Abstract
Insect-borne diseases have experienced a troubling resurgence in recent years. Emergence of resistance to pesticides greatly hampers control efforts. Paratransgenesis, or the genetic transformation of bacterial symbionts of disease vectors, is an alternative to traditional approaches. Previously, we developed paratransgenic lines of Rhodnius prolixus, a vector of Chagas disease in Central America. Here, we report identification of a Corynebacterial species as a symbiont of Triatoma infestans, a leading vector of Chagas disease in South America. We have modified this bacterium to produce an immunologically active single chain antibody fragment, termed rDB3. This study establishes the basis for generating paratransgenic T. infestans as a strategy for control of Chagas disease.
Index Descriptors: Paratransgenesis, Chagas disease, Triatoma infestans, Corynebacteria
1. Introduction
Insect-borne diseases remain a leading cause of human illness throughout the world. Strategies for control of these diseases rely principally on chemical insecticides that eliminate or reduce numbers of vector insects. Chagas disease and its main vector, Triatoma infestans, are widely prevalent in the Gran Chaco, a semi-arid landscape extending over Argentina, Bolivia, Paraguay and southwestern Brazil. Several vector control programs, such as the Southern Cone Initiative for control of Chagas disease, are mostly based on the residual application of pyrethroid insecticides (Dias et al., 2002; Schofield and Dias, 1999). In the Argentine Chaco, however, symptomatic acute cases of Chagas disease are increasingly reported since 2001. Disorganized decentralization of vector control programs in the early 1980s followed by diminishing operational capacity since the 1990s, further compounded by the Argentine economic crisis in late 2001, contributed to the present scenario of persistent peridomestic infestation with recurrent domestic recolonization by T. infestans and renewed transmission to humans in the most affected regions (Gürtler et al., 2005).
Most insecticides cause environmental toxicity and many classes of these chemicals harm humans. Insects have evolved resistance to many of these agents (World Health Organization, 1992) and insecticide failure is common. Resistance of triatomine populations to a variety of insecticides has been documented in other Chagas endemic regions of the world (Picollo et al., 2005). The poor effects of pyrethroid insecticides against peridomestic T. infestans and other triatomines are mainly caused by their short-lasting residual effects in outdoor sites exposed to sunlight, high temperatures, rain and dust (Gürtler et al., 2004). The suite of several insecticide intervention trials and the background experience of Chagas vector control services in the Gran Chaco clearly demonstrate that current tactics and procedures fail to eliminate populations of T. infestans in rural areas and need to be revised.
Control of vector-borne diseases may potentially be achieved by modifying insects, using foreign genes, to reduce their ability to transmit pathogens. This can involve direct germline transformation (Coates et al., 1998; Jasinskiene et al., 1998; Olson et al., 1996), or paratransgenic manipulation (Richards, 1993; Beard et al., 1993; Conte, 1997; Beard et al., 1998; Beard et al., 2000). In the reduviid bug vector of Chagas disease, Rhodnius prolixus, we have expressed the peptide, cecropin A, via an engineered symbiotic bacterium, Rhodococcus rhodnii, at concentrations that eliminate the parasite, Trypanosoma cruzi (Durvasula et al., 1997). We have demonstrated stable expression of an active antibody fragment in R. prolixus via genetically altered R. rhodnii (Durvasula et al., 1999a,b). Furthermore, we have shown under simulated field conditions that engineered bacteria can be delivered to populations of newly emerging nymphs of R. prolixus. These transformed bacteria predominate in the host insect throughout its life cycle (Durvasula et al., 1999a,b).
A wide variety of bacteria—including Nocardia, Cory-nebacteria and Streptococci—have been isolated from the midgut of T. infestans (Figueiro et al., 1995). These organisms play an important role in insect development and survival by providing essential nutrients such as pantothenic acid to nymphs (Dasch et al., 1984). Here we report the identification of a Corynebacterial species as a symbiont of T. infestans (Argentina) strain and the genetic transformation of this bacterium to produce an immunologically active single chain antibody fragment. Our test antibody fragment, termed DB3 VH-Kappa (rDB3), is a murine antibody fragment that binds progesterone with affinity in the order of 1 × 109 l/mol (He et al., 1991). It is used as a model for expression of single chain antibodies that have activity against T. cruzi. This study establishes the basis for generating paratransgenic T. infestans—insects that carry genetically altered symbionts—as a strategy for control of Chagas disease transmission.
