CRY1 by RTqPCR in CD19+ cell samples. The mean ± 2 SEM relative concentration in unmutated and mutated samples were: 0.311±0.081 and 0.034±0.023, respectively, p<0.0001 (Online Supplementary Figure S1).
The optimal predictive model based on CRY1 expression in relation to IgVH mutational status yielded an area under ROC curve of 0.963. A cut-off value that best segregates IgVH unmutated from mutated cases for CRY1 relative concentration of 0.090 was selected (95% sensitivity and 92% specificity). Our data show 92.8% of concordant results between CRY1 expression and IgVH mutational status (Online Supplementary Table S1).
We have also analyzed CRY1 expression in 18 samples without B-cell selection. The results showed that CRY1 was able to distinguish between IgVH unmutated and mutated samples (mean values: 0.380 and 0.051, for unmutated and mutated groups respectively, p=0.007). Therefore, our results reveal that CRY1 mRNA expression can be analyzed in PBMC as well as CD19+ selected B cells. CRY1 expression was higher in unmutated CLLs than in mutated ones, but the reasons for this differential expression are still unknown.
CRY1 expression in relation to ZAP70, CD38 or LPL expression and cytogenetic abnormalities
ZAP70 expression was measured by FC and RTqPCR in leukemic B cells, CD38 was determined by FC and LPL by RTqPCR. ROC curves were used to evaluate the performance of each assay. The areas under the curves were 0.835 and 0.880 for ZAP70 measured by FC and RTqPCR respectively, 0.963 for LPL and 0.666 for CD38. Taking into account these results, CRY1 and LPL are the markers that better assess IgVH mutational status.
Next, we analyzed correlations between continuous variables using Spearman’s test, and strong significance was found for CRY1 and LPL or ZAP70 (p<0.001).
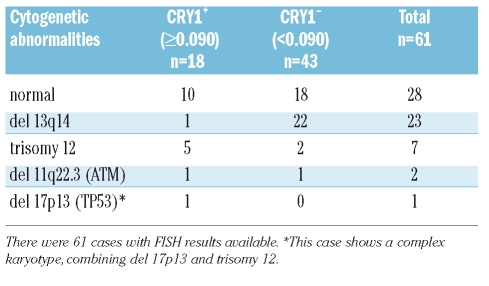
Other independent prognostic markers in CLL are cytogenetic abnormalities. We performed FISH analysis on 61 CLL samples. Abnormalities were seen in 54.1% (33/61) of patients. The correlation of CRY1 expression and the presence of the different chromosomal aberrations is shown in Table 1. Remarkably, loss of 13q14, which as a single abnormality is associated with a favourable prognosis,20 was associated with low expression of CRY1, except in one case. Deletion of 17p13 which is associated with bad prognosis was detected in one case in combination with trisomy 12, this case was IgVH unmutated, with high expression of CRY1 and positive values of CD38 and ZAP70.
Table 1.
Cryptochrome-1 gene expression in relation to cytogenetic abnormalities.
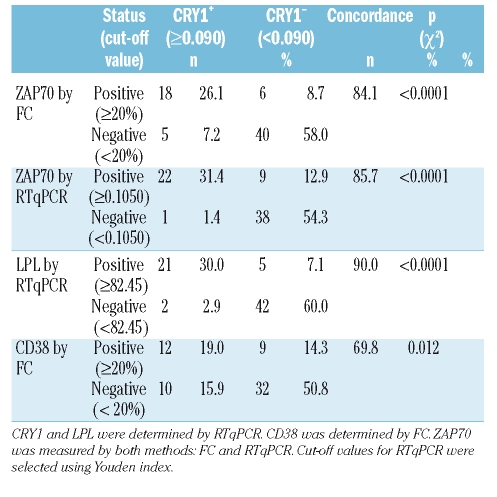
In order to compare variables, we chose a cut-off value that best segregates IgVH unmutated from mutated cases for each variable, therefore cases were scored as positive (+) or negative (−) according to this criteria. Table 2 shows concordance percentages between expression of CRY1 and ZAP70, CD38 or LPL. Both methods for ZAP70 estimation displayed strong concordance with CRY1 expression, with slightly better results in the case of RTqPCR. A better correlation was obtained between CRY1 and LPL expression, with a concordance ratio of 90.0% whereas CD38 show the lowest percentage of concordant results (69.8%).
