Abstract
Understanding the mechanisms behind malarial anemia should lead to new approaches to the management and treatment of children. In this perspective article Drs. Robson and Weatherall examine the pathophysiology of this condition. See related article on page 195.
Malaria infection places a huge economic burden on the developing world. It has been estimated that annually there are over 500 million episodes and it is responsible for 18% of childhood deaths in sub-Saharan Africa.1
Infection in the human host is initiated by a bite from a female anopheline mosquito during which sporozoites enter the body and home to the liver initiating the hepatic phase of the disease. Merozoites emerge from ruptured liver schizonts and initiate the erythrocytic phase of the parasite life-cycle. The parasites in infected red cells can follow one of two developmental pathways, one which is asexual and is associated with the classical fever and the other sexual, allowing transmission of disease through future mosquito bites. In the asexual phase the invading merozoite develops into a ring-stage parasite that matures to a trophozoite and then into a schizont, which, on rupturing, releases numerous merozoites thus re-initiating the cycle of infection in red cells. In P. falciparum this cycle takes 48 hours and is a synchronous process. As parasites continue to infect further red cells the parasitemia increases unrestricted unless the individual has had a previous bout of malaria and can mount an immune response. It is the synchronous rupture of schizonts that produces cytokine-inducing toxins resulting in the fever associated with malaria.
Approximately 1% of malaria episodes in P. falciparum infections in African children result in complications requiring hospital admission.2 These include profound anemia, cerebral malaria, hypoglycemia, jaundice, renal failure, pulmonary edema and clotting problems. Clearly genetic host and parasite factors are involved. For example, in African children there are geographical differences, suggesting that in regions where there are high rates of malarial transmission the incidence of cerebral malaria is low, but there is a greater prevalence of anemia.3 The major problem in tropical countries is that the anemia in children, chronically infected with malaria, is extremely debilitating and occasionally fatal. As in the acute forms of malaria it is due to hemolysis combined with ineffective erythropoiesis.
Depending on the degree of host immunity, infected red cells can be destroyed before the schizonts mature and release merozoites. There is some evidence to suggest that ten times as many uninfected red cells are removed from the circulation for each parasitized red cell.4 Several studies support the observation that both infected and uninfected red cells have a shortened life span in both the acute and convalescent phases of a malaria infection.5,6
Infected red cells are removed by macrophages. Children with acute malaria infections have high levels of interferon (IFN)-γ and tumor necrosis factor (TNF)-α,7 both of which activate macrophages. Both infected and uninfected red cells are removed by the spleen. Removal of uninfected red cells has been attributed to decreased deformability; the mechanism responsible is not fully understood. Studies in a murine P. berghei model confirm that the removal of uninfected red cells by macrophages is one of the underlying causes of anemia. However, pro-inflammatory cytokines may play a significant role in this process.
Immune-complex mediated hemolysis is another contributory factor to malarial anemia in some populations (reviewed by Weatherall et al.8). Some patients with malaria develop a positive direct Coombs’ antiglobulin test. Red cell sensitization is usually associated with C3, but can occur with IgG alone or combinations of C3, C4 and IgG. Recent data suggest that it is not just host proteins on the surface of uninfected red cells that result in hemolysis. Immunoglobulin-antigen complexes containing the P. falciparum ring surface protein (RSP-2) have been reported. Soon after merozoites have infected a red cell, RSP-2 is expressed on the cell surface and may play a role in adhesion to endothelial cells. This protein is also found on the surfaces of uninfected red cells and erythroblasts in bone marrow from patients infected by P. falciparum, suggesting a role for RSP-2 in the development of anemia.9
The other half of the equation is clearly what is happening to erythropoiesis during malaria infection. It is well known that the reticulocytosis that results as a consequence of the decreased hematocrit is inappropriately low and delayed.
Particularly in chronic malarial anemia there are marked dyserythropoietic changes in the red-cell precursors and increased erythrophagocytosis. It has been suggested that these changes may be mediated, at least in part, by high levels of TNF-α.10 The bone marrow response is clearly defective and may include a defective erythropoietin response in the chronic forms of anemia. As part of the acute phase response, the peptide hepcidin is synthesized and released by hepatocytes resulting in increased serum ferritin and a reduction of bio-available iron. Hepcidin binds to the iron exporter ferroportin, which is expressed on the surface of macrophages, resulting in the internalization of the complex and its degradation.11 Hence, iron is retained by macrophages, rather than being released into the circulation, bound to transferrin and transported to the bone marrow or spleen where it can be re-cycled in erythropoiesis. It is clear from mouse models that there is a fine balance between erythropoietin and hepcidin levels in regulating erythropoiesis.12 Hepcidin is clearly implicated in the anemia of inflammation or chronic disease.13 It is not yet clear the extent to which hepcidin has a role to play in malarial anemia. In part, this is because a quantitative serum hepcidin assay has only just been developed.14 During the acute phase of malaria infection the serum iron level rapidly decreases and iron is sequestered in the marrow. Serum ferritin levels are high mimicking what is seen in other acute infections.