2. Materials and methods
2.1. Identification of Corynebacterium
Adult T. infestans (Argentina) were kindly provided by Dr. Vaclav Hitsva. These insects were initially collected from the Gran Chaco region and had matured for generations under insectary conditions. Fecal droplets from 10 adult T. infestans were collected. Individual droplets were diluted in 100 µl of PBS and plated on BHI agar (Difco, Detroit, MI) for 24 h at 28 °C. Individual colonies of bacteria from a monoculture were identified by 16S rDNA sequence analysis. A 1.4 kb fragment was amplified using standard 16S primers, 27F (AGAGTTTGATCMTGGCTCAG) and 1492R (TACGGYTACCTTGTTACGACTT) (Lane, 1991) and the GeneAmp XL PCR Kit (PE Applied Biosystems, Foster City, CA) that contains the rTth DNA polymerase XL, a polymerase with proofreading activity. The resulting product was purified using the Wizard PCR Prep kit (Promega, Madison, WI). Both strands of the fragment were sequenced using the above primers and the internal primers 530F(GTGCCAGCMGCCGCGG) and 685R (TCTACGRATTTCACCYCTAC) (Lane, 1991) and the ABI Prism BigDye Terminator Cycle sequencing kit. Unincorporated dyes were removed from reaction mix with Centri-sep columns (Princeton Separations, Inc., Adelphia, NJ). The fluorescent dye-labelled fragments were electrophoresed on 5% Long Ranger polyacrylamide gel using an ABI 377 automated sequencer. Sequence alignment and contig assembly were done using Sequence Navigator (PE Applied Biosystems) and further analysis and sequence comparisons were done with the Wisconsin Genetics Computer Group (GCG) package.
2.2. Establishing Corynebacterium as the symbiont of T. infestans
Aposymbiotic first instar nymphs of T. infestans were generated as described (Durvasula and Taneja, 1999). Hundred and eighty nymphs were divided into four groups. Group 1 (n = 60) were kept in containers lined with filter paper stained by the feces of the adult T. infestans. Group 2 (n = 40) were maintained in a sterile container but fed two blood meals supplemented with the Corynebacterium at a concentration of 1 × 106 CFU/ml. Group 3 (n = 40) were kept in a sterile container and fed two blood meals supplemented with R. rhodnii at a concentration of 1 × 106 CFU/ ml. Group 4 (n = 40) were kept in a sterile container until the sixth month. At that time, Group 4 was again divided. Group 4a (n = 20) received a blood meal supplemented with the Corynebacterium at a concentration of 1 × 106 CFU/ml; Group 4b (n = 20) received a sterile blood meal. All insects in the study were maintained on monthly meals of defibrinated sterile sheep’s blood. The colonies were monitored for growth, mortality and egg production. At 12 months, a fecal droplet was collected from the living insects and cultured. Infection with the Corynebacterium was established as described above. Gut contents of insects that died during the course of the experiment were similarly cultured within 24 h of their death to assay for presence of the Corynebacterium or contaminating bacteria.
2.3. Construction of the shuttle plasmid, pRrMDWK6, and genetic transformation of Corynebacterium
Construction of the shuttle plasmid, pRrMDWK6, is described elsewhere (Durvasula et al., 1999a,b). Briefly, the replication origin fragment of R. rhodnii was cloned into the EcoR1 restriction site of the DNA vector pBlue-script SK+ (Stratagene). The alpha antigen gene of Myco-bacterium kansasii (MKα), used to express a heterologous promoter-signal peptide, was ligated in frame to the rDB3 VH-Kappa fragment. The Mkα/rDB3 fragment was cloned into the Xba1 site of the shuttle vector. Cloning of a kanamycin resistance gene (Pharmacia) as a BamHI fragment yielded the final shuttle plasmid pRrMDWK6. Generation of electrocompetent Corynebacterium and transformation with pRrMDWK6 were done as previously described (Durvasula et al., 1999a,b). Transformed cells were selected on Luria Bertani (LB) agar that contained 50 µg/ml of kanamycin.
2.4. Measurement of DB3 activity in transformed Corynebacterium
Individual colonies of the Corynebacterium that were genetically transformed with pRrMDWK6 were inoculated into 100 ml of LB broth containing kanamycin (50 µg/ml) and grown for 16 h with gentle agitation at 28 °C. Cells were pelleted and lysed by incubation in a buffer containing 50 mM Tris–Cl (pH 8.0), 150 mM NaCl, 100 µg/ml phenylmethylsulfonyl fluoride and 1% Triton X-100 (Sigma) at 4°C for 2 h. The lysate was centrifuged at 12,000g for 5 min at 4°C. Serial dilutions of the supernatant were used for ELISA. All assays were performed in triplicate.