Table 2.
Relation between CRY1 and CD38, ZAP70 or LPL expression.
Given the strong prognostic value shown by CRY1, ZAP70 and IgVH mutational status, we studied the correlation between all of them, as well as the discordant results. A perfect correlation was obtained with all double-negative patients, ZAP70- by RTqPCR and CRY1-, (n=38) showing in all cases mutated IgVH genes; whereas, in the case of all double-positive patients, ZAP70+ by RTqPCR and CRY1+ (n=22), 3 samples expressed mutated IgVH genes, in which one of these samples was ZAP70− by FC as well as CD38<20% and the other 2 samples were ZAP70+ and CD38>20% by FC in both cases. The strong correlation accounted for these markers is remarkable, but still discordant profiles represent a 14.3% of CLL patients in our study. They included 9 patients expressing ZAP70+ by RTqPCR and CRY1−, and one patient expressing ZAP70− and CRY1+. Among these discordant patients with ZAP70+/CRY1− profiles, 8 had mutated IgVH gene, whereas one was unmutated (this sample was ZAP70− by FC). On the other hand, the discordant patient with ZAP70−/CRY1+ displayed mutated IgVH gene, but this patient had high CD38 expression. Notably, from the 10 CRY1/ZAP70 discordant patients, in 8 cases CRY1 was useful to assign IgVH mutational status correctly (Online Supplementary Table S2).
Interestingly, with IgVH mutational status as the comparison method, CRY1 expression showed 92.8% agreement in terms of results, a much better performance than ZAP70 measured by FC and RTqPCR (82.6% and 84.3% of agreement respectively) which is until now the most used surrogate marker for IgVH mutations.
We have also compared the performance of CRY1 against LPL which is one of the newest prognostic markers proposed in CLL.10,11,13,21 The concordance between CRY1 and LPL expression was high, with only 7 discordant results. All double-positive samples, except 2, were unmutated for IgVH. Among the discordant results, there were 2 cases CRY1+/LPL−, and both were IgVH mutated, thus LPL would assign mutational status correctly in those cases. In the case of the other 5 discordant samples (CRY1−/LPL+), 4 of them were IgVH mutated, therefore CRY1 behaves as a better surrogate marker than LPL in this group of samples (Online Supplementary Table S2).
CRY1 expression and survival
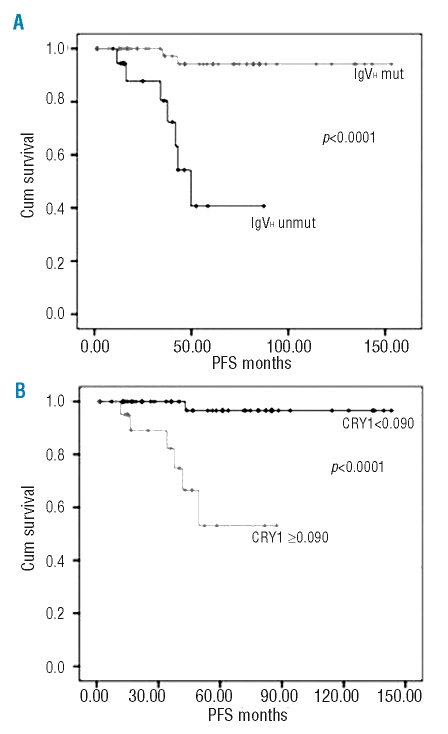
We evaluated CRY1 as a new marker of disease progression in our group of early-stage CLL patients in comparison with other prognostic markers. The median PFS was 49.67 months (95% CI, 38.79–69.55 months) in CLLs displaying unmutated IgVH genes whereas the median PFS was not achieved in mutated patients, p<0.0001 (Figure 1A). The estimated mean survival for patients with CRY1 values ≥0.090 was 63.20 months (95% CI, 48.17–78.23), and 139 months (95% CI, 133.12–146.43) for patients with CRY1 values below 0.090, p<0.0001 (Figure 1B). In PFS curves for ZAP70 measured by FC and RTqPCR there was a better distinction between groups in the case of RTqPCR determinations (p=0.001 and p=0.054 for RTqPCR and FC respectively). LPL was also a strong predictor for PFS in our group of patients. Thus, LPL positive cases have a significantly shorter median PFS than negative cases, p<0.0001 (Online Supplementary Figure S2). Among the parameters studied, CRY1, LPL and IgVH mutational status were those which better exhibited association with PFS in early-stage CLL.
