This Decision Making and Problem Solving article reports recommendations for distinguishing hypocellular acute myeloid leukemia from hypocellular myelodysplastic syndromes and aplastic anemia.
Keywords: hypocellular myelodysplasia, hypocellular myeloid leukemia, abnormal localization of immature precursors, aplastic anemia
Abstract
Members of the French-American-British Cooperative Leukemia Working Group met to review cases of aplastic anemia, hypocellular myelodysplastic syndrome and hypocellular acute myeloid leukemia. Criteria were proposed and modified following three workshops. Additional input was obtained from another hematopathologist with a special interest in bone marrow histology and immunohistochemistry. Guidelines were recommended based on the workshop results as well as additional studies including selective immunohistochemistry, flow cytometry and cytogenetics.
Introduction
Hypocellular or hypoplastic Acute Myeloid Leukemia (H-AML) and hypocellular Myelodysplastic Syndromes (H-MDS) represent a small (10–15%) but significant number of patients diagnosed with myeloid malignancies.1,2 H-AML mainly affects the elderly and accounts for 5–7% of de novo AML. H-MDS is more frequent in women and occurs with an age-related frequency which is similar to that seen in primary MDS. In children refractory cytopenia is the commonest form of marrow failure that is recognized, accounting for 50% of all MDS cases.3 Previous genotoxic exposure or therapy needs to be excluded since hypocellular marrows can be seen in cases of therapy-related-MDS/AML.
Both H-AML and H-MDS are associated with pronounced cytopenia, a finding which may suggest a clinical diagnosis of acquired aplastic anemia (AA). Moreover, in cases where the bone marrow cellularity is extremely low (i.e.<20%), it may be very difficult to separate these disorders from AA utilizing standard morphological criteria applied to bone marrow aspirate smears such as the percentage of blasts and degree of morphological dysplasia. Since the majority of the H-MDS cases by both FAB and WHO classification systems4 fall within the category of refractory anemia, their blast count is not increased and overlaps what is seen in AA. Although dysplasia can be more easily appreciated in cases of H-MDS than H-AML, even in the former group it may be minimal. Since most of the H-MDS cases belong to the category of RA, they may display only mild dyserythropoiesis, a finding which is not specific for MDS since it can also be observed in a proportion of cases of AA.
All these issues are further compounded by the paucicellularity of bone marrow aspirates which are typically obtained in patients with severe marrow hypocellularity. Thus a bone marrow biopsy is critical and necessary to diagnose these variants in all patients, children included.
Aim of this study
Although there have been criteria published for the diagnosis of aplastic anemia,5 H-AML and H-MDS have not been defined satisfactorily and compared to AA by a group of investigators in an attempt at establishing concordance.
Design and Methods
During the first workshop a series of publications were reviewed1,2,5–9 and a small number of cases examined. Criteria were proposed and applied to a series of cases in the subsequent workshops (Table 1).
Table 1.
Guidelines for diagnosis.
Bone marrow cellularity and the impact of age correction
An important criterion which allows for a more reproducible diagnosis of H-MDS and H-AML has been to establish the impact of age and make an appropriate correction before determining and recording the degree of cellularity as done by Tuzuner et al.6 In that paper the original data on normal cellularity published by Hartsock7 was compared against a large data base of AML and MDS cases. Thirteen percent of patients with AML and 29% with MDS fell below the lower range corrected for age.
Results
Three workshops were held by members of the French-American-British (FAB) Cooperative Leukemia Working Group over a two year period with 63 cases reviewed. These included 38 cases of hypocellular MDS (diagnosed at one center and previously published),2 14 cases of hypocellular AML1 and 11 cases of AA (provided from the files of two of the members). All cases had bone marrow cellularity of <20%.
The materials reviewed included peripheral blood smears, bone marrow aspirate smears, iron stains to identify ring sideroblasts and cytochemical peroxidase stains to identify myeloid precursors. Conventionally processed bone marrow biopsies were also reviewed. All cases were coded and laboratory/clinical information was made available only after the initial diagnoses were recorded.