2.5. ELISA
Microtiter plates were coated with progesterone 3-(O-carboxymethyl)oxime:BSA conjugate (Sigma) using 2.0 µg of conjugate per well. A volume of 150 µl lysate of Corynebacterium were incubated in the progesterone-coated wells for 16 h at 4°C. After washing the wells five times with PBS, the bound VH/K was detected using alkaline phosphatase-conjugated sheep anti-mouse IgG (Boehringer Mannheim) at 1:1000 dilution followed by p-nitrophenyl-phosphate (Sigma). Absorbance at 405 nm was read using an MRX-HD automated kinetic plate reader (Dynex).
For reactions that involved excess progesterone-CMO as a competitive inhibitor, 100 µl of the cell lysate were mixed with 50 µl of a 260 mM solution of progesterone 3-(O-carboxymethyl)oxime (Sigma).
3. Results
3.1. Identification of Corynebacterium
FASTA and NetBlast searches of the GenBank databases were used to find sequences similar to the 1.4 kb sequence fragment (Accession No. AF322369). No sequences with 100% identity were found. The highest sequence identities (99%) observed were with Corynebacterium sp. CNJ954 PL04 (Accession No. DQ448694), with a single gap in the sequence and with Corynebacterium sp. BBH8 (Accession No. AM183334), where three separate gaps in the sequence was observed. This sequence also showed 98.8% sequence identity with Corynebacterium variabilis (Accession No. AJ222815) although a 10 bp gap had to be inserted around base 410 in C. variabilis for the alignment. Sequence similarity of 98.1, 96.6 and 96.1 were observed with Corynebacterium terpenotabidum (AB004730), Corynebacterium falsenii (Y13024) and Corynebacterium jeikeium (X84250), respectively.
3.2. Tests for symbiosis
Of the 60 first instar nymphs in Group 1 (bugs exposed to feces of adult T. infestans), 50 survived to month 12 (see Table 1). Faecal droplets collected from half of the insects at 12 months were positive by culture for the Corynebacterium. No other bacteria were isolated. By month 11, nine sexually mature females had produced 100 eggs. Thirty eight of the 40 insects in Group 2 (bugs fed the Corynebacterium via initial blood meals) survived to month 12. At month 11, 9 sexually mature females had produced 80 eggs.
Table 1.
Percent survival of experimental T. infestans at months 6 and 12 and percent of insects infected with the Corynebacterium at month 12
| Number surviving to 6 months (%) | Number surviving to 12 months (%) | Percentage of sampled insects infected with Corynebacterium (n) | |
|---|---|---|---|
| GROUP 1 (n = 60) | 58 (96.6) | 50 (83.3) | 100% (20) |
| GROUP 2 (n = 40) | 38 (95.0) | 38 (95.0) | 100% (19) |
| GROUP 3 (n = 40) | 36 (90.0) | 0 | N/A |
| GROUP 4a (n = 20) | 20 (100) | 0 | 0 (10) |
| GROUP 4b (n = 20) | 20 (100) | 20 (100) | 100% (10) |
Group 1 were kept in containers lined with filter paper stained by the feces of the adult T. infestans.
Group 2 were maintained in a sterile container but fed two blood meals supplemented with the Corynebacterium at a concentration of 1 × 106 CFU/ml.
Group 3 were kept in a sterile container and fed two blood meals supplemented with R. rhodnii at a concentration of 1 × 106 CFU/ml.
Group 4 were kept in a sterile container until the sixth month. Group 4a received a blood meal supplemented with the Corynebacterium at a concentration of 1 × 106 CFU/ml; Group 4b received a sterile blood meal.
None of the 40 nymphs in Group 3 (bugs fed R. rhodnii via initial blood meals) survived to month 12. About 90% of these insects reached the third or fourth instar stage by month 6 but all were dead by month 9. No eggs were produced. Cultures of the gut contents of these insects within 24 h of their death yielded no bacteria.