Figure 1.
Kaplan–Meier plots. (A) PFS according to IgVH mutational status. (B) PFS according to CRY1 gene expression.
In summary, it is well known that IgVH mutational status is a reliable prognostic marker in CLL,5,6 but its determination is laborious and time-consuming, It is tempting, therefore, to search for other markers. Here, we showed that CRY1 quantification by RTqPCR may constitute a new prognostic marker in CLL. CRY1 is a key component of the circadian clock. Recent epidemiological studies have raised the possibility that disruption of the circadian clock may increase the risk of developing cancer in humans and adversely affect prognosis in cancer patients.22 A cyclic and significant expression of circadian clock genes in peripheral mononuclear blood cells of healthy individuals23 and an anomalous expression of CRY1 (among other components of the biological clock) has been documented during chronic and blastic phases of chronic myeloid leukemia.24 In our study, CRY1 show a similar or better behavior than other well established biomarkers in CLL, such as ZAP70, CD38, LPL or cytogenetic abnormalities, and an excellent correlation with IgVH mutational status. Our observation, along with the easiness of CRY1 determination by RTqPCR on B lymphocytes or unselected PBMC, indicate that CRY1 may be used as a new marker of disease progression for the initial prognostic assessment of early-stage CLL. In addition, it would be interesting to introduce this marker in clinical trials for its evaluation in larger cohorts of patients.
Footnotes
Authorship and Disclosures
EJL is responsible for study design, performed the research, analyzed of data, wrote and reviewed the paper; CRM: performed part of the research, analyzed data, and critically reviewed the paper; CGB: performed the FISH analysis, collected patient’s data and reviewed the paper; DM: is responsible for biostatistic analysis and reviewed the paper; RF: is responsible for study design, data analysis and reviewed the paper; JRM is responsible for data collection and reviewed the paper; JGC is responsible for co-ordination and study design, and reviewed the paper.
The authors reported no potential conflicts of interest.
The online version of this paper contains a supplementary appendix. Funding: supported by grants from Instituto de Salud Carlos III (PI020889 and PI051001). Rosa Farràs is supported by Fondo de Investigaciones Sanitarias (CP03/00106).