The following approaches were agreed upon; (i) the presence of unequivocal blasts in the peripheral blood was considered indicative of MDS or AML. Because of significant leukopenia, a precise determination of blast percentage was often impossible, but an effort was made to count at least 100 cells, including lymphocytes; (ii) hypogranular neutrophils or pseudo-Pelger neutrophils were considered indicative of either MDS or AML if more than 10% were identified. Fewer numbers raised the suspicion but were not felt to be definitive; (iii) the presence of >1% to 20% blasts in the marrow aspirates was considered diagnostic of MDS if dysplasia was recognized; (iv) morphological marrow dysplasia of either granulocytes or megakaryocytes was considered as abnormal and inconsistent with AA. Erythroid dysplasia had to be moderate to severe, if the sole finding (bi or trinucleated forms, numerous Howell-Jolly bodies, nuclear budding or bridging);10 (v) the presence of any abnormal sideroblasts (>5 granules surrounding the nuclear membrane or occupying at least 1/3 of the circumference) was considered as evidence of dyserythropoiesis and excluded a diagnosis of AA; (vi) a 1–2 cm. core biopsy was preferred for all patients being evaluated for a potential diagnosis of either hypoplastic myeloid disorder or aplastic anemia. Being able to visualize 4–5 undistorted fields under 100× magnification was considered as adequate. Bone marrow cellularity was estimated utilizing an ocular grid as published previously;8 (vii) the presence of two or more clusters of immature precursors (minimum of three blasts/clusters) in the bone marrow biopsy was indicative of either MDS or AML;11 and (viii) consensus diagnosis required agreement by at least 5/7 participants.
Aplastic anemia
There was a very high degree of concordance, with 100% agreement on 10 cases and 71% (5/7 investigators) concordance in one case. In the latter, the finding of focal granulocytic hyperplasia was considered as most consistent with early (pre) MDS rather than AA by one participant. Median cellularity was 10% (range; <5–80%). In the case with 80% cellularity there was a mixture of lymphocytes, plasma cells and occasional granulocytes but no erythroid precursors or megakaryocytes. Among the different investigators the estimate of cellularity based on reading the biopsy, clot section or particles on the marrow aspirates, varied less than 25%. The use of a pictorial representation of degrees of cellularity8 proved to be most helpful.
Hypocellular myelodysplastic syndromes
There was complete agreement on these cases. The presence of unequivocal dysplasia and/or excess blasts was the central component that provided for the observed high concordance.
Hypocellular acute myeloid leukemia
This group proved to be the most contentious for the panel. Of the 14 cases submitted as having H-AML there was 100% agreement on 8 of these. Of the remaining 6 cases 4 were considered to have AA by 4 observers and 2 were considered to have H-MDS. Therefore concordance was 57%. Even the presence of occasional clusters of immature precursors (ALIP) were not considered as diagnostic of AML, since this was also a well recognized finding in patients with MDS. The most important feature was the identification of 20% or more blasts on the aspirate which was possible only in adequately cellular, optimally stained aspirates. With inadequate aspirates one was dependent on the presence of ALIP in the biopsy. With the absence of sufficient numbers of granulocytes, AML was established. We suggest that such cases should be reviewed by, at least, two observers when there is doubt raised.
Discussion
Bone marrow cellularity may be an important prognostic factor in H-MDS. There is some controversy on survival differences between H-MDS and normo/hypercellular MDS, but patients appear to do at least as well or better.2,12 The distinction from AA is, however, clinically relevant, because the risk of progression to acute leukemia is much greater in H-MDS. In contrast to AA where immunosuppressive therapy has been utilized extensively only a small number of series have been published in cases of MDS, where both hypocellular and normo/hypercellular cases have been included. Results have been mixed, at best, with the data from NIH having very good outcomes.13 Obviously, the distinction from AA is even more important in cases of H-AML. The latter patients require appropriate acute leukemia treatment; if properly managed, they share the same prognosis of normo/hypercellar AML with comparable cytogenetics.
The identification of hypoplastic myeloid neoplasms is compounded by the lack of clear cut diagnostic criteria, a fact that is responsible for serious diagnostic inconsistencies. These are both in relation to the definition of a case as being truly hypocellular as well as the separation between H-MDS, H-AML, and aplastic anemia.