At month 6, all of the 40 nymphs in Group 4 (bugs maintained in sterile environment for 6 months) were alive and had reached the third to fourth instar stages. The 20 nymphs removed and fed the Corynebacterium (Group 4a) all survived to month 12; of these, 10 insects were sampled and found to be culture positive for the Corynebacterium. By month 12, 4 sexually mature females had produced 40 eggs. In contrast, insects that were not fed the Corynebacterium (Group 4b) died by month 12. No eggs were produced and all of the 10 insects sampled were negative by culture for the Corynebacterium.
There was no significant difference in survival to month 12 between the control group (n = 60) and the groups fed Corynebacterium at month 0 or month 6 (p = .085). The difference in survival to month 12 between the aposymbiotic group and the control group was highly significant (p ≪ .0001). Also significant was the difference in survival to month 12 between the group that was fed R. rhodnii and the control group (p ≪ .0001).
3.3. Measurement of DB3 activity in transformed Corynebacterium
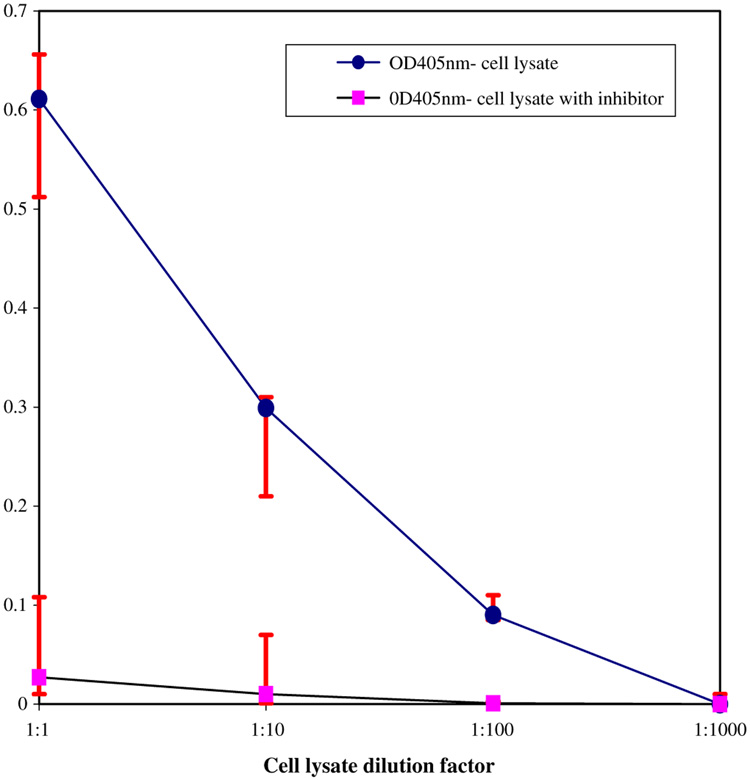
Fig. 1 provides evidence that rDB3 is being produced by the transformed Corynebacterium. The cell lysate binds strongly to the progesterone-coated plate, giving rise to an OD405nm of .59 ± .073. Serial dilutions of the lysate yield decreased binding to the progesterone-coated plate. When a competitive inhibitor, progesterone-3-CMO, is added to the lysate, binding to the progesterone-coated plate is drastically reduced. The lysate of the untransformed Corynebacterium does not bind to the progesterone-coated plate.
Fig. 1.
Binding of serial dilutions of the cell lysate from the Corynebacterium transformed with pRrMDWK6 to progesterone-coated wells. OD405 nm denotes readings obtained from an ELISA plate-reader. Inhibitor used was a 260 µM solution of progesterone-3-CMO. Binding of lysates to progesterone 3-CMO on the plates was markedly reduced in the presence of free progesterone as evidenced by greatly reduced OD405 nm values. Cell lysate of untransformed Corynebacterium did not bind progesterone, giving rise to an OD405 nm of 0.0 (not shown).
4. Discussion
This study establishes a Corynebacterial species as a symbiont of the T. infestans (Argentina) strain. This organism was isolated from an insectary colony of T. infestans as a monoculture, and is required for the maturation of the triatomine bug. It is therefore highly likely that this Corynebacterim sp. represents the intrinsic flora of T. infestans. The DNA sequence encoding the 16S ribosomal subunit of this bacterium shares 99% homology to corresponding 16S rRNA gene sequences of Corynebacterium sp. CNJ954 PL04 (Accession No. DQ448694), and Corynebacterium sp. BBH8 (Accession No. AM183334). It is interesting to note that both organisms were cultured from marine sediments. The 16S rRNA gene sequence of this Corynebacterium is available (GenBank Accession No. AF322369).
