Abstract
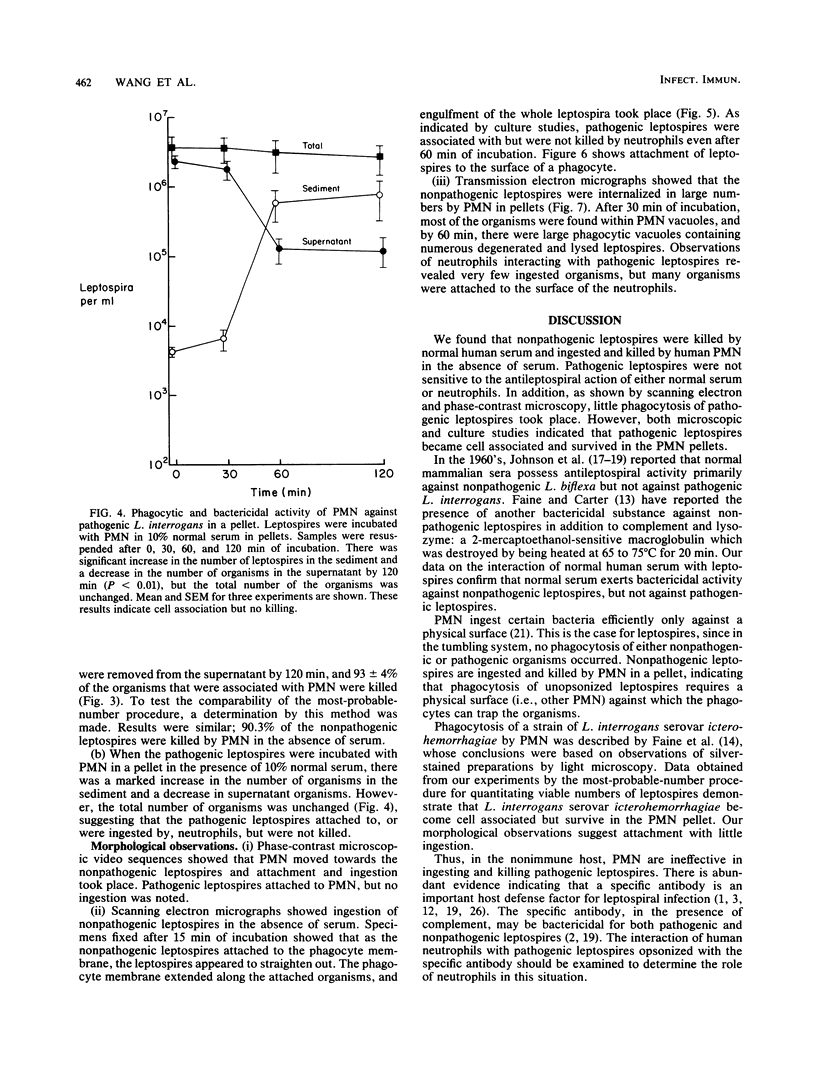
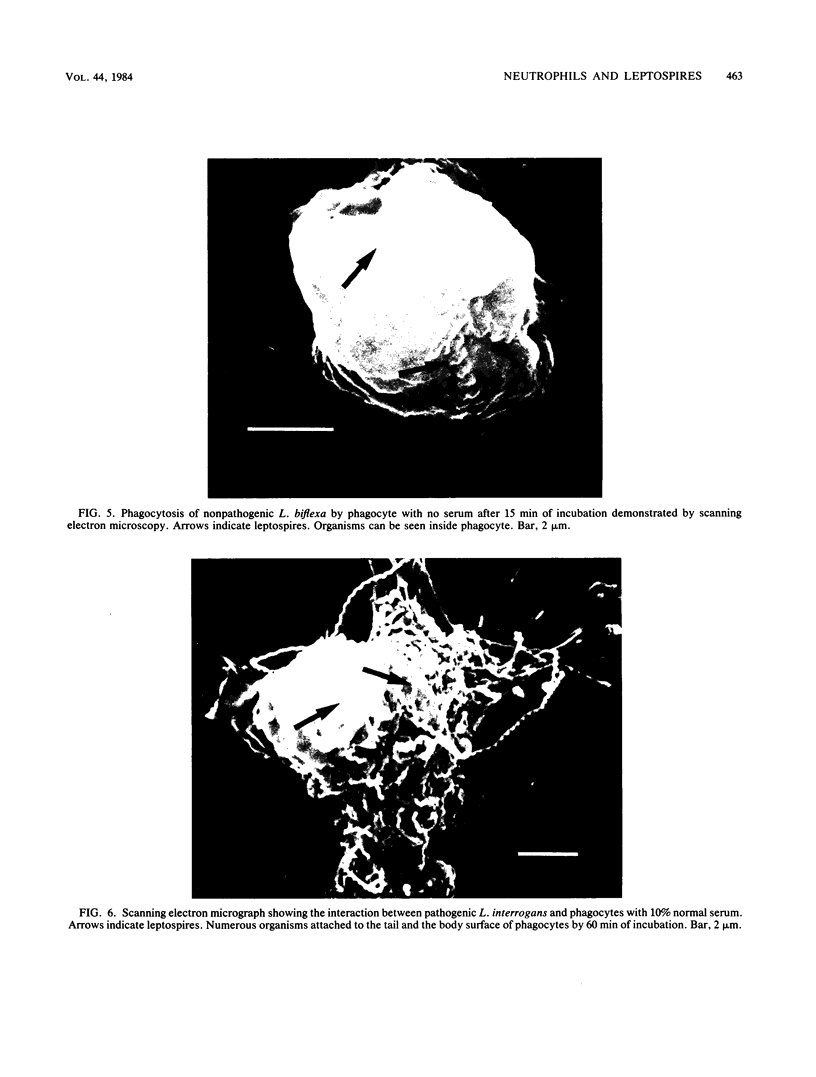
The role of the polymorphonuclear neutrophils (PMN) in defense against leptospires has not been adequately studied, in part, because of difficulty in quantitating pathogenic leptospires. By using pour plates to quantitate nonpathogenic leptospires and the most-probable-number procedure to quantitate the pathogenic leptospires, we examined the interactions of nonpathogenic Leptospira biflexa and pathogenic Leptospira interrogans serovar icterohemorrhagiae with human neutrophils. Phase-contrast, scanning electron, and transmission electron microscopic observations were made. Leptospires were incubated with PMN at 37 degrees C and tumbled together. There was no ingestion or killing of nonpathogenic leptospires (with no serum) or of pathogenic organisms (with 10% normal serum). However, when leptospires were incubated with PMN (without serum) in a pellet and then resuspended, 91 +/- 6% of nonpathogenic leptospires were removed from the supernatant, and 93 +/- 4% of these organisms were killed. The pathogenic leptospires became cell associated in a pellet, but were not killed by PMN even in the presence of 10% normal serum. Observations of morphological interactions indicated that PMN phagocytized the nonpathogenic leptospires in the absence of serum and that the pathogenic leptospires attached to but were not ingested by neutrophils in the presence of 10% normal serum. PMN do not seem to be an efficient defense factor for pathogenic leptospires in nonimmune hosts. The virulence of leptospires appears to be related to their ability to resist killing by serum and to resist ingestion and killing by neutrophils.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B., Faine S. Host immunological mechanisms in the resistance of mice to leptospiral infections. Infect Immun. 1977 Jul;17(1):67–72. doi: 10.1128/iai.17.1.67-72.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler B., Faine S., Muller H. K., Green D. E. Maturation of humoral immune response determines the susceptibility of guinea-pigs to leptospirosis. Pathology. 1980 Oct;12(4):529–538. doi: 10.3109/00313028009086806. [DOI] [PubMed] [Google Scholar]