There is still much to understand about the mechanisms of the ineffective marrow response. It is possible that the suppression in the bone marrow is a consequence of the high levels of TNF-α that result from a malaria infection8. This cytokine is implicated in the anemia of chronic infection. TNF-α suppresses the growth of erythroid progenitor cells in human bone marrow cultures, though this effect lessens as the cells differentiate. However, TNF-α stimulates fibroblasts to secrete hematopoietic growth factors in vitro. The pigment resulting from the malaria parasite is ingested by resident macrophages in the spleen and marrow, resulting in the production of TNF-α at the site of erythropoiesis. Work in mice supports a role for TNF-α in the dyserythropoietic changes resulting from malaria infection. Other cytokines, such as interleukin (IL)-10, may contribute to dyserythropoiesis. IL-10 is an inhibitory cytokine that acts in opposition to TNF-α. It was shown in a study of African children with severe malarial anemia that the TNF-α:IL-10 ratio was high in plasma, suggesting that low levels of IL-10 contribute to the TNF-α suppression of erythropoiesis.15 Another cytokine, IL-12, protects against severe anemia in a mouse model.16 The parasite by-product of hemoglobin digestion, hemozoin also plays a role in the suppression of erythropoiesis.
What other factors may be involved in modulating erythropoiesis in malaria? The paper by Thawani et al., in this issue of the journal, describes the STAT6-mediated suppression of erythropoiesis in an experimental animal model of malarial anemia.17 STAT6 is a member of the signal transducer and activator of transcription (STAT) family of proteins of which there are six other members (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b)18. Studies using STAT6-deficient mice have revealed the key role that this protein plays in IL-4 and IL-13 signaling and in Th2 polarization of the immune system. STAT6 is ubiquitously expressed.
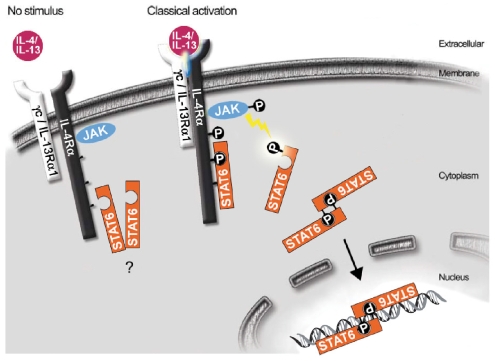
The STAT6 signaling cascade is activated when IL-4 or IL-13 engage with their corresponding receptor (Figure 1). The major receptor for IL-4 consists of IL-4R· and the common γ chain (γc).19 Signaling is initiated by the binding of IL-4 to IL-4Rα which then dimerises with γc (Figure 1). The type II IL-4 receptor is composed of IL-4Rα and IL-13Rα1 chains and can, consequently, transmit signals from IL-4 and IL-13. Once the particular cytokine is engaged, this leads to the phosphorylation of a specific Janus kinase (JAK) and the consequent phosphorylation of three tyrosine residues on IL-4Rα which then provides the necessary docking site for STAT6 (Figure 1). JAK then phosphorylates STAT6 which in turn dimerizes, and is translocated to the nucleus where it binds to DNA regulating the transcription of certain genes (Figure 1). STAT6 can also be activated by other pathways18.
Figure 1.
The classical STAT6 signaling pathway (adapted from a paper by Hebenstreit et al.18).
STAT6 binds to a highly conserved DNA sequence motif; the consensus is TTC(N)2–4GAA. This was identified in response to IFN-γ signaling and so is called the gamma-activated sequence (GAS) motif. STAT6 on its own is insufficient to upregulate transcription of a specific gene. It is the association of STAT6 with a combination of regulatory co-factors and the basal transcription complex that is the key to its activation of specific target genes. Other transcription factors can positively or negatively affect STAT6-regulated transcription. For example, nuclear factor-κB and STAT6 can act synergistically in some systems but antagonistically in others. About 35 STAT6-regulated loci have been described18.
In STAT6-deficient mice, the ability of a T cell to differentiate into normal Th2 cells is greatly reduced. This has thus allowed the detailed analysis of Th1 and Th2 responses in various disease models. STAT6-deficient mice have increased numbers of myeloid progenitor cells as well as increased cell cycling. IL-4 and IL-13 have been shown to increase iron uptake and storage by macrophages such that iron availability in the bone marrow restricts erythropoiesis.20
Thawani and colleagues exploited STAT6-deficient mice to investigate malarial anemia in the P. chabaudi AS animal model.17 These malaria-infected mice had an earlier and higher reticulocytosis despite having a higher peak parasitemia and a similar degree of anemia when compared to infected control mice. Exogenous erythropoietin treatment enhanced this reticulocytosis. These results suggested a role for STAT6 in malarial anemia.