In regard to the first point, by using anatomic comparisons, Tuzuner and co-workers6 demonstrated that 63% of patients with AML and 35% with MDS had hypercellular marrows; 24% of AML patients and 36% with MDS were normocellular and 13% of AML and 29% with MDS were hypocellular. Correcting for age, however, lowered the percentage of hypocellular marrows to 2.2% and 7% respectively. Age correction is therefore necessary and should be considered as one of the defining criteria for such diagnoses.
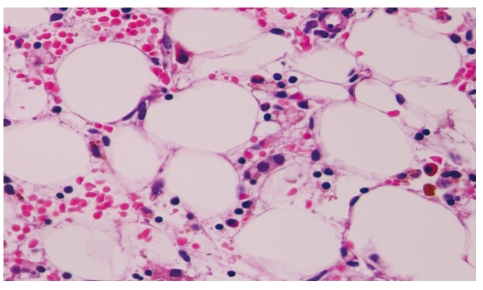
Figure 2.
Aplastic anemia. H&E. ×400
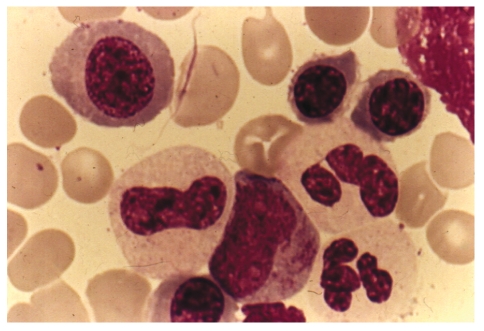
Figure 3.
Bone marrow aspirate from a case of hypocellular myelodysplastic syndromes: note the megaloblastic erythroid precursors and agranular granulocytes with one abnormal promyelocyte. Wright-Giemsa Stain. ×1000.
The separation between H-MDS, H-AML, and AA can be problematic as illustrated by our own experience (JMB and Tuzuner N, unpublished observations, 1993–95): 17/270 cases referred to our center as having MDS were reclassified as AML (6.7%) and 3/71 cases considered to have AA were reclassified as hypocellular AML (4.2%). In most of these cases the original blast count was inaccurate. It has been shown that unless a large number of peripheral blood or marrow cells are counted the reliability of the blast count percentage can be questioned.14 For example a 5% blast count has a 95% confidence interval between 1.6 and 11.3% for a 100 cell count but narrows considerably with a 500 cell differential (3.3–7.3%). Since we have established a 5% minimal blast percentage in the marrow for MDS in the absence of significant dysplasia15 and with the percentage of blasts being below 1% in AA, the use of a 500 cell differential count as also recommended by the WHO system appears quite reasonable.
Even by histological analysis, the morphological differences may be subtle. Separation from AA is compounded by the high proportion of cases showing nonspecific mesenchymal reactions, including the presence of an increase in mast cells, lymphocytes, and reactive lymphoid follicles, features similar to those observed in bone marrow biopsies obtained from patients with AA. The presence of easily identifiable megakaryocytes within an architecturally disorganized marrow and the presence of reticulin fibrosis favor MDS over aplastic anemia.9 An important feature provided by the bone marrow biopsy, particularly valuable in cases with fatty marrow and paucicellular aspirates, is its capacity to identify blasts. Blasts which are normally scattered or adjacent to the bone trabeculae or blood vessels in normal or reactive marrows in patients with leukemic disorders display a tendency to form aggregates or clusters in abnormal central marrow cavity location. The presence of such aggregates or clusters of blasts, previously defined as abnormally localized immature myeloid precursor cells (ALIP), is mainly seen in the aggressive MDS subtypes and is associated with a poor prognosis and an increased incidence of progression to acute leukemia. Cases are classified as ALIP positive if at least three aggregates (more than five myeloid precursors) or clusters (three to five myeloid precursors) are identified in a histology section.11 The identification of blasts in tissue sections may be compromised by using paraffin sections of excessive thickness or otherwise suboptimal morphology. Additionally, other immature cells (i.e. proerythroblasts, subsets of lymphoid cells) may be mistaken for blasts by morphology alone.