- Adler B., Faine S. Susceptibility of mice treated with cyclophosphamide to lethal infection with Leptospira interrogans Serovar pomona. Infect Immun. 1976 Sep;14(3):703–708. doi: 10.1128/iai.14.3.703-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi E., Cinco M., Bellini M., Soranzo M. R. The role of antibodies and serum complement in the interaction between macrophages and leptospires. J Gen Microbiol. 1982 Apr;128(4):813–816. doi: 10.1099/00221287-128-4-813. [DOI] [PubMed] [Google Scholar]
- Banfi E., Cinco M., Bellini M., Soranzo M. R. The role of antibodies and serum complement in the interaction between macrophages and leptospires. J Gen Microbiol. 1982 Apr;128(4):813–816. doi: 10.1099/00221287-128-4-813. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COX C. D., LARSON A. D. Colonial growth of leptospirae. J Bacteriol. 1957 Apr;73(4):587–589. doi: 10.1128/jb.73.4.587-589.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAINE S. RETICULOENDOTHELIAL PHAGOCYTOSIS OF VIRULENT LEPTOSPIRES. Am J Vet Res. 1964 May;25:830–835. [PubMed] [Google Scholar]
- FAINE S., SHAHAR A., ARONSON M. PHAGOCYTOSIS AND ITS SIGNIFICANCE IN LEPTOSPIRAL INFECTION. Aust J Exp Biol Med Sci. 1964 Oct;42:579–588. doi: 10.1038/icb.1964.54. [DOI] [PubMed] [Google Scholar]
- Faine S., Adler B., Ruta G. A mechanism of immunity to leptospirosis. Aust J Exp Biol Med Sci. 1974 Apr;52(2):301–310. doi: 10.1038/icb.1974.28. [DOI] [PubMed] [Google Scholar]
- Faine S., Carter J. N. Natural antibody in mammalian serum reacting with an antigen in some leptospires. J Bacteriol. 1968 Feb;95(2):280–285. doi: 10.1128/jb.95.2.280-285.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Brush J., Ravdin J. I., Sullivan J. A., Mandell G. L. Interaction between Entamoeba histolytica and human polymorphonuclear neutrophils. J Infect Dis. 1981 Jan;143(1):83–93. doi: 10.1093/infdis/143.1.83. [DOI] [PubMed] [Google Scholar]