Th1 cytokines have previously been reported to have a role in erythropoietic suppression. IFN-γ, lipopolysaccharide, and TNF-α upregulate the expression of divalent metal transporter 1, thus increasing the uptake of iron into macrophages13. Hepcidin expression is induced by lipopolysaccharide and IL-6 but is inhibited by TNF-α. As already mentioned, hepcidin blocks release of iron from macrophages by binding to ferroportin resulting in its internalization and degradation thus explaining its role in the anemia of chronic disease. The results of Thawani and colleagues suggest a role for the Th2 cytokine, IL-4, in malarial anemia. Both the Th2 cytokines IL-4 and IL-13 contribute to the observed hypoferremia seen during chronic inflammation by increasing iron uptake by macrophages through transferrin receptor 1 (CD71).20
It should be remembered that the spleen is a major site of extra-medullary erythropoiesis in malaria-infected mice thus enabling the analysis of erythroid progenitor cells in wild type and STAT6-deficient mice in response to malaria infection, with and without erythropoietin treatment. Thawani and colleagues provide evidence to suggest that a deficiency in STAT6 restricted the diversion of transferrin receptor 1 from erythroid to non-erythroid cells, thus maintaining iron availability to erythropoietin-responsive erythroid precursors.17 In P. chabaudi-infected mice, they also found increased proliferation of erythropoietin-treated splenocytes in STAT6-deficient animals when compared to in wild-type controls. The question they then posed was whether STAT6 played a role in IFN-γ production by natural killer and natural killer T cells in malarial anemia. Data from IL-4-depleted mice support the idea that IL-4 may be involved in inducing a protective Th1 response to control acute malarial infection and to regulate erythropoiesis. They found that malaria-infected STAT6-deficient mice had significantly lower IFN-γ levels than infected wild-type mice. Consequently, they suggested that there may be a link between IFN-γ levels and IL-4-dependent STAT6 signaling in malarial anemia. The relationship of these novel mechanisms in a murine model to the pathogenesis of malarial anemia in humans remains to be determined. Understanding the mechanism behind malarial anemia should lead to new approaches to managing the treatment of children, in particular those requiring hospital admission due to severe malarial anemia.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood BM, Bradley AK, Greenwood AM, Byass P, Jammeh K, Marsh K, et al. Mortality and morbidity from malaria among children in a rural area of the Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81:478–86. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- 3.Slutsker L, Taylor TE, Wirima JJ, Steketee RW. In-hospital morbidity and mortality due to malaria-associated severe anaemia in two areas of Malawi with different patterns of malaria infection. Trans R Soc Trop Med Hyg. 1994;88:548–51. doi: 10.1016/0035-9203(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 4.Jakeman GN, Saul A, Hogarth WL, Collins WE. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitol. 1999;119:127–33. doi: 10.1017/s0031182099004564. [DOI] [PubMed] [Google Scholar]
- 5.Looareesuwan S, Merry AH, Phillips RE, Pleehachinda R, Wattanagoon Y, Ho M, et al. Reduced erythrocyte survival following clearance of malarial parasitaemia in Thai patients. Br J Haematol. 1987;67:473–8. doi: 10.1111/j.1365-2141.1987.tb06171.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg EB, Strickland GT, Yang SL, Whalen GE. IgM antibodies to red cells and autoimmune anemia in patients with malaria. Am J Trop Med Hyg. 1973;22:146–52. doi: 10.4269/ajtmh.1973.22.146. [DOI] [PubMed] [Google Scholar]
- 7.Kwiatkowski D, Hill AV, Sambou I, Twumasi P, Castracane J, Manogue KR, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–4. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 8.Weatherall D, Kwiatkowski D, Roberts D. Hematologic manifestations of systemic diseases in children of the developing world. In: Orkin SH, Ginsburg D, Nathan DG, Look TA, Fisher DE, Lux SE, editors. Nathan and Oski’s Hematology of Infancy and Childhood. 7th ed. Elsevier; 2009. in press. [Google Scholar]
- 9.Layez C, Nogueira P, Combes V, Costa FT, Juhan-Vague I, da Silva LH, et al. Plasmodium falciparum rhoptry protein RSP2 triggers destruction of the erythroid lineage. Blood. 2005;106:3632–8. doi: 10.1182/blood-2005-04-1574. [DOI] [PubMed] [Google Scholar]
- 10.McGuire W, Knight JC, Hill AV, Allsopp CE, Greenwood BM, Kwiatkowski D. Severe malarial anemia and cerebral malaria are associated with different tumor necrosis factor promoter alleles. J Infect Dis. 1999;179:287–90. doi: 10.1086/314533. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–42. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 12.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbagen.2008.08.006. Aug 22 Epub. [DOI] [PubMed] [Google Scholar]
- 14.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–7. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 15.Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis. 1999;179:279–82. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 16.Mohan K, Stevenson MM. Interleukin-12 corrects severe anemia during blood-stage Plasmodium chabaudi AS in susceptible A/J mice. Exp Hematol. 1998;26:45–52. [PubMed] [Google Scholar]
- 17.Thawani N, Tam M, Stevenson MM. STAT6-mediated suppression of erythropoiesis in an experimental model of malarial anemia. Haematologica. 2009;94:195–204. doi: 10.3324/haematol.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–88. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 20.Weiss G, Bogdan C, Hentze MW. Pathways for the regulation of macrophage iron metabolism by the anti-inflammatory cytokines IL-4 and IL-13. J Immunol. 1997;158:420–5. [PubMed] [Google Scholar]