In the laboratory colonies of T. infestans used in this study, this bacterium is required for sexual maturation of nymphs. Nymphs maintained in the aposymbiotic state failed to mature beyond the fourth instar stage. Administration of the Corynebacterium to aposymbiotic nymphs at the first instar stage, either via a blood meal or via natural feces, allowed sexual maturation within 8 months, a rate comparable to the development of first instar nymphs in our insectary which are not aposymbiotic. Furthermore, administration of this bacterium to third and fourth instar aposymbiotic nymphs which were arrested in their development allowed completion of sexual maturation. R. rhodnii, a related nocardiform actinomycete which is a symbiont of R. prolixus (Baines, 1956), did not serve as a symbiont in this strain of T. infestans. Aposymbiotic first instar nymphs which were fed R. rhodnii via a blood meal suffered a growth arrest at the third and fourth instar stages and subsequently died, suggesting a specific relationship between the Corynebacterium and the Argentina strain of T. infestans.
The Corynebacterium transformed with the shuttle plasmid pRrMDWK6 was resistant to kanamycin and expressed an immunologically active single chain antibody, rDB3. Both the origin of replication, derived from R. rhodnii, and the MKα promoter for the rDB3 gene, derived from M. kansasii, were functional in the transformed Corynebacterium. Though we could not detect progesterone binding activity in cell-free supernatants of cultures of transformed Corynebacterium, assays of cell lysates were positive. Furthermore, binding activity decreased with serial dilutions of the cell lysate and was virtually eliminated by the addition of the competitive inhibitor, progesterone-3-CMO. No progesterone binding was detected in cell lysates from the untransformed Corynebacterium. These findings suggest that the progesterone binding activity observed in the transformed symbionts is due to expression of the functional single chain antibody, rDB3. We are currently modifying the signal peptidase recognition region of the MKα fragment to allow cleavage of the signal peptide and secretion of rDB3 from the transformed Corynebacterium.
This study establishes the foundation for generation of paratransgenic T. infestans. In vivo transgene expression by the engineered Corynebacterium in laboratory colonies of T. infestans is currently being assessed. We are also working on the transformation of symbiotic bacteria of other strains of T. infestans as well as the delivery of transformed symbionts to populations of T. infestans in simulated field settings. Comprehensive microbiology studies in the Argentine Chaco are underway to better understand the bacterial flora of T. infestans. Furthermore, single chain antibodies targeting key surface antigens of T. cruzi are in development; the rDB3 fragment is used in this study as a model for single chain antibody expression. These studies aim to develop a safe and effective strategy for delivery of molecules with anti-trypanosomal activity to field populations of T. infestans. Such a strategy could serve as a powerful tool to complement current insecticide-based control programs.
The use of trade names does not constitute endorsement by the US Public Health Service or The Centers for Disease Control and Prevention.
Abbreviations
- PCR
polymerase chain reaction
- rDNA
ribosomal deoxyribonucleic acid
- PBS
phosphate buffered saline
- BHI
brain–heart infusion
References
- Baines S. The role of the symbiotic bacteria in the nutrition of Rhodnius prolixus (Hemiptera) Journal of Experimental Biology. 1956;33:533–541. [Google Scholar]
- Beard CB, O’Neill SL, Tesh RB, Richards FF, Aksoy S. Modification of arthropod vector competence via symbiotic bacteria. Parasitology Today. 1993;9:179–183. doi: 10.1016/0169-4758(93)90142-3. [DOI] [PubMed] [Google Scholar]
- Beard CB, Durvasula RV, Richards FF. Bacterial symbiosis in arthropods and the control of disease transmission. Emerging Infectious Disease. 1998;4:581–591. doi: 10.3201/eid0404.980408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CB, Durvasula RV, Richards FF. Bacterial symbiont transformation in Chagas disease vectors. In: Handler AM, James AA, editors. Insect Transgenesis: Methods and Applications. Boca Raton: CRC Press; 2000. pp. 289–303. [Google Scholar]
- Coates CJ, Jasinskiene N, Miyashiro L, James AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proceedings of the National Academy of Sciences (USA) 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte J. A novel approach to preventing insect-borne diseases. The New England Journal of Medicine. 1997;337:785–786. doi: 10.1056/NEJM199709113371112. [DOI] [PubMed] [Google Scholar]
- Dasch GA, Weiss E, Chang K. Endosymbionts of insects. In: Krieg NR, editor. Bergey’s Manual of Systematic Bacteriology. vol. 1. Baltimore: Williams and Wilkins; 1984. pp. 811–833. [Google Scholar]
- Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Memórias do Instituto Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Gumbs A, Panackal A, Kruglov O, Aksoy S, Merrifield RB, Richards FF, Beard CB. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proceedings of the National Academy of Sciences (USA) 1997;94:3274–3278. doi: 10.1073/pnas.94.7.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula RV, Gumbs A, Panackal A, Kruglov O, Taneja J, Kang A, Cordon-Rosales C, Richards FF, Whitham R, Beard CB. Expression of a functional antibody fragment in the gut of Rhodnius prolixus via transgenic bacterial symbiont Rhodococcus rhodnii. Medical and Veterinary Entomology. 1999a;13:115–119. doi: 10.1046/j.1365-2915.1999.00175.x. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Kroger A, Goodwin M, Panackal A, Kruglov O, Taneja J, Gumbs A, Richards FF, Beard CB, Cordon-Rosales C. Strategy for introduction of foreign genes into field populations of Chagas disease vectors. Annals of the Entomological Society of America. 1999b;92:937–943. [Google Scholar]
- Durvasula RV, Taneja J. Maintenance of the Triatomine bugs Rhodnius prolixus and Triatoma dimidiata under laboratory conditions. In: Maramorosch K, Mahmood F, editors. Maintenance of Human Animal, and Plant Pathogen Vectors. Enfield: Science Publishers Inc.; 1999. pp. 139–157. [Google Scholar]
- Figueiro AR, Nunes ZG, Silvia AAL, Giordano-Dias CMG, Conra JR, Hofer E. Isolation of microorganisms of triatomines maintained in artificial and sylvatic conditions. Memorias do Instituto Oswaldo Cruz (Rio de Janeiro) 1995;90:228. [Google Scholar]
- Gürtler RE, Canale DM, Spillmann C, Stariolo R, Salomon OD, Blanco S, Segura EL. Effectiveness of residual spraying with deltamethrin and permethrin on peridomestic populations of Triatoma infestans in rural Western Argentina: a district-wide randomized trial. Bulletin of the World Health Organization. 2004;82:196–205. [PMC free article] [PubMed]
- Gürtler RE, Cecere MC, Lauricella MA, Petersen RM, Canale DM, Castañera MB, Chuit R, Segura EL, Cohen JE. Incidence of Trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural northwestern Argentina. American Journal of Tropical Medicine and Hygiene. 2005;73:95–103. [PMC free article] [PubMed] [Google Scholar]
- He M, Kang A, Hamon M, Humphreys AS, Gani M, Taussig MJ. Characterization of a progesterone binding, three-domain antibody fragment (VH/K) expressed in Escherichia coli. Immunology. 1991;84:662–668. [PMC free article] [PubMed] [Google Scholar]
- Jasinskiene N, Coates CJ, Benedict MQ, Cornel AJ, Rafferty CS, James AA, Collins FH. Stable transformation of the yellow fever mosquito, Aedes aegyptii, with the Hermes element from the housefly. Proceedings of the National Academy of Sciences (USA) 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematics. New York: John Wiley and Sons; 1991. pp. 116–163. [Google Scholar]
- Olson KE, Higgs S, Gaines PJ, Powers AM, Davis BS, Kamrud KI, Carlson JO, Blair CD, Beaty BJ. Genetically engineered resistance to dengue-2 virus transmission in mosquitoes. Science. 1996;272:884–886. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- Picollo MI, Vassena C, Santo Orihuela P, Barrios S, Zaidemberg M, Zerba E. High resistance to pyrethroid insecticides associated with ineffective field treatments in Triatoma infestans (Hemiptera: Reduviidae) from North Argentina. Journal of Medical Entomology. 2005;42:637–642. doi: 10.1093/jmedent/42.4.637. [DOI] [PubMed] [Google Scholar]
- Richards FF. An approach to reducing arthropod vector competence. American Society of Microbiology News. 1993;59:509–514. [Google Scholar]
- Schofield CJ, Dias JC. The southern cone initiative against Chagas disease. Advances in Parasitology. 1999;42:1–27. doi: 10.1016/s0065-308x(08)60147-5. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Vector resistance to pesticides. 15th Report of the WHO Expert Committee on Vector Biology and Control. World Health Organization Technical Report Series. 1992;818 [PubMed]