The introduction of immunohistological markers capable of reacting with routinely fixed-paraffin-embedded bone marrow material has provided additional assistance in the blast identification. CD34, an antigen expressed in progenitor and early precursor marrow cells, has been proved among the most useful ones in this regard.16 A significant amount of published evidence has demonstrated that CD34 can be used as a tool for counting blasts in tissue sections.17 Other antibodies that may be useful include CD117, myeloperoxidase, lysozyme and CD68.
Immunohistochemistry may help in distinguishing H-MDS and H-AML from acquired aplastic anemia.17 Both an increase in the percentage of CD34 positive cells and a tendency of positive cells to form aggregates have been shown to be useful in distinguishing these hypoplastic myeloid neoplasms from cases of aplastic anemia (Figures 1–4).
Figure 1.
Normocellular bone marrow (no evidence for myelodys-plastic syndromes). Hematoxylin & Eosin. ×400
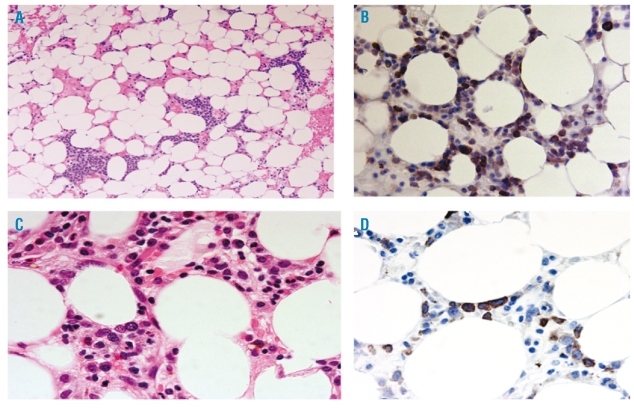
Figure 4.
(A) Numerous clusters of immature precursors in a hypocellular setting. Hypocellular acute myeloid leukemia. H&E.×200. (B) CD34 immunostain revealing many positive blasts. ×400. (C) Hypocellular myelodysplastic syndromes: note maturing cells with rare immature cell. H&E.×400. (D) Hypocellular myelodysplastic syndromes: CD34 immunostain with rare positive cells. ×400.
In an attempt at improving this situation, our paper proposes guidelines which should allow for a reproducible identification of cases of myeloid neoplasms with fatty marrow such H-MDS and H-AML. It needs to be stressed, however, that this diagnosis may be very difficult particularly in cases with a bone marrow cellularity of less than 10%. Cytogenetic studies may be of particular value in this group, as abnormalities of chromosomes 5 and/or 7 may be identified following therapy for AA utilizing standard Giemsa banding cytogenetics or by fluorescence in situ hybridization (FISH) on paraffin embedded tissues.18 It must be emphasized, however, that these markedly fatty marrows are frequently cytogenetic failures. In some cases, peripheral blood FISH studies for the above mentioned abnormalities can be positive and can act as a quality control to prevent false negative marrow karyotype results.
When MDS is suspected, flow cytometry is often requested to rule out excess of blasts or acute blastic transformation (i.e. to AML). In all these cases a comprehensive panel of antibodies which include myeloid, myelomonocytic and lymphoid antibodies is generally used. Qualitative aberrations have been noted in patients with MDS but would likely to be positive in the progeny of cases of AML as well.19
However, in cases with low CBC counts, absence of an increased number of blasts and in general in all cases with markedly hypocellular bone marrow aspirates, the value of this technique has not been proven. Although there is often good correlation between CD34 percentage as determined by flow cytometry and morphology, the percentage of blasts as determined by flow may be influenced by hemodilution of the specimen at the time of collection, as seen in cases with fatty or fibrotic marrows. The result from flow cytometry should never be used in lieu of morphological inspection of the peripheral blood film, bone marrow aspirate smears, and bone marrow biopsy.20
Finally, AA and hypocellular MDS have a number of overlapping features, such as the appearance of a clone of PNH cells or evidence of T-cell mediated myelosuppression, suggesting that they share a common patho-physiologic pathway. However, sensitive assays for the presence of PIG-A mutations have demonstrated their presence in sufficient numbers of patients with either AA or MDS to not allow these assays to be considered definitive.21
Novel cytogenetic and molecular genetic approaches are likely to revolutionize the way we classify these diseases in the near future. However, it is highly unlikely that these new techniques will be capable, on their own, of adequately stratifying all patients for purpose of treatment. Subgroups like the hypoplastic myeloid neoplasms will still benefit from a multipara-metric diagnostic approach only possible through a close collaboration between the clinical hematologist and hematopathologist with expertise in these relatively uncommon conditions.
Acknowledgments
We appreciate very much the participation of members of the French-American-British (F.A.B.) Cooperative Leukemia Working Group (J.M. Bennett, D. Catovsky, M-T. Daniel, G. Flandrin, D.A.G. Galton, H.R. Gralnick and C. Sultan) in three workshops and for careful review of the manuscript by D. Catovsky and G. Flandrin, We are indebted for the secretarial support of Ms. Phoebe Downing. No governmental or industry support was provided to the authors.
Footnotes
Authorship and Disclosures
The authors equally contributed to the manuscript.
References
- 1.Tuzuner N, Cox C, Rowe JM, Bennett JM. Hypocellular acute myeloid leukemia: the Rochester (New York) experience. Hematol Pathol. 1995;9:195–203. [PubMed] [Google Scholar]
- 2.Tuzuner N, Cox C, Rowe JM, Watrous D, Bennett JM. Hypocellular myelodysplastic syndrome: new proposals. Br J Haematol. 1995;91:612–7. doi: 10.1111/j.1365-2141.1995.tb05356.x. [DOI] [PubMed] [Google Scholar]
- 3.Niemeyer CM, Baumann I. The myelodysplastic syndromes in children and adolescents. Seminars in Hematology. 2008;45:60–70. doi: 10.1053/j.seminhematol.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Goyal R, Qawi H, Ali I, Dar S, Dar S, Mundle S, Shetty V, et al. Biologic characteristics of patients with hypocellular myelodysplastic syndromes. Leuk Res. 1999;23:357–64. doi: 10.1016/s0145-2126(98)00187-8. [DOI] [PubMed] [Google Scholar]
- 5.Young NS. Aplastic Anemia. In: Young NS, editor. The Bone marrow failure syndromes. Philadelphia, Pa: WB Saunders; 2000. pp. 1–46. [Google Scholar]
- 6.Tuzuner N, Cox C, Rowe JM, Bennett JM. Bone marrow cellularity in myeloid stem cell disorders; impact of age correction. Leuk Res. 1994;18:559–64. doi: 10.1016/0145-2126(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 7.Hartsock RJ, Smith EP, Petty CS. Normal variation with aging of the hematopoietic tissue in bone marrow from anatomic iliac crest. Am J Clin Path. 1965;43:326–31. doi: 10.1093/ajcp/43.4.326. [DOI] [PubMed] [Google Scholar]
- 8.Tuzuner N, Bennett JM. Reference standards for bone marrow cellularity. Leuk Res. 1994;18:645–7. doi: 10.1016/0145-2126(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 9.Fohlmeister I, Fischer R, Mödder B, Rister M, Schaefer HE. Aplastic anemia and the hypocellular myelodys-plastic syndrome: histomorphological, diagnostic and prognostic features. J Clin Pathol. 1985;38:1218–24. doi: 10.1136/jcp.38.11.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goasguen JE, Matsuo T, Cox C, Bennett JM. Evaluation of the dysmyelopoiesis in 336 patients with De Novo acute myeloid leukemia: major importance of dysgranulopoiesis for remission and survival. Leukemia. 1992;6:520–5. [PubMed] [Google Scholar]
- 11.De Wolf-Peeters C, Stessens R, Desmet V, Tricot G, Verwilghen RL. The histological characterization of ALIP in the myelodysplastic syndromes. Pathol Res Pract. 1986;181:402–7. doi: 10.1016/S0344-0338(86)80075-9. [DOI] [PubMed] [Google Scholar]
- 12.Yue G, Hao S, Fadare O, Baker S, Pozdnyakova O, Galili N, et al. Hypocellularity in myelodysplastic syndrome is an independent factor which predicts a favorable outcome. Leuk Res. 2008;32:553–8. doi: 10.1016/j.leukres.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplastic syndrome treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–11. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koepke JA, editor. Practical laboratory Haematology. Churchill Livingston; Philadelphia, Pa, USA: 1991. p. 134. [Google Scholar]