- Halvorson H. O., Ziegler N. R. Application of Statistics to Problems in Bacteriology: I. A Means of Determining Bacterial Population by the Dilution Method. J Bacteriol. 1933 Feb;25(2):101–121. doi: 10.1128/jb.25.2.101-121.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., MUSCHEL L. H. ANTILEPTOSPIRAL ACTIVITY OF NORMAL SERUM. J Bacteriol. 1965 Jun;89:1625–1626. doi: 10.1128/jb.89.6.1625-1626.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. 5-FLUOROURACIL AS A SELECTIVE AGENT FOR GROWTH OF LEPTOSPIRAE. J Bacteriol. 1964 Feb;87:422–426. doi: 10.1128/jb.87.2.422-426.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Antileptospiral activity of serum. II. Leptospiral virulence factor. J Bacteriol. 1967 Feb;93(2):513–519. doi: 10.1128/jb.93.2.513-519.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Muschel L. H. Antileptospiral activity of serum. I. Normal and immune serum. J Bacteriol. 1966 Apr;91(4):1403–1409. doi: 10.1128/jb.91.4.1403-1409.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. M., Varela-Díaz V. M. Selective isolation of leptospiras from contaminated material by incorporation of neomycin to culture media. Appl Microbiol. 1973 May;25(5):781–786. doi: 10.1128/am.25.5.781-786.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A. Application of the most-probable-number procedure to suspensions of Leptospira autumnalis Akiyami A. Appl Microbiol. 1973 Feb;25(2):235–239. doi: 10.1128/am.25.2.235-239.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A. Survivor curves for Leptospira autumnalis Akiyami A based on most-probable-number values. Appl Microbiol. 1973 Feb;25(2):240–243. doi: 10.1128/am.25.2.240-243.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. W., Mandell G. L. Role of neutrophil degranulation in streptococcal leukotoxicity. Infect Immun. 1981 Jul;33(1):267–274. doi: 10.1128/iai.33.1.267-274.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu V., Adler B., Faine S. The role of macrophages in the protection of mice against leptospirosis: in vitro and in vivo studies. Pathology. 1982 Oct;14(4):463–468. doi: 10.3109/00313028209092128. [DOI] [PubMed] [Google Scholar]
- YANAGAWA R., HIRAMUNE T., FUJITA J. EFFECTS OF CARBON DIOXIDE ON THE COLONIAL GROWTH OF PATHOGENIC LEPTOSPIRAE. J Bacteriol. 1963 Apr;85:875–878. doi: 10.1128/jb.85.4.875-880.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]