References
- 1.Garand R, Robillard N. Immunophenotypic characterization of acute leukemias and chronic lymphoproliferative disorders: practical recommendations and classifications. Hematol Cell Ther. 1996;38:471–86. doi: 10.1007/s00282-996-0471-4. [DOI] [PubMed] [Google Scholar]
- 2.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–34. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 3.Binet JL, Lepoprier M, Dighiero G, Charron D, D’Athis P, Vaugier G, et al. A clinical staging system for chronic lymphocytic leukemia: prognostic significance. Cancer. 1977;40:855–64. doi: 10.1002/1097-0142(197708)40:2<855::aid-cncr2820400239>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Waselenko JK, Keating M, Rai K, Grever MR. Novel therapies for chronic lymphocytic leukemia in the 21st century. Semin Oncol. 2000;27:587–97. [PubMed] [Google Scholar]
- 5.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–7. [PubMed] [Google Scholar]
- 6.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–54. [PubMed] [Google Scholar]
- 7.Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–47. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cymbalista F. Assessment of ZAP70 and other prognostic factors. Leuk Lymphoma. 2007;48(Suppl 1):S9. [Google Scholar]
- 9.Reinoso-Martin C, Jantus-Lewintre E, Ballesteros CG, Campos CB, Ferrer JR, Garcia-Conde J. ZAP-70 mRNA expression provides clinically valuable information in early-stage chronic lymphocytic leukemia. Haematologica. 2008;93:1422–4. doi: 10.3324/haematol.12710. [DOI] [PubMed] [Google Scholar]
- 10.van’t Veer MB, Brooijmans AM, Langerak AW, Verhaaf B, Goudswaard CS, Graveland WJ, et al. The predictive value of lipoprotein lipase for survival in chronic lymphocytic leukemia. Haematologica. 2006;91:56–63. [PubMed] [Google Scholar]
- 11.Van Bockstaele F, Pede V, Janssens A, Callewaert F, Offner F, Verhasselt B, et al. Lipoprotein lipase mRNA expression in whole blood is a prognostic marker in B cell chronic lymphocytic leukemia. Clin Chem. 2007;53:204–12. doi: 10.1373/clinchem.2006.076331. [DOI] [PubMed] [Google Scholar]
- 12.Jantus Lewintre E, Reinoso Martín C, Montaner D, Marín M, Terol MJ, Farràs R, et al. Analysis of CLL transcriptomic profile: differences between molecular subgroups. Leuk Lymphoma. 2008 doi: 10.1080/10428190802541807. In press. [DOI] [PubMed] [Google Scholar]
- 13.Abruzzo LV, Barron LL, Anderson K, Newman RJ, Wierda WG, O’Brien S, et al. Identification and validation of biomarkers of IgV(H) mutation status in chronic lymphocytic leukemia using microfluidics quantitative real-time polymerase chain reaction technology. J Mol Diagn. 2007;9:546–55. doi: 10.2353/jmoldx.2007.070001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasconcelos Y, De Vos J, Vallat L, Reme T, Lalanne AI, Wanherdrick K, et al. Gene expression profiling of chronic lymphocytic leukemia can discriminate cases with stable disease and mutated Ig genes from those with progressive disease and unmutated Ig genes. Leukemia. 2005;19:2002–5. doi: 10.1038/sj.leu.2403865. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7. [PubMed] [Google Scholar]
- 16.van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 17.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–75. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 18.Catherwood MA, Matthews C, Niblock R, Dobbin E, Morris TC, Alexander HD. ZAP-70 mRNA quantification in B-cell chronic lymphocytic leukaemia. Eur J Haematol. 2006;76:294–8. doi: 10.1111/j.1600-0609.2005.00619.x. [DOI] [PubMed] [Google Scholar]
- 19.Matthews C, Catherwood M, Morris TC, Alexander HD. Routine analysis of IgVH mutational status in CLL patients using BIOMED-2 standardized primers and protocols. Leuk Lymphoma. 2004;45:1899–904. doi: 10.1080/10428190410001710812. [DOI] [PubMed] [Google Scholar]
- 20.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 21.Oppezzo P, Vasconcelos Y, Settegrana C, Jeannel D, Vuillier F, Legarff-Tavernier M, et al. The LPL/ADAM29 expression ratio is a novel prognosis indicator in chronic lymphocytic leukemia. Blood. 2005;106:650–7. doi: 10.1182/blood-2004-08-3344. [DOI] [PubMed] [Google Scholar]
- 22.Gauger MA, Sancar A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 2005;65:6828–34. doi: 10.1158/0008-5472.CAN-05-1119. [DOI] [PubMed] [Google Scholar]
- 23.Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–5. doi: 10.1182/blood-2003-03-0779. [DOI] [PubMed] [Google Scholar]
- 24.Yang MY, Chang JG, Lin PM, Tang KP, Chen YH, Lin HY, et al. Downregulation of circadian clock genes in chronic myeloid leukemia: alternative methylation pattern of hPER3. Cancer Sci. 2006;97:1298–307. doi: 10.1111/j.1349-7006.2